Island vs. Mainland: Genetic Divergence of Calotes versicolor (Daudin, 1802) (Squamata: Agamidae) in Thailand
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
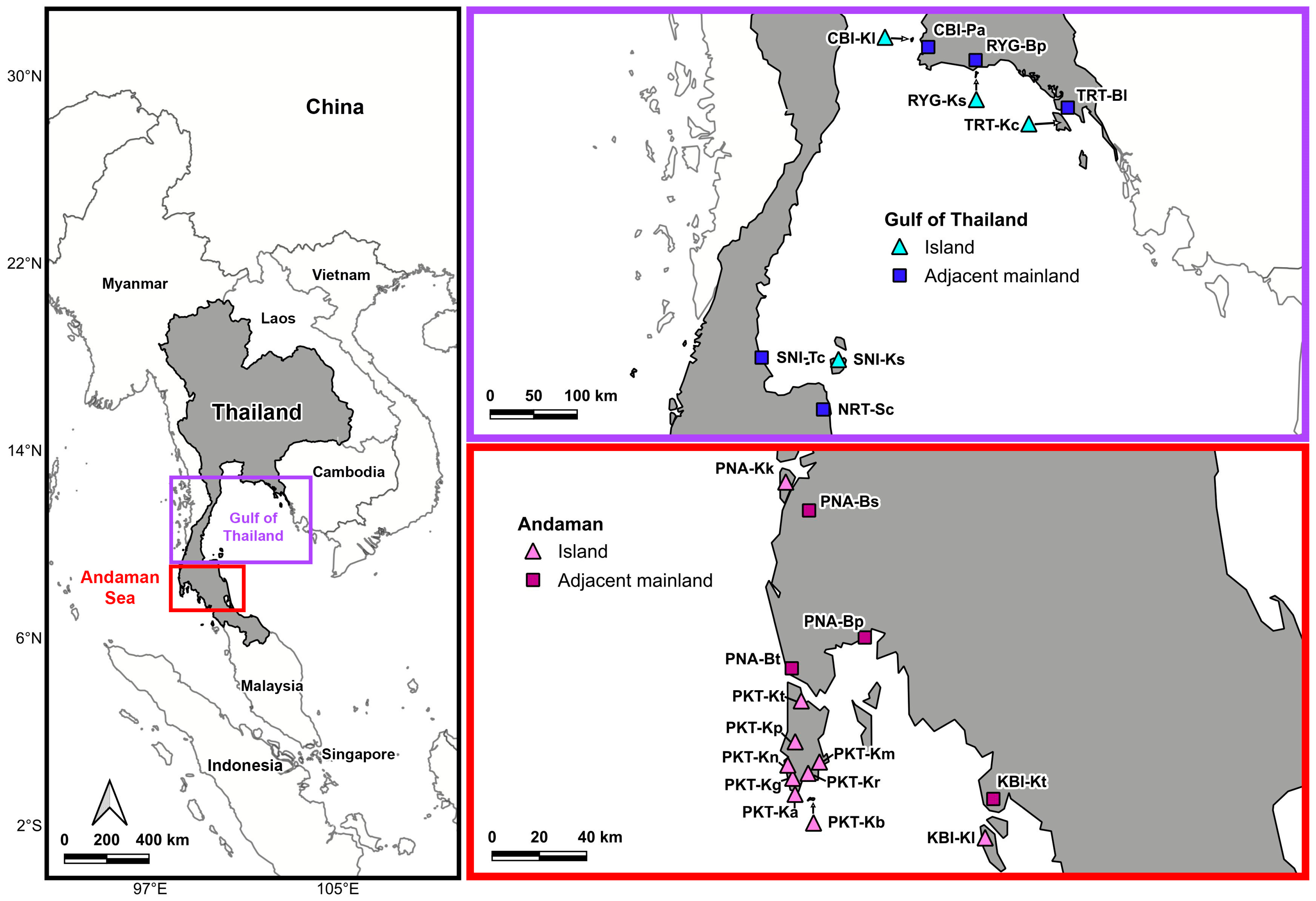
2.1. Sample Collection
2.2. Molecular Analysis
2.3. Data Analyses
2.4. Time-Calibrated Phylogenetic Tree
2.5. Species Delimitation Analysis
3. Results
3.1. CO1 Sequence Variation
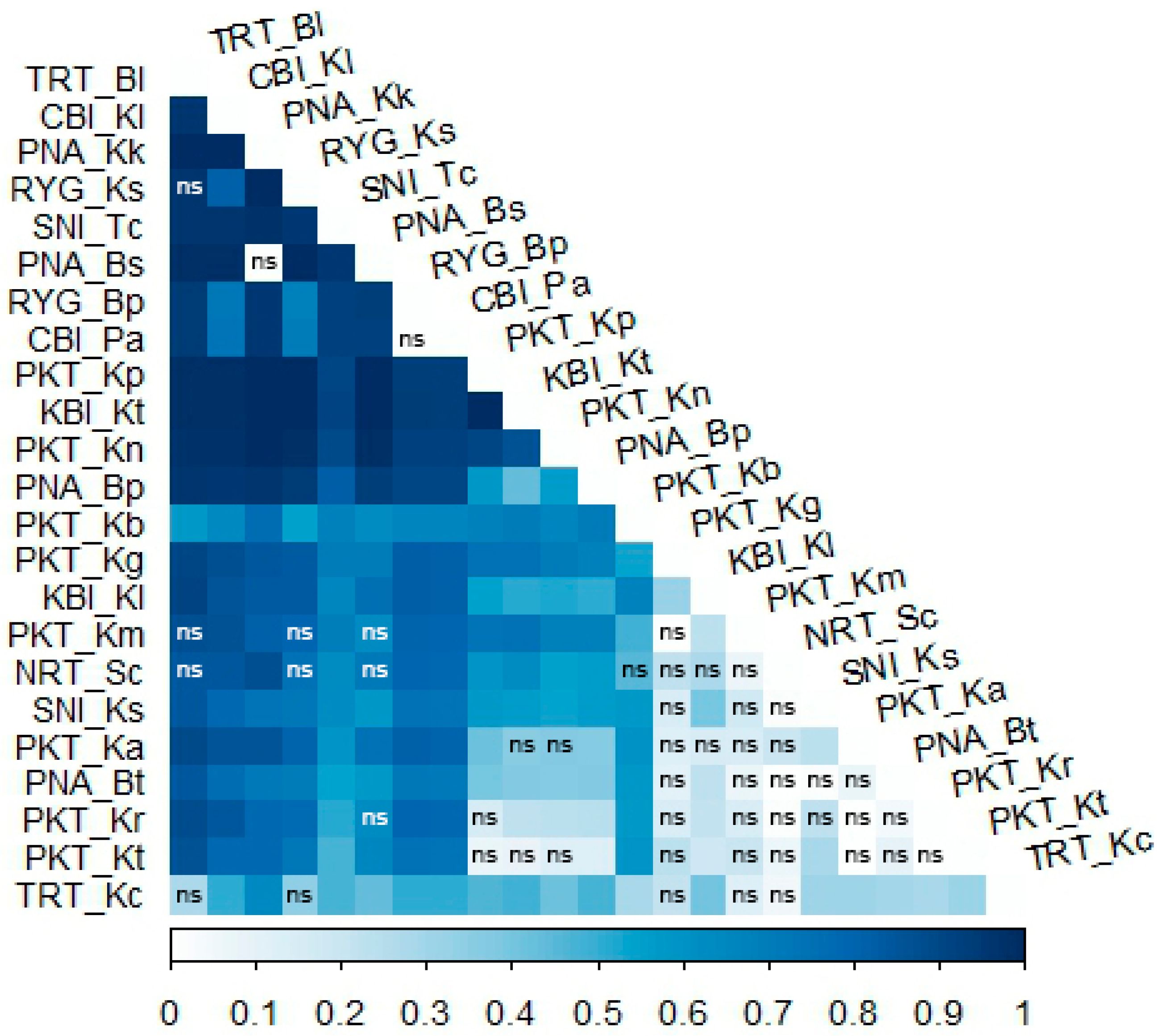
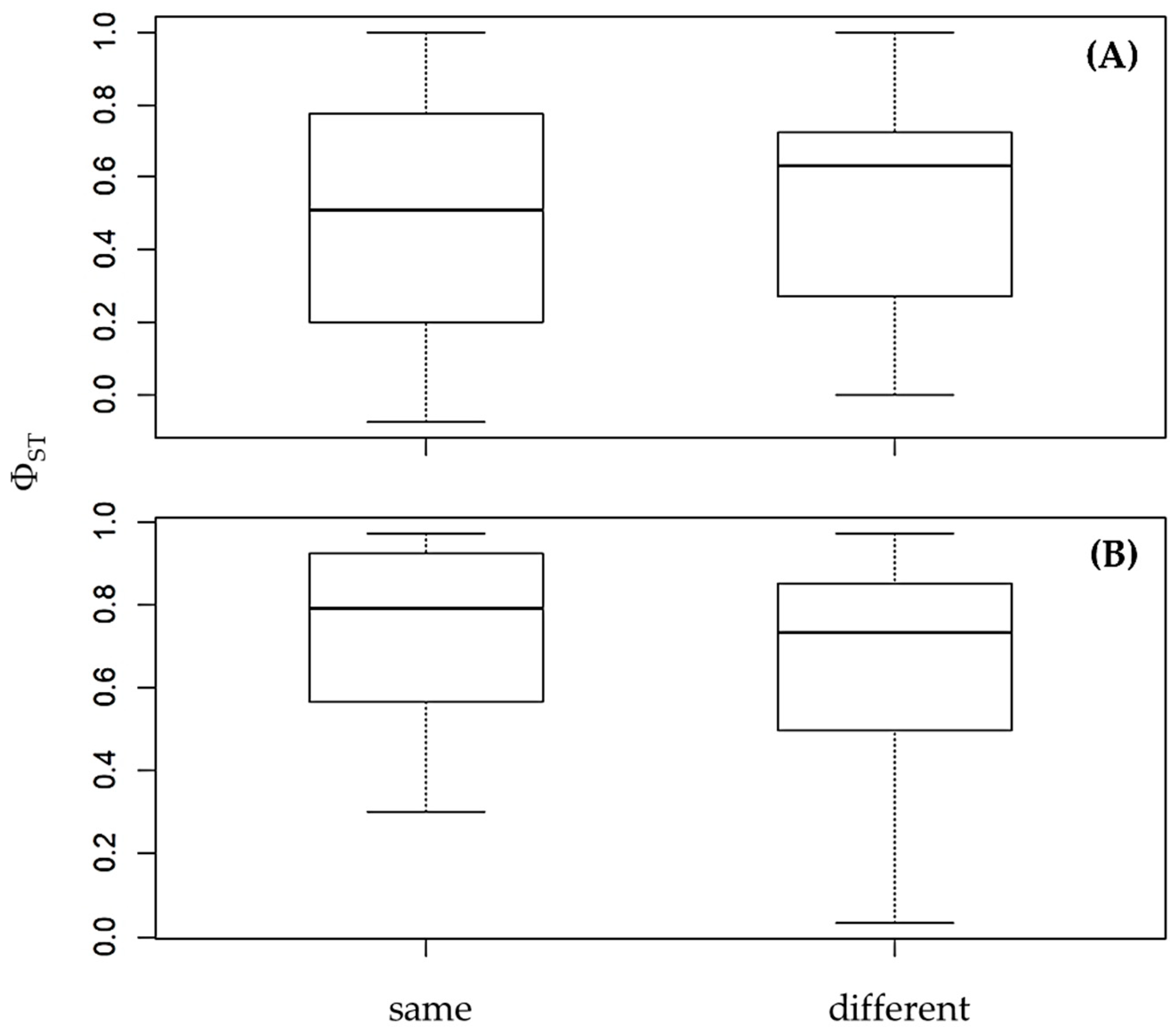
3.2. Genetic Differentiation
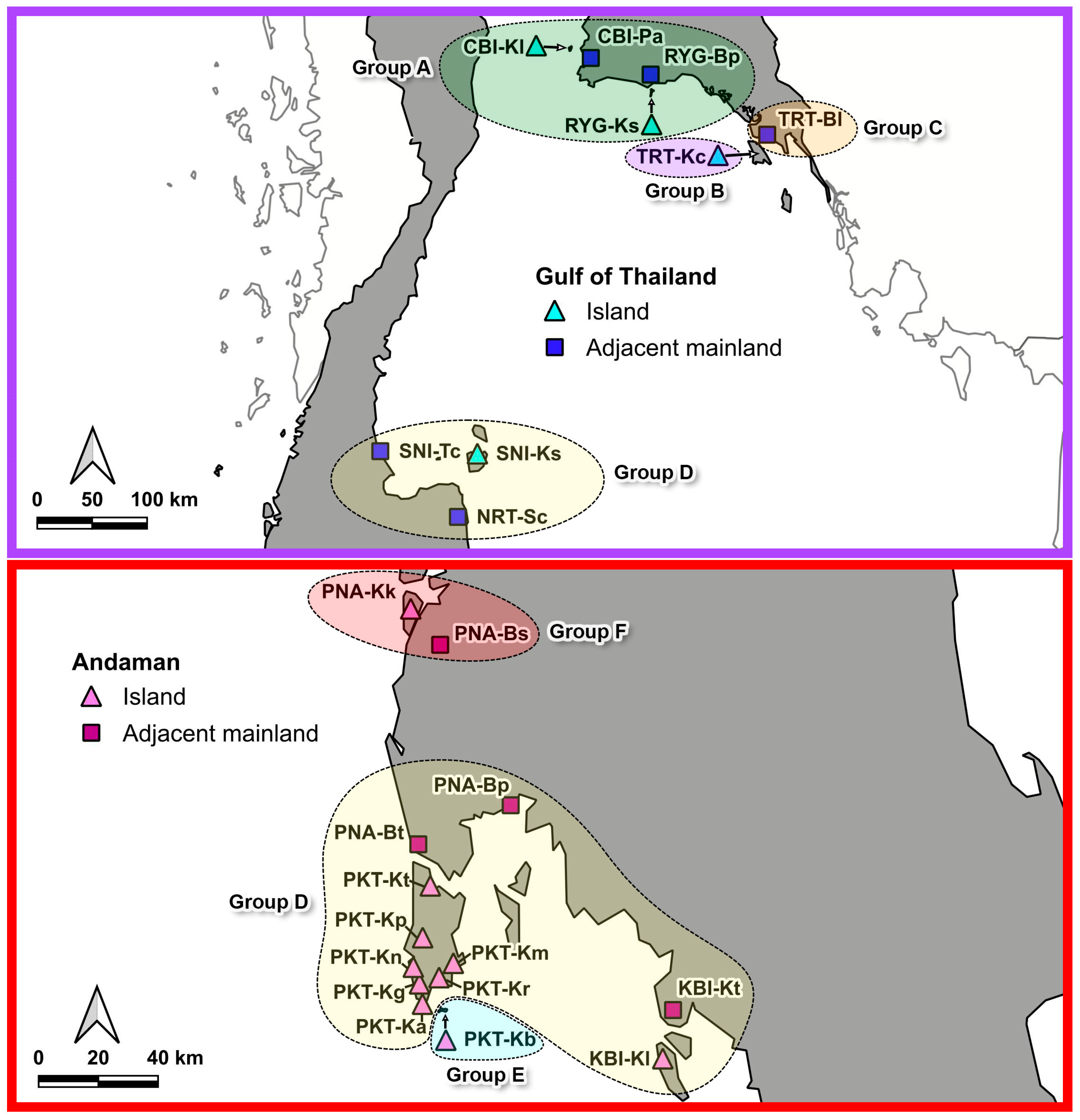
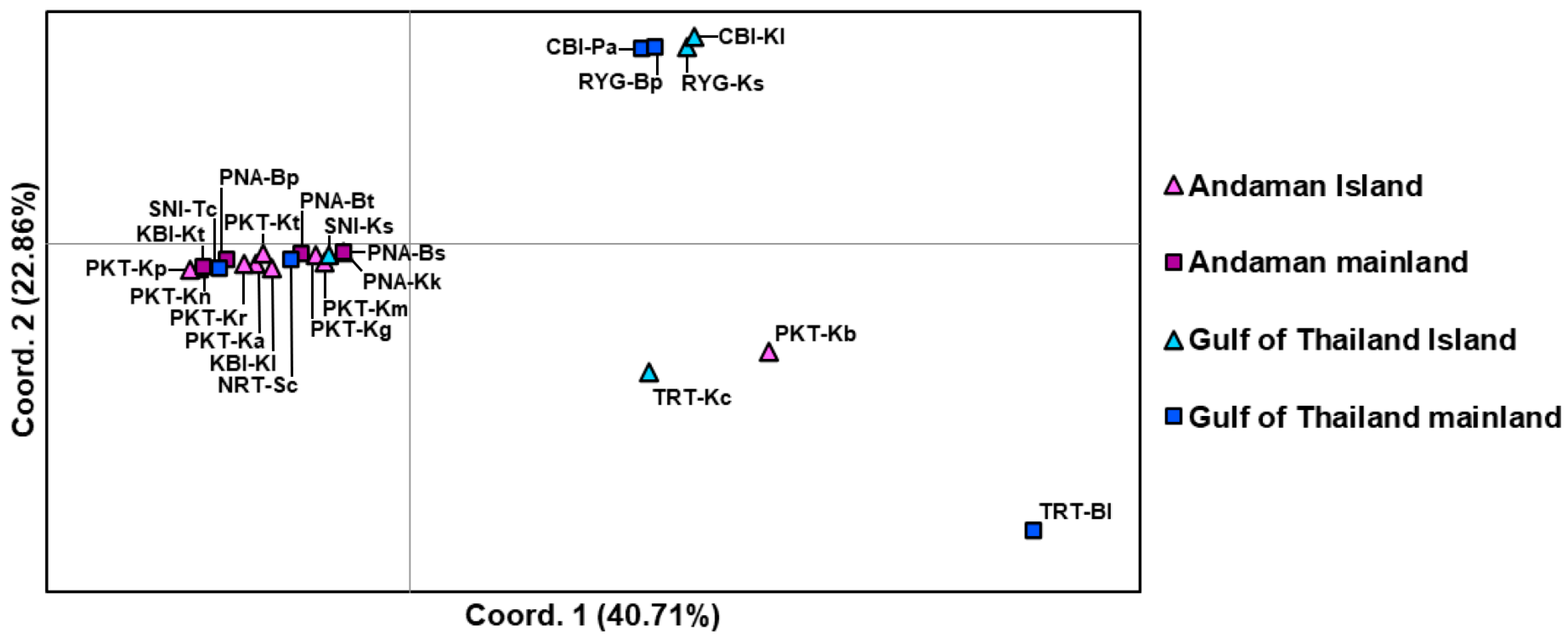
3.3. Genetic Structure
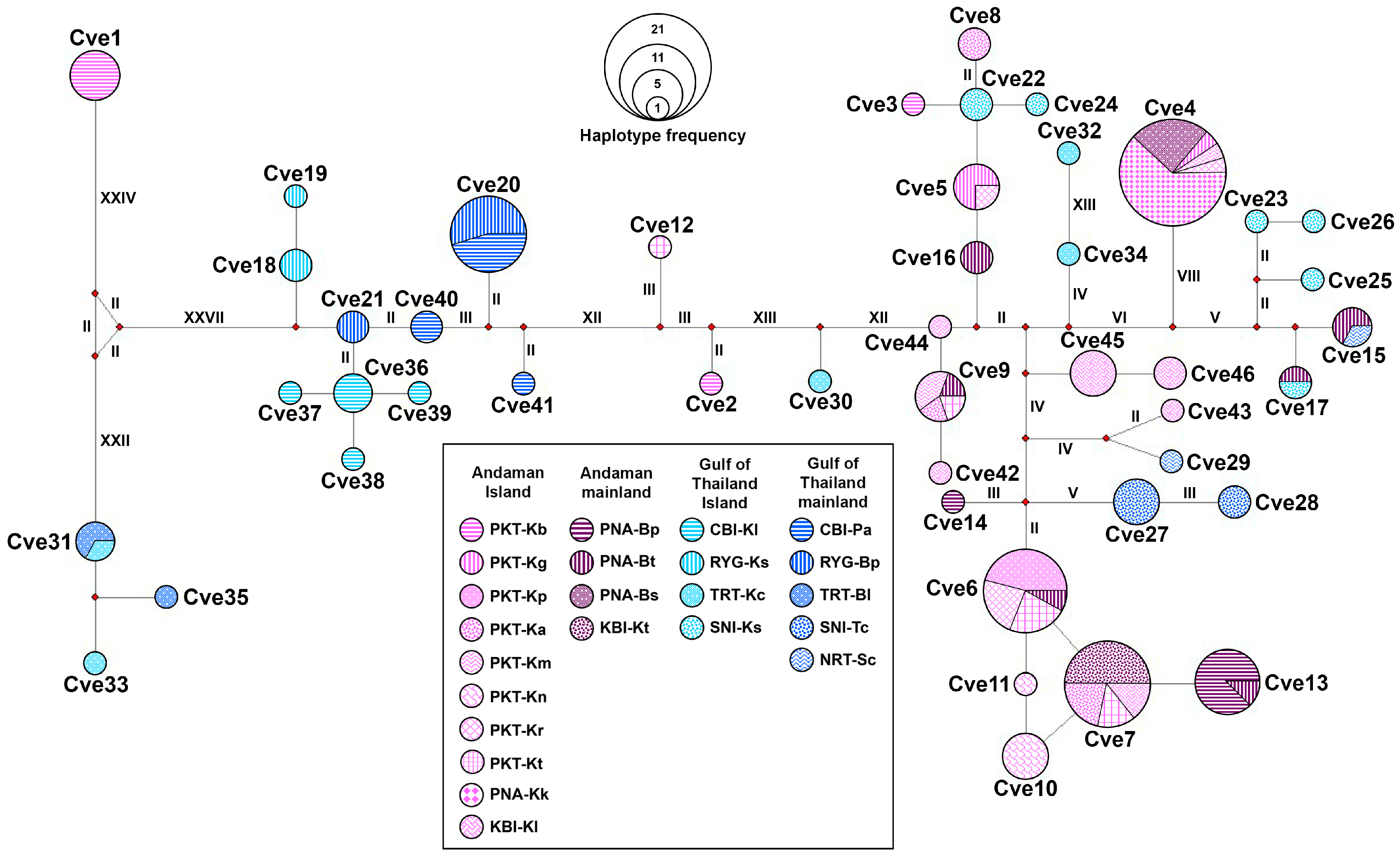
3.4. Haplotype Network
3.5. Divergence Time and Species Delimitation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, K.P.; Adler, F.R.; Cherry, J.L. Genetic and Phylogenetic Consequences of Island Biogeography. Evolution 2000, 54, 387–396. [Google Scholar] [CrossRef]
- Losos, J.B.; Ricklefs, R.E. Adaptation and Diversification on Islands. Nature 2009, 457, 830–836. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; 13th printing; Princeton University Press: Princeton, NJ, USA, 2001; ISBN 978-0-691-08836-5. [Google Scholar]
- Clegg, S.M.; Degnan, S.M.; Kikkawa, J.; Moritz, C.; Estoup, A.; Owens, I.P.F. Genetic Consequences of Sequential Founder Events by an Island-Colonizing Bird. Proc. Natl. Acad. Sci. USA 2002, 99, 8127–8132. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, R.S.; Surget-Groba, Y.; Johansson, H. The Relative Importance of Ecology and Geographic Isolation for Speciation in Anoles. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 3071–3081. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.B.; Round, P.D.; Woodruff, D.S. The Indochinese–Sundaic Faunal Transition at the Isthmus of Kra: An Analysis of Resident Forest Bird Species Distributions. J. Biogeogr. 2003, 30, 569–580. [Google Scholar] [CrossRef]
- Grismer, L.L.; Quah, E.S.H.; Duzulkafly, Z.; Yambun, P. On the Taxonomy of Lygosoma bampfyldei Bartlett, 1895 (Squamata: Scincidae) with Descriptions of New Species from Borneo and Peninsular Malaysia and the Resurrection of Lygosoma schneideri Werner, 1900. Zootaxa 2018, 4438, 528–550. [Google Scholar] [CrossRef]
- Borah, P.K.; Grismer, L.L.; Das, A.; Purkayastha, J.; Deuti, K.; Lalremsanga, H.T.; Datta-Roy, A. What Is Sphenomorphus maculatus (Blyth, 1854 “1853”) (Reptilia: Squamata: Scincidae)? Validating Cryptic Diversity and the Designation of a Neotype. Zootaxa 2024, 5543, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Gowande, G.; Mishra, A.; Mirza, Z.A. Neotype Designation for Calotes versicolor Daudin, 1802 (Sauria: Agamidae) with Notes on Its Systematics. Zootaxa 2016, 4126, 271–279. [Google Scholar] [CrossRef]
- Radder, R.S. An Overview of Geographic Variation in the Life History Traits of the Tropical Agamid Lizard, Calotes versicolor. Curr. Sci. 2006, 91, 1354–1363. [Google Scholar]
- Baig, K.J.; Wagner, P.; Ananjeva, N.B.; Böhme, W. A Morphology-Based Taxonomic Revision of Laudakia Gray, 1845 (Squamata: Agamidae). Vertebr. Zool. 2012, 62, 213–260. [Google Scholar] [CrossRef]
- Gowande, G.; Pal, S.; Jablonski, D.; Masroor, R.; Phansalkar, P.U.; Dsouza, P.; Jayarajan, A.; Shanker, K. Molecular Phylogenetics and Taxonomic Reassessment of the Widespread Agamid Lizard Calotes versicolor (Daudin, 1802) (Squamata, Agamidae) across South Asia. Vertebr. Zool. 2021, 71, 669–696. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, X.; Ho, S.Y.W.; Shi, H.; Li, J.; Li, J.; Cai, B.; Wang, Y. Diversification and Demography of the Oriental Garden Lizard (Calotes versicolor) on Hainan Island and the Adjacent Mainland. PLoS ONE 2013, 8, e64754. [Google Scholar] [CrossRef]
- Hussain, J.; Afzal, G.; Haider, M.Z.; Qadeer, I.; Perveen, S.; Ahmad, H.I.; El-Mansi, A.A.; Gadallah, A.A.; Abass, K.S. Genetic Diversity and Phylogenetic Relationships of Calotes and Uromastyx in the Cholistan Desert, Pakistan, Based on COI Gene Analysis. PLoS ONE 2025, 20, e0324053. [Google Scholar] [CrossRef]
- Tantrawatpan, C.; Thongnetr, W.; Pilap, W.; Suksavate, W.; Agatsuma, T.; Tawong, W.; Petney, T.N.; Saijuntha, W. Genetic Diversity and Population Structure of the Oriental Garden Lizard, Calotes versicolor Daudin, 1802 (Squamata: Agamidae) along the Mekong River in Thailand and Lao PDR. Asian Herpetol. Res. 2021, 12, 49–57. [Google Scholar]
- Zhan, L.; Chen, Y.; He, J.; Guo, Z.; Wu, L.; Storey, K.B.; Zhang, J.; Yu, D. The Phylogenetic Relationships of Major Lizard Families Using Mitochondrial Genomes and Selection Pressure Analyses in Anguimorpha. Int. J. Mol. Sci. 2024, 25, 8464. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, H.; Wang, Y.; Li, M.; Hou, M.; Cai, B. Taxonomic Review of the Calotes versicolor Complex (Agamidae, Sauria, Squamata) in China, with Description of a New Species and Subspecies. ZooKeys 2023, 1187, 63–89. [Google Scholar] [CrossRef]
- Bennett, D. Expedition Field Techniques: Reptiles and Amphibians; Geography Outdoors; Royal Geographical Society with IBG: London, UK, 1999; pp. 35–40. ISBN 978-0-907649-81-6. [Google Scholar]
- Koutsokali, M.; Dianni, C.; Valahas, M. Buccal Swabs as an Effective Alternative to Traditional Tissue Sampling Methods for DNA Analyses in Chamaeleonidae. Wildl. Biol. 2023, 2023, e01052. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X Version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Librado, P.; Rozas, J. DnaSP v5: A Software for Comprehensive Analysis of DNA Polymorphism Data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Rohl, A. Median-Joining Networks for Inferring Intraspecific Phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin Suite Ver 3.5: A New Series of Programs to Perform Population Genetics Analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Dupanloup, I.; Schneider, S.; Excoffier, L. A simulated annealing approach to define the genetic structure of populations. Mol. Ecol. 2002, 11, 2571–2581. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. genalex 6: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: http://www.R-project.org/ (accessed on 25 July 2025).
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest v2; Program distributed by the author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Rambaut, A. Figtree ver 1.4.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2018. [Google Scholar]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef]
- Vences, M.; Miralles, A.; Brouillet, S.; Ducasse, J.; Fedosov, A.; Kharchev, V.; Kostadinov, I.; Kumari, S.; Patmanidis, S.; Scherz, M.D.; et al. iTaxoTools 0.1: Kickstarting a specimen-based software toolkit for taxonomists. Megataxa 2021, 6, 77–92. [Google Scholar] [CrossRef]
- Saijuntha, W.; Khumkratok, S.; Wongpakam, K.; Thanonkeo, S.; Senakhun, C.; Appamaraka, S.; Yodsiri, S.; Thongnetr, W.; Pilap, W.; Kongbuntad, W.; et al. Genetic Diversity and Population Structure of Blue-Crested Lizard, Calotes mystaceus Duméril & Bibron, 1837 (Squamata: Agamidae) in Thailand. J. Genet. 2017, 96, 377–382. [Google Scholar] [CrossRef]
- Saijuntha, W.; Tantrawatpan, C.; Pilap, W.; Sedlak, S.; Sakdakham, K.; Sripirom, P.; Suksavate, W.; Kongbuntad, W.; Tawong, W. Genetic Differentiation of the Forest Crested Lizards, Calotes emma alticristatus Schmidt, 1925 and C. emma emma Gray, 1845 (Squamata: Agamidae) Relating to Climate Zones in Thailand. Asian Herpetol. Res. 2020, 11, 19–27. [Google Scholar]
- Voris, H.K. Maps of Pleistocene Sea Levels in Southeast Asia: Shorelines, River Systems and Time Durations. J. Biogeogr. 2000, 27, 1153–1167. [Google Scholar] [CrossRef]
- Sathiamurthy, E.; Voris, H.K. Maps of Holocene Sea Level Transgression and Submerged Lakes on the Sunda Shelf. Trop. Nat. His. 2006, 6, 1–44. [Google Scholar] [CrossRef]
- Welton, L.J.; Wood, P.L.; Oaks, J.R.; Siler, C.D.; Brown, R.M. Fossil-Calibrated Phylogeny and Historical Biogeography of Southeast Asian Water Monitors (Varanus salvator Complex). Mol. Phylogenetics Evol. 2014, 74, 29–37. [Google Scholar] [CrossRef]
- Aowphol, A.; Yodthong, S.; Rujirawan, A.; Thirakhupt, K. Mitochondrial Diversity and Phylogeographic Patterns of Gekko gecko (Squamata- Gekkonidae) in Thailand. Asian Herpetol. Res. 2019, 10, 158–169. [Google Scholar] [CrossRef]
- Qin, X.-M.; Li, H.-M.; Zeng, Z.-H.; Zeng, D.-L.; Guan, Q.-X. Genetic Variation and Differentiation of Gekko gecko from Different Populations Based on Mitochondrial Cytochrome b Gene Sequences and Karyotypes. Zool. Sci. 2012, 29, 384–389. [Google Scholar] [CrossRef]
- Zug, G.R.; Mulcahy, D.G.; Vindum, J.V. Resurrection of Bronchocela burmana Blanford, 1878 for the Green Crested Lizard (Squamata, Agamidae) of Southern Myanmar. ZooKeys 2017, 657, 141–156. [Google Scholar] [CrossRef][Green Version]
- Calsbeek, R.; Smith, T.B. Ocean Currents Mediate Evolution in Island Lizards. Nature 2003, 426, 552–555. [Google Scholar] [CrossRef]
- Rocha, S.; Perera, A.; Silva, A.; Posada, D.; Harris, D.J. Evolutionary History of Trachylepis Skinks in the Seychelles Islands: Introgressive Hybridization, Morphological Evolution and Geographic Structure. Biol. J. Linn. Soc. 2016, 119, 15–36. [Google Scholar] [CrossRef]
- McGUIRE, J.A.; Heang, K.B. Phylogenetic Systematics of Southeast Asian Flying Lizards (Iguania: Agamidae: Draco) as Inferred from Mitochondrial DNA Sequence Data. Biol. J. Linn. Soc. 2001, 72, 203–229. [Google Scholar] [CrossRef][Green Version]
- McGuire, J.A.; Alcala, A.C. A Taxonomic Revision of the Flying Lizards (Iguania: Agamidae: Draco) of the Philippine Islands, with a Description of a New Species. Herpetol. Monogr. 2000, 14, 81. [Google Scholar] [CrossRef]
- Moritz, C. Defining ‘Evolutionarily Significant Units’ for Conservation. Trends Ecol. Evol. 1994, 9, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R.; Briscoe, D.A.; Ballou, J.D. Introduction to Conservation Genetics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010; pp. 365–392. ISBN 978-0-521-70271-3. [Google Scholar]

| Sea | Island | Adjacent Mainland | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Province | Locality | Code | N | Latitude | Longitude | Province | Locality | Code | N | Latitude | Longitude | |
| Andaman | Phuket | Banana beach | PKT-Kb | 7 | 7.744250 N | 98.382056 E | Phang-nga | Bang Pat | PNA-Bp | 8 | 8.363444 N | 98.577556 E |
| Kata beach | PKT-Kg | 4 | 7.817139 N | 98.299611 E | Ban Tha Noon | PNA-Bt | 8 | 8.207417 N | 98.295056 E | |||
| Patong beach | PKT-Kp | 6 | 7.885417 N | 98.277472 E | ||||||||
| Promthep Cape | PKT-Ka | 6 | 7.761278 N | 98.309889 E | ||||||||
| Muaeng district | PKT-Km | 3 | 7.865389 N | 98.398889 E | ||||||||
| Karon beach | PKT-Kn | 5 | 7.850500 N | 98.292861 E | ||||||||
| Rawai beach | PKT-Kr | 5 | 7.770861 N | 98.319750 E | ||||||||
| Talang district | PKT-Kt | 7 | 8.120278 N | 98.333694 E | ||||||||
| Phang-nga | Ko Kho Khao | PNA-Kk | 13 | 8.959778 N | 98.274194 E | Phang-nga | Bang Saphan | PNA-Bs | 5 | 11.313972 N | 99.467389 E | |
| Krabi | Ko Lanta | KBI-Kl | 11 | 7.685361 N | 99.109583 E | Krabi | Klong Tom | KBI-Kt | 7 | 7.920472 N | 99.251694 E | |
| Gulf of Thailand | Chonburi | Ko Lan | CBI-Kl | 6 | 12.914111 N | 100.777861 E | Chonburi | Pattaya | CBI-Pa | 8 | 12.840167 N | 100.948722 E |
| Rayong | Ko Samed | RYG-Ks | 3 | 12.551417 N | 101.447389 E | Rayong | Ban Phe | RYG-Bp | 8 | 12.631500 N | 101.444694 E | |
| Trat | Ko Chang | TRT-Kc | 5 | 12.078250 N | 102.368194 E | Trat | Ban Laem Kho | TRT-Bl | 3 | 12.171976 N | 102.394995 E | |
| Surat Thani | Ko Samui | SNI-Ks | 7 | 9.568056 N | 100.010611 E | Surat Thani | Tha Chana | SNI-Tc | 6 | 9.588056 N | 99.207361 E | |
| Nakhon Sri Thammarat | Sichon | NRT-Sc | 2 | 9.044722 N | 99.849361 E | |||||||
| Total | 88 | 55 | ||||||||||
| Populations | n | S | H | Uh | Hd ± SD | π ± SD |
|---|---|---|---|---|---|---|
| Group A | 25 | 18 | 10 | 10 | 0.797 ± 0.076 | 0.0043 ± 0.0003 |
| Group B | 5 | 82 | 5 | 4 | 1.000 ± 0.126 | 0.0355 ± 0.0072 |
| Group C | 3 | 5 | 2 | 1 | 0.667 ± 0.314 | 0.0027 ± 0.0013 |
| Group D | 85 | 71 | 27 | 26 | 0.932 ± 0.013 | 0.0076 ± 0.0006 |
| Group E | 7 | 76 | 3 | 3 | 0.524 ± 0.209 | 0.0245 ± 0.0092 |
| Group F | 18 | 0 | 1 | 0 | 0.000 ± 0.000 | 0.0000 ± 0.0000 |
| Total | 143 | 161 | 46 | 36 | 0.948 ± 0.008 | 0.0180 ± 0.0014 |
| Source of Variation | d.f. | Ss | Vc | %Va | Fi |
|---|---|---|---|---|---|
| Island vs. Mainland (Andaman Sea) | |||||
| Among groups | 1 | 19.812 | 0.59221 | 7.40 | FCT = 0.07395 |
| Among populations within groups | 12 | 467.403 | 5.37169 | 67.08 | FSC = 0.62462 * |
| Within populations | 81 | 261.490 | 3.22827 | 40.31 | FST = 0.59686 * |
| Island vs. Mainland (Gulf of Thailand) | |||||
| Among groups | 1 | 25.902 | 2.04137 | 14.83 | FCT = 0.14827 |
| Among populations within groups | 7 | 442.639 | 11.5359 | 83.79 | FSC = 0.72971 * |
| Within populations | 39 | 166.646 | 4.27299 | 31.04 | FST = 0.68963 * |
| Six groups estimated by SAMOVA | |||||
| Among groups | 5 | 956.288 | 10.4062 | 66.88 | FCT = 0.66885 * |
| Among populations within groups | 17 | 224.554 | 1.58446 | 10.18 | FSC = 0.30753 * |
| Within populations | 120 | 428.136 | 3.56780 | 22.93 | FST = 0.77068 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomontean, B.; Pilap, W.; Jaroenchaiwattanachote, C.; Laotongsan, P.; Pliankham, P.; Saijuntha, J.; Tawong, W.; Tantrawatpan, C.; Saijuntha, W. Island vs. Mainland: Genetic Divergence of Calotes versicolor (Daudin, 1802) (Squamata: Agamidae) in Thailand. Animals 2025, 15, 3028. https://doi.org/10.3390/ani15203028
Gomontean B, Pilap W, Jaroenchaiwattanachote C, Laotongsan P, Pliankham P, Saijuntha J, Tawong W, Tantrawatpan C, Saijuntha W. Island vs. Mainland: Genetic Divergence of Calotes versicolor (Daudin, 1802) (Squamata: Agamidae) in Thailand. Animals. 2025; 15(20):3028. https://doi.org/10.3390/ani15203028
Chicago/Turabian StyleGomontean, Bhuvadol, Warayutt Pilap, Chavanut Jaroenchaiwattanachote, Panida Laotongsan, Pichit Pliankham, Jatupon Saijuntha, Wittaya Tawong, Chairat Tantrawatpan, and Weerachai Saijuntha. 2025. "Island vs. Mainland: Genetic Divergence of Calotes versicolor (Daudin, 1802) (Squamata: Agamidae) in Thailand" Animals 15, no. 20: 3028. https://doi.org/10.3390/ani15203028
APA StyleGomontean, B., Pilap, W., Jaroenchaiwattanachote, C., Laotongsan, P., Pliankham, P., Saijuntha, J., Tawong, W., Tantrawatpan, C., & Saijuntha, W. (2025). Island vs. Mainland: Genetic Divergence of Calotes versicolor (Daudin, 1802) (Squamata: Agamidae) in Thailand. Animals, 15(20), 3028. https://doi.org/10.3390/ani15203028






