Screening and Functional Transformation Analysis of Genes Related to Skeletal Muscle Development in Supplemental-Fed Oula Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Selection of Animals
2.2. Library Construction and Sequencing
2.3. Data Quality Control and Sequence Alignment
2.4. Differential Gene Analysis
2.5. qPCR Validation
3. Results and Analysis
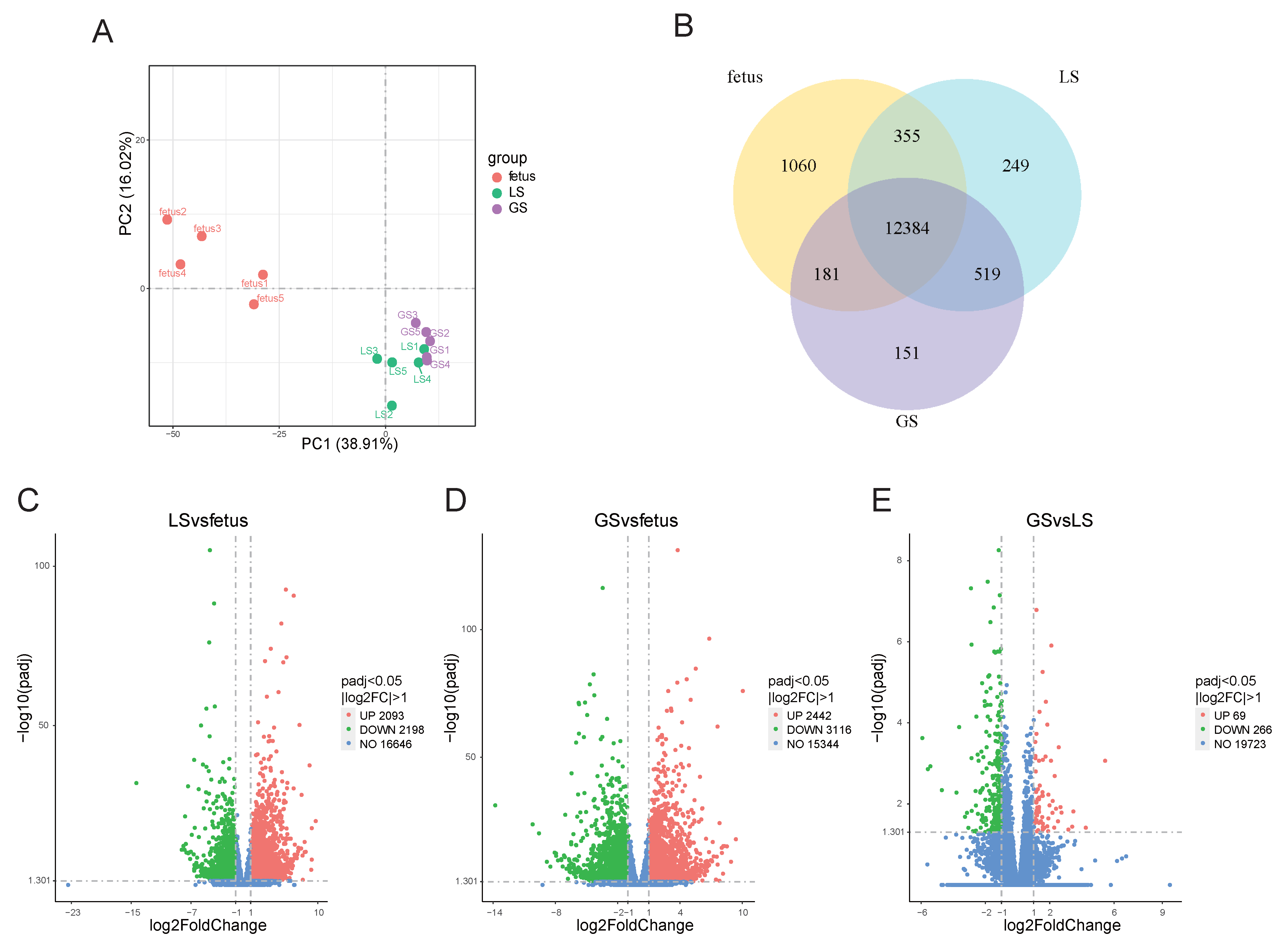
3.1. Analysis of Differentially Expressed Genes in Oula Sheep at Different Ages
3.2. STEM Analysis of Differential Genes in the Longissimus Dorsi Muscle of Oula Sheep
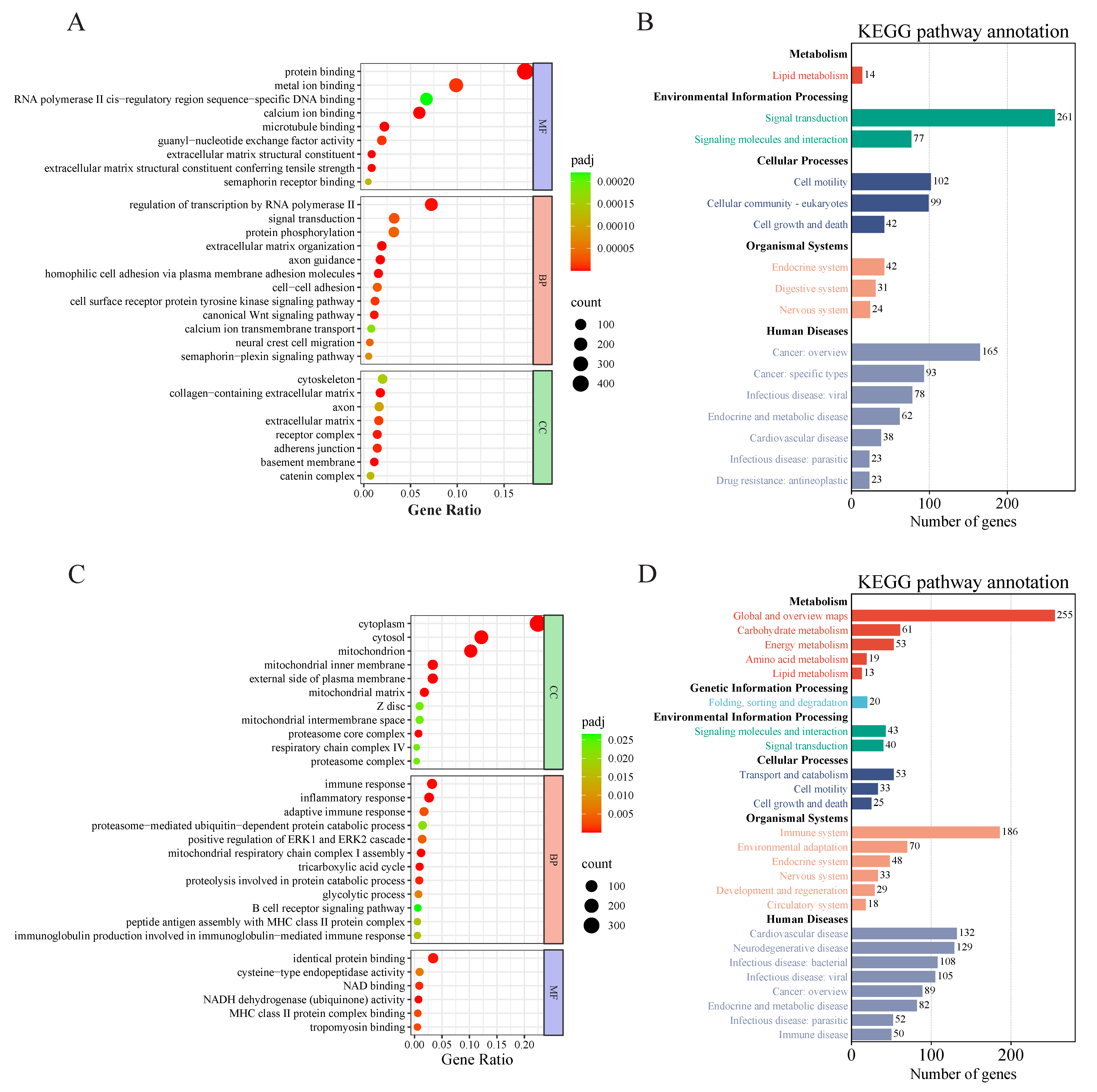
3.3. Differential Gene Enrichment Analysis of LS vs. Fetus
3.4. Differential Gene Enrichment Analysis of GS vs. Fetus
3.5. Differential Gene Enrichment Analysis of GS vs. LS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Zhao, L.; Li, Q.; Chen, H.; Zhou, H.; Xu, S.; Dong, Q.; Wu, G.; He, Y. Using balance of seasonal herbage supply and demand to inform sustainable grassland management on the Qinghai–Tibetan Plateau. Front. Agric. Sci. Eng. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Gui, L.-S.; Raza, S.H.A.; Ahmed Allam, F.A.E.; Zhou, L.; Hou, S.; Khan, I.; Kakar, I.U.; Abd El-Aziz, A.H.; Jia, J.; Sun, Y.; et al. Altered Milk Yield and Rumen Microbial Abundance in Response to Concentrate Supplementation during the Cold Season in Tibetan Sheep. Electron. J. Biotechnol. 2021, 53, 80–86. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.Z.; Zhang, J.S.; Gong, J.X.; Wang, Y.H.; Zhang, C.L.; Chen, H.; Fang, X.T. Effects of microRNAs on Skeletal Muscle Development. Gene 2018, 668, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Liu, A.; Wang, Q.; Wang, H.; Dong, D.; Liu, L. Transcriptome Analysis of Embryonic Muscle Development in Chengkou Mountain Chicken. BMC Genomics 2021, 22, 431. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Li, S.; Bao, G.; Wang, J.; Liu, X.; Hu, J.; Zhao, F.; Zhao, Z.; Shi, B.; Luo, Y. Comparative Transcriptome Analysis Reveals the Mechanism Associated With Dynamic Changes in Meat Quality of the Longissimus Thoracis Muscle in Tibetan Sheep at Different Growth Stages. Front. Vet. Sci. 2022, 9, 926725. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.-S.; Cheng, J.; Tuggle, C.; Dyck, M.; Canada, P.; Fortin, F.; Harding, J.; Plastow, G.; Dekkers, J. Genetic Analysis of the Blood Transcriptome of Young Healthy Pigs to Improve Disease Resilience. Genet. Sel. Evol. 2023, 55, 90. [Google Scholar] [CrossRef]
- Wu, C.; Li, J.; Xu, X.; Xu, Q.; Qin, C.; Liu, G.; Wei, C.; Zhang, G.; Tian, K.; Fu, X. Effect of the FA2H Gene on Cashmere Fineness of Jiangnan Cashmere Goats Based on Transcriptome Sequencing. BMC Genomics 2022, 23, 527. [Google Scholar] [CrossRef]
- Xu, L.; Liu, C.; Na, R.; Zhang, W.; He, Y.; Yuan, Y.; Zhang, H.; Han, Y.; Zeng, Y.; Si, W.; et al. Genetic Basis of Follicle Development in Dazu Black Goat by Whole-Transcriptome Sequencing. Animals 2021, 11, 3536. [Google Scholar] [CrossRef]
- Duckett, S.K.; Greene, M.A. Identification of microRNA Transcriptome Involved in Bovine Intramuscular Fat Deposition. Front. Vet. Sci. 2022, 9, 883295. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, H.; Liu, X.; Yang, Q.; Pan, C.; Lei, C.; Dang, R.; Chen, H.; Lan, X. The Muscle Development Transcriptome Landscape of Ovariectomized Goat. R. Soc. Open Sci. 2017, 4, 171415. [Google Scholar] [CrossRef]
- Yue, B.; Wang, J.; Ru, W.; Wu, J.; Cao, X.; Yang, H.; Huang, Y.; Lan, X.; Lei, C.; Huang, B.; et al. The Circular RNA CircHUWE1 Sponges the miR-29b-AKT3 Axis to Regulate Myoblast Development. Mol. Ther.-Nucleic Acids 2020, 19, 1086–1097. [Google Scholar] [CrossRef]
- Ali, M.; Baek, K.H.; Lee, S.-Y.; Kim, H.C.; Park, J.-Y.; Jo, C.; Jung, J.H.; Park, H.C.; Nam, K.-C. Comparative Meat Qualities of Boston Butt Muscles (M. Subscapularis) from Different Pig Breeds Available in Korean Market. Food Sci. Anim. Resour. 2021, 41, 71–84. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Li, L.; Ren, M.; Zhou, M.; Li, S. Transcriptome Profiling of Different Developmental Stages on Longissimus Dorsi to Identify Genes Underlying Intramuscular Fat Content in Wannanhua Pigs. Genes 2023, 14, 903. [Google Scholar] [CrossRef]
- Arora, R.; Siddaraju, N.K.; Manjunatha, S.S.; Sudarshan, S.; Fairoze, M.N.; Kumar, A.; Chhabra, P.; Kaur, M.; Sreesujatha, R.M.; Ahlawat, S.; et al. Muscle Transcriptome Provides the First Insight into the Dynamics of Gene Expression with Progression of Age in Sheep. Sci. Rep. 2021, 11, 22360. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhang, H. Extracellular Matrix: An Important Regulator of Cell Functions and Skeletal Muscle Development. Cell Biosci. 2021, 11, 65. [Google Scholar] [CrossRef]
- Huang, G.; Ge, G.; Wang, D.; Gopalakrishnan, B.; Butz, D.H.; Colman, R.J.; Nagy, A.; Greenspan, D.S. A3(V) Collagen Is Critical for Glucose Homeostasis in Mice Due to Effects in Pancreatic Islets and Peripheral Tissues. J. Clin. Investig. 2011, 121, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, Y.; Xu, J.; Yue, B.; Wen, Y.; Wang, X.; Lei, C.; Chen, H. Pleomorphic Adenoma Gene 1 (PLAG1) Promotes Proliferation and Inhibits Apoptosis of Bovine Primary Myoblasts through the PI3K-Akt Signaling Pathway. J. Anim. Sci. 2022, 100, skac098. [Google Scholar] [CrossRef] [PubMed]
- Deries, M.; Gonçalves, A.B.; Vaz, R.; Martins, G.G.; Rodrigues, G.; Thorsteinsdóttir, S. Extracellular Matrix Remodeling Accompanies Axial Muscle Development and Morphogenesis in the Mouse. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2012, 241, 350–364. [Google Scholar] [CrossRef]
- Stearns-Reider, K.M.; D’Amore, A.; Beezhold, K.; Rothrauff, B.; Cavalli, L.; Wagner, W.R.; Vorp, D.A.; Tsamis, A.; Shinde, S.; Zhang, C.; et al. Aging of the Skeletal Muscle Extracellular Matrix Drives a Stem Cell Fibrogenic Conversion. Aging Cell 2017, 16, 518–528. [Google Scholar] [CrossRef]
- Berger, J.; Hall, T.E.; Currie, P.D. Novel Transgenic Lines to Label Sarcolemma and Myofibrils of the Musculature. Zebrafish 2015, 12, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Al Barashdi, M.A.; Ali, A.; McMullin, M.F.; Mills, K. Protein Tyrosine Phosphatase Receptor Type C (PTPRC or CD45). J. Clin. Pathol. 2021, 74, 548–552. [Google Scholar] [CrossRef]
- LaBerge, G.S.; Duvall, E.; Grasmick, Z.; Haedicke, K.; Galan, A.; Leverett, J.; Baswan, S.; Yim, S.; Pawelek, J. Recent Advances in Studies of Skin Color and Skin Cancer. YALE J. Biol. Med. 2020, 93, 69–80. [Google Scholar]
- Li, Y.; Chen, X.; Zhang, R.; Chen, M.; Shen, J.; Wu, J.; Yu, J.; Sun, Q. Correlation Analysis of Lipid Metabolism Genes with the Immune Microenvironment in Gastric Cancer and the Construction of a Novel Gene Signature. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2023, 25, 1315–1331. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, Metaflammation and Immunometabolic Disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Gonzalez, M.B.; Lane, M.; Knight, E.J.; Robker, R.L. Inflammatory Markers in Human Follicular Fluid Correlate with Lipid Levels and Body Mass Index. J. Reprod. Immunol. 2018, 130, 25–29. [Google Scholar] [CrossRef]
- de-Lima-Júnior, J.C.; Souza, G.F.; Moura-Assis, A.; Gaspar, R.S.; Gaspar, J.M.; Rocha, A.L.; Ferrucci, D.L.; Lima, T.I.; Victório, S.C.; Bonfante, I.L.P.; et al. Abnormal Brown Adipose Tissue Mitochondrial Structure and Function in IL10 Deficiency. EBioMedicine 2019, 39, 436–447. [Google Scholar] [CrossRef]
- Zhang, R.; Hou, T.; Cheng, H.; Wang, X. NDUFAB1 Protects against Obesity and Insulin Resistance by Enhancing Mitochondrial Metabolism. FASEB J. 2019, 33, 13310–13322. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhou, K.; Zhang, J.; Ling, X.; Zhang, X.; Li, P.; Zhang, L.; Wei, Q.; Zhang, T.; Xie, K.; et al. Transcriptome Integration Analysis at Different Embryonic Ages Reveals Key lncRNAs and mRNAs for Chicken Skeletal Muscle. Front. Vet. Sci. 2022, 9, 908255. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, Y.; Zhang, S.; Wu, Z.; Lu, X.; Liu, W.; Liu, B.; Zhou, X. Smartphone-based Digital Phenotyping for Genome-wide Association Study of Intramuscular Fat Traits in Longissimus Dorsi Muscle of Pigs. Anim. Genet. 2024, 55, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Zhang, R.; Jian, C.; Ding, W.; Wang, Y.; Ling, S.; Ma, Q.; Hu, X.; Cheng, H.; Wang, X. NDUFAB1 Confers Cardio-Protection by Enhancing Mitochondrial Bioenergetics through Coordination of Respiratory Complex and Supercomplex Assembly. Cell Res. 2019, 29, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; You, W.; Wang, Y.; Shan, T. The Regulatory Role of Myomaker and Myomixer–Myomerger–Minion in Muscle Development and Regeneration. Cell. Mol. Life Sci. 2020, 77, 1551–1569. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.-M.; You, C.; Byun, Y.J.; Kang, H.W.; Noh, J.; Lee, J.; Lee, H.Y.; Kim, K.; Kim, W.T.; Yun, S.J.; et al. Prognostic Value of BUB1 for Predicting Non-Muscle-Invasive Bladder Cancer Progression. Int. J. Mol. Sci. 2021, 22, 12756. [Google Scholar] [CrossRef]
- Zhou, X.; Yuan, Y.; Kuang, H.; Tang, B.; Zhang, H.; Zhang, M. BUB1B (BUB1 Mitotic Checkpoint Serine/Threonine Kinase B) Promotes Lung Adenocarcinoma by Interacting with Zinc Finger Protein ZNF143 and Regulating Glycolysis. Bioengineered 2022, 13, 2471–2485. [Google Scholar] [CrossRef]
- Yoshida, S.; Ikedo, A.; Yanagihara, Y.; Sakaue, T.; Saeki, N.; Imai, Y. Bub1 Suppresses Inflammatory Arthritis–Associated Bone Loss in Mice through Inhibition of TNFα–Mediated Osteoclastogenesis. J. Bone Miner. Res. 2024, 39, 341–356. [Google Scholar] [CrossRef]
- Elowe, S.; Bolanos-Garcia, V.M. The Spindle Checkpoint Proteins BUB1 and BUBR1: (SLiM)Ming down to the Basics. Trends Biochem. Sci. 2022, 47, 352–366. [Google Scholar] [CrossRef]
- Singh, P.; Pesenti, M.E.; Maffini, S.; Carmignani, S.; Hedtfeld, M.; Petrovic, A.; Srinivasamani, A.; Bange, T.; Musacchio, A. BUB1 and CENP-U, Primed by CDK1, Are the Main PLK1 Kinetochore Receptors in Mitosis. Mol. Cell 2021, 81, 67–87.e9. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Tanaka, T.; Mulati, M.; Ochi, H.; Sato, S.; Kaldis, P.; Yoshii, T.; Okawa, A.; Inose, H. Cyclin-Dependent Kinase 1 Is Essential for Muscle Regeneration and Overload Muscle Fiber Hypertrophy. Front. Cell Dev. Biol. 2020, 8, 564581. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.; Pang, J.S.; Lin, L.; Yu, T.; Tsai, W. Leukocyte-poor Platelet-rich Plasma and Leukocyte-rich Platelet-rich Plasma Promote Myoblast Proliferation through the Upregulation of Cyclin A, Cdk1, and Cdk2. J. Orthop. Res. 2024, 42, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, J.; Yu, S.; Yu, H.; Kuraz, A.B.; Jilo, D.D.; Cheng, G.; Li, A.; Jia, C.; Zan, L. Knockdown of NFIC Promotes Bovine Myoblast Proliferation through the CENPF/CDK1 Axis. J. Agric. Food Chem. 2024, 72, 12641–12654. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Wang, M.; Wu, J.; Shi, X. Tceal7 Regulates Skeletal Muscle Development through Its Interaction with Cdk1. Int. J. Mol. Sci. 2023, 24, 6264. [Google Scholar] [CrossRef]
- Gerassimov, N.; Crain, C.; Bilyeu, C.; Jacob, A.; Fan, C. Examining the Lineage Autonomous Role of Β3-integrin in Muscle Regeneration. FASEB J. 2022, 36, e22385. [Google Scholar] [CrossRef]
- Yu, D.; Li, Z.; Cao, J.; Shen, F.; Wei, G. microRNA-25-3p Suppresses Osteogenic Differentiation of BMSCs in Patients with Osteoporosis by Targeting ITGB3. Acta Histochem. 2022, 124, 151926. [Google Scholar] [CrossRef]
- De Las Heras-Saldana, S.; Chung, K.Y.; Kim, H.; Lim, D.; Gondro, C.; Van Der Werf, J.H.J. Differential Gene Expression in Longissimus Dorsi Muscle of Hanwoo Steers—New Insight in Genes Involved in Marbling Development at Younger Ages. Genes 2020, 11, 1381. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Niu, A.; Chen, S.-E.; Li, Y.-P. Beta3-Integrin Mediates Satellite Cell Differentiation in Regenerating Mouse Muscle. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 1914–1921. [Google Scholar] [CrossRef]
- Li, G.; Qiu, J.; Lu, X.; Jin, Y. ITGB2 Fosters the Cancerous Characteristics of Ovarian Cancer Cells through Its Role in Mitochondrial Glycolysis Transformation. Aging 2024, 16, 3007–3020. [Google Scholar] [CrossRef] [PubMed]




| Ingredients | Contents % | Nutrient Levels (2) | Contents % |
|---|---|---|---|
| Corn | 39.92 | CP | 15.09 |
| Soybean meal | 12.99 | ME (MJ/kg) | 2.62 |
| Wheat bran | 7.73 | Ca | 0.79 |
| Rapeseed cake | 6.93 | TP | 0.4 |
| Limestone | 1.42 | Lys | 0.67 |
| Premix (1) | 1.01 | Met | 0.26 |
| Dried corn stalk | 30.00 | NDF | 33.34 |
| Total | 100.00 | ADF | 19.79 |
| Sample | Raw Reads Number | Cleanreads Number | Cleanreadsrate (%) | Clean Q30 Basesrate (%) | GC Content (%) | Mappingratio (%) | Uniquemappingratio (%) |
|---|---|---|---|---|---|---|---|
| fetus1 | 47,639,868 | 45,509,926 | 95.53 | 93.84 | 52.58 | 96.06 | 87.25 |
| fetus2 | 47,164,860 | 44,679,420 | 94.73 | 92.00 | 52.62 | 94.36 | 87.82 |
| fetus3 | 47,697,680 | 45,048,138 | 94.45 | 92.34 | 52.31 | 94.73 | 87.94 |
| fetus4 | 45,614,790 | 42,633,336 | 93.46 | 91.94 | 51.86 | 94.38 | 87.19 |
| fetus5 | 43,742,352 | 40,576,428 | 92.76 | 92.94 | 51.73 | 94.94 | 85.76 |
| LS1 | 41,212,104 | 38,519,372 | 91.71 | 91.99 | 51.8 | 94.64 | 83.34 |
| LS2 | 46,013,906 | 43,545,542 | 93.24 | 92.17 | 49.36 | 95.43 | 81.43 |
| LS3 | 45,748,468 | 43,155,138 | 98.15 | 92.39 | 52.42 | 94.87 | 84.31 |
| LS4 | 45,241,120 | 41,792,798 | 98.15 | 92.5 | 49.01 | 94.89 | 80.92 |
| LS5 | 46,274,008 | 43,830,938 | 97.26 | 92.06 | 51.92 | 94.95 | 83.81 |
| GS1 | 43,475,578 | 39,881,436 | 91.73 | 93.4 | 51.23 | 95.01 | 82.66 |
| GS2 | 48,228,642 | 45,179,514 | 93.68 | 91.42 | 50.91 | 94.66 | 81.91 |
| GS3 | 47,582,042 | 45,289,166 | 95.18 | 92.23 | 51.92 | 95.32 | 84.98 |
| GS4 | 48,922,814 | 45,936,318 | 93.90 | 92.41 | 50.03 | 94.93 | 80.61 |
| GS5 | 50,433,518 | 49,632,898 | 98.41 | 93.39 | 52.25 | 96.45 | 85.27 |
| Gene | Accession No. | Primer Sequence (5′-3′) | Tm/°C | Size/bp |
|---|---|---|---|---|
| PTPRC | XM_060396233.1 | F CTGCAGAACCCAAAGAATTGGTC R GGATGAGAAGAGGCACATTCCTG | 61 | 111 |
| IL10 | XM_060395938.1 | F GGGAAGGAGACCTCCAGGAT R AGGGGAGAGGCACAGTAGAG | 60 | 122 |
| NDUFAB1 | XM_027961453.2 | F CAGTGCATCCTGGGTCGG R GAAGAGGGTAGTGCTGAGCG | 60 | 136 |
| BUB1 | XM_012173898.5 | F TCAGAGGGTCTTCCCCATTAC R GATCACAGTAAGCTGTCTATCCTG | 59 | 284 |
| CDK1 | NM_001142508.1 | F CCTGCCAAACGAATTTCTGGC R CTGCTCTTGACACAACACAGG | 60 | 117 |
| BUB1B | XM_042252264.1 | F GGAACAAGGAAGGTCTAAGGGT R ACATGAGTGGTTATGACTTTCTGT | 60 | 299 |
| ITGB3 | XM_060395754.1 | F CATGTATGCGCCATCCTCCT R CGTTCAGCAAGCCTCATCCT | 60 | 128 |
| ITGB2 | NM_001009485.1 | F GAGAAGCTGAGGAACCCCTG R GCTTCCCGACTTCTGTCTCG | 60 | 118 |
| GAPDH | XM_015097299.4 | F ACGACCACCCTATCCAGGTT R AGGTCCTGGATGGAGGCTT | 60 | 271 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, Y.; Yan, M.; Wu, S.; Liu, M.; Khan, R. Screening and Functional Transformation Analysis of Genes Related to Skeletal Muscle Development in Supplemental-Fed Oula Sheep. Animals 2025, 15, 3040. https://doi.org/10.3390/ani15203040
Li Y, Wang Y, Yan M, Wu S, Liu M, Khan R. Screening and Functional Transformation Analysis of Genes Related to Skeletal Muscle Development in Supplemental-Fed Oula Sheep. Animals. 2025; 15(20):3040. https://doi.org/10.3390/ani15203040
Chicago/Turabian StyleLi, Yumeng, Yanhao Wang, Mingyi Yan, Sen Wu, Meng Liu, and Rajwali Khan. 2025. "Screening and Functional Transformation Analysis of Genes Related to Skeletal Muscle Development in Supplemental-Fed Oula Sheep" Animals 15, no. 20: 3040. https://doi.org/10.3390/ani15203040
APA StyleLi, Y., Wang, Y., Yan, M., Wu, S., Liu, M., & Khan, R. (2025). Screening and Functional Transformation Analysis of Genes Related to Skeletal Muscle Development in Supplemental-Fed Oula Sheep. Animals, 15(20), 3040. https://doi.org/10.3390/ani15203040






