Insight into the Skin Mycobiota of Myotis myotis: How Age, Sex, and Biometric Traits Correlate with Fungal Diversity
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Methods
2.3. Isolation of Fungi from Samples
2.4. Identification of Fungi
2.5. Data Analyses
3. Results
3.1. Fungal Isolation and Identification
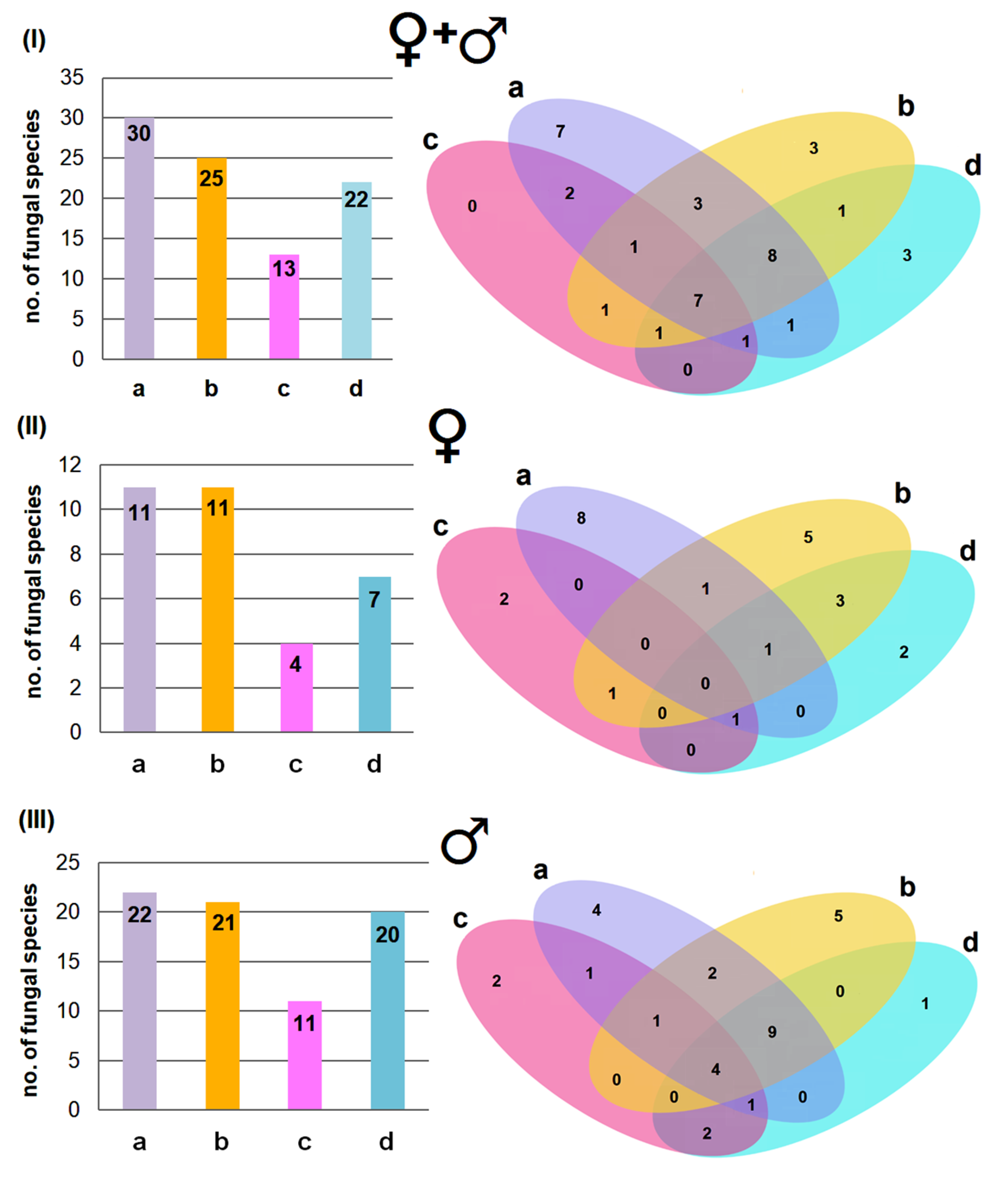
3.2. Fungal Diversity Across Body Regions and the Effect of Age and Sex
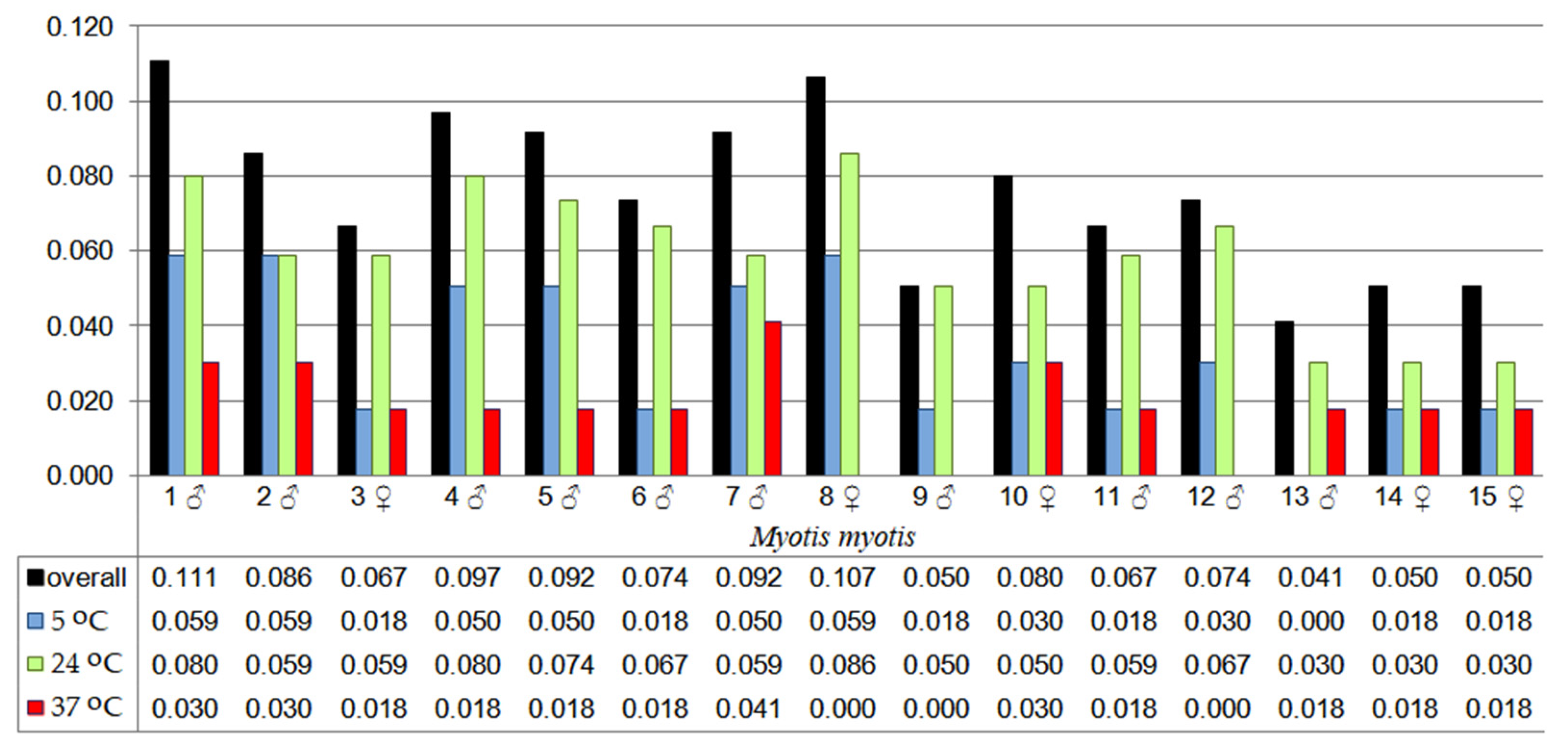
3.3. Effect of Incubation Temperature on Fungal Isolation
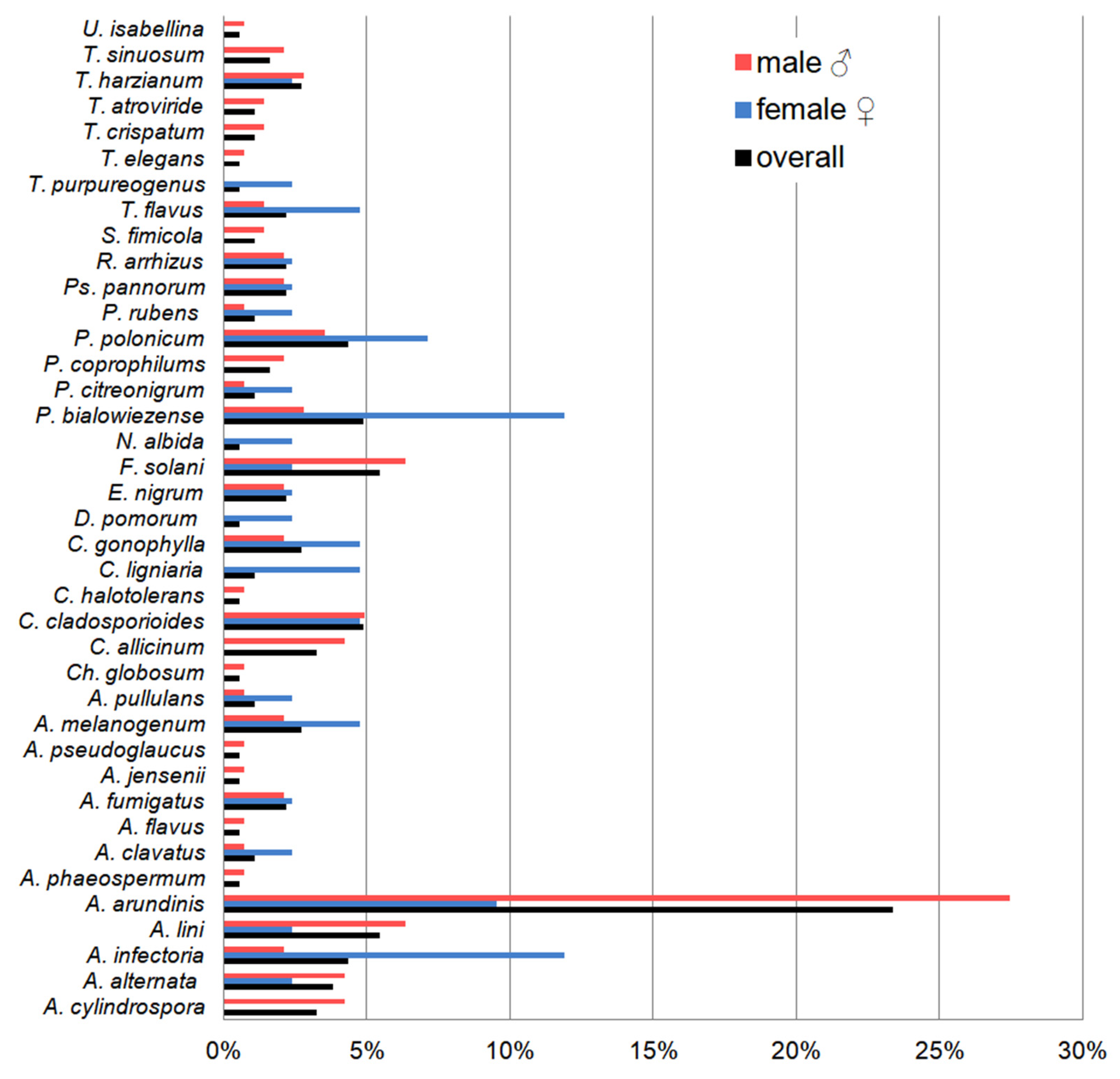
3.4. Dominant Fungal Species and the Effect of Biometric Features on Fungal Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Fungal Species | Myotis myotis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 ♂ | 2 ♂ | 3 ♀ | 4 ♂ | 5 ♂ | 6 ♂ | 7 ♂ | 8 ♀ | 9 ♂ | 10 ♀ | 11 ♂ | 12 ♂ | 13 ♂ | 14 ♀ | 15 ♀ | |
| Absidia cylindrospora | – | – | – | – | 24 °C (c, d) | 24 °C (a, b, c, d) | – | – | – | – | –1 | – | – | – | – |
| Alternaria alternata | 5 °C (a) | – | – | – | – | – | 24 °C (a, b, d); 37 °C (a, b) | 5 °C (b) | – | – | – | – | – | – | – |
| Alternaria infectoria | – | – | 37 °C (a) | – | 24 °C (d) | – | 37 °C (d) | 24 °C (b) | – | 37 °C (a) | 24 °C (c) | – | – | – | 37 °C (b, d) |
| Alternaria lini | 24 °C (b, d) | – | – | – | 24 °C (d) | 24 °C (d) | 24 °C (a, b, d) | – | 24 °C (a) | – | – | 24 °C (b) | – | – | 24 °C (a) |
| Apiospora arundinis | 5 °C (b); 24 °C (a, c, d) | 5 °C (b); 24 °C (a, d) | 5 °C (b); 24 °C (b) | 5 °C (a, b, d); 24 °C (a, b, d) | 5 °C (b); 24 °C (d) | 5 °C (b); 24 °C (a, b, c) | 5 °C (a, d); 24 °C (a, d) | 24 °C (c, d) | 5 °C (a, b, c, d); 24 °C (b, c, d) | – | – | 5 °C (a, c, d); 24 °C (a, b, c, d) | 24 °C (c, d) | – | – |
| Arthrinium phaeospermum | – | – | – | – | – | – | 24 °C (d) | – | – | – | – | – | – | – | – |
| Aspergillus clavatus | 5 °C (c) | – | 24 °C (a) | – | – | – | – | – | – | – | – | – | – | – | – |
| Aspergillus flavus | – | – | – | – | – | – | – | – | – | – | – | – | 37 °C (b) | – | – |
| Aspergillus fumigatus | 24 °C (b) | – | – | – | – | – | – | 24 °C (c) | – | – | 24 °C (a); 37 °C (d) | – | – | – | – |
| Aspergillus jensenii | 24 °C (d) | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Aspergillus pseudoglaucus | 24 °C (d) | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Aureobasidium melanogenum | – | 37 °C (d) | 24 °C (d) | 24 °C (a, d) | – | 37 °C (d) | – | – | – | – | – | – | – | 37 °C (d) | – |
| Aureobasidium pullulans | – | – | – | – | – | – | – | 5 °C (a) | – | – | 24 °C (b) | – | – | – | – |
| Chaetomium globosum | 37 °C (a) | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Cladosporium allicinum | 5 °C (b) | 5 °C (d) | – | 5 °C (a, d) | 5 °C (b) | – | 5 °C (b) | – | – | – | – | – | – | – | – |
| Cladosporium cladosporioides | – | 5 °C (b, d) | – | 5 °C (b, d) | – | – | 5 °C (a, b, d) | – | – | 5 °C (b, d) | – | – | – | – | – |
| Cladosporium halotolerans | – | – | – | – | – | – | – | – | – | – | 24 °C (b) | – | – | – | – |
| Coniochaeta ligniaria | – | – | – | – | – | – | – | 24 °C (a) | – | – | – | – | – | 5 °C (a) | – |
| Coprinopsis gonophylla | 24 °C (c) | – | – | – | 24 °C (c) | – | 24 °C (b, c) | 24 °C (d) | – | 24 °C (b) | – | – | – | – | – |
| Didymella pomorum | – | – | 24 °C (a) | – | – | – | – | – | – | – | – | – | – | – | – |
| Epicoccum nigrum | – | – | – | – | 24 °C (b) | – | 5 °C (a, b) | 5 °C (a) | – | – | – | 5 °C (a) | – | – | – |
| Fusarium solani | 24 °C (a, b) | 24 °C (d) | – | 24 °C (a, c); 37 °C (b) | – | 24 °C (d) | – | – | – | 24 °C (b) | 24 °C (a, b) | – | – | – | – |
| Naganishia albida | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 24 °C (a) |
| Penicillium bialowiezense | – | 5 °C (b); 24 °C (c) | – | 24 °C (a) | – | 24 °C (d) | – | 5 °C (a); 24 °C (a, c) | 24 °C (d) | – | – | – | – | 24 °C (c, d) | – |
| Penicillium citreonigrum | 24 °C (b) | – | – | – | – | – | – | 24 °C (d) | – | – | – | – | – | – | – |
| Penicillium coprophilums | – | – | – | 24 °C (a) | – | 24 °C (a) | – | – | – | – | – | 24 °C (a) | – | – | – |
| Penicillium polonicum | – | 5 °C (c); 24 °C (b) | – | – | 5 °C (b); 37 °C (a) | – | 37 °C (b) | 5 °C (c) | – | 5 °C (b) | – | – | – | – | 5 °C (c) |
| Penicillium rubens | 5 °C (a) | – | – | – | – | – | – | – | – | 24 °C (b) | – | – | – | – | – |
| Pseudogymnoascus pannorum | – | – | – | – | – | – | – | 24 °C (b) | – | – | 5 °C (a, b, d) | – | – | – | – |
| Rhizopus arrhizus | – | 37 °C (b) | 24 °C (b) | 5 ° C (d) | – | – | – | – | 24 °C (a) | – | – | – | – | – | – |
| Sordaria fimicola | – | – | – | – | – | – | – | – | – | – | 24 °C (a, b) | – | – | – | |
| Talaromyces flavus | – | – | – | 24 °C (c, d) | – | – | – | – | – | 37 °C (a) | – | – | – | 24 °C (b) | – |
| Talaromyces purpureogenus | – | – | – | – | – | – | – | – | – | 24 °C (a) | – | – | – | – | – |
| Thamnidium elegans | – | – | – | – | 5 °C (a) | – | – | – | – | – | – | – | – | – | – |
| Trichocladium crispatum | – | – | – | 24 °C (c) | – | – | – | – | – | – | – | – | 24 °C (a) | – | – |
| Trichoderma atroviride | – | – | – | – | 24 °C (c, d) | – | – | – | – | – | – | 24 °C (a) | – | – | – |
| Trichoderma harzianum | 37 °C (b, d) | 24 °C (a, d) | – | – | – | – | – | 24 °C (d) | – | – | – | – | – | – | – |
| Trichoderma sinuosum | – | – | – | – | – | – | – | – | – | – | – | 24 °C (a, b, d) | – | – | – |
| Umbelopsis isabellina | – | – | – | 24 °C (b) | – | – | – | – | – | – | – | – | – | – | – |
References
- Sakoui, S.; Derdak, R.; Addoum, B.; Serrano-Delgado, A.; Soukri, A.; El Khalfi, B. The life hidden inside caves: Ecological and economic importance of bat guano. Int. J. Ecol. 2020, 2020, 9872532. [Google Scholar] [CrossRef]
- Bandouchova, H.; Zukal, J.; Linhart, P.; Berkova, H.; Brichta, J.; Kovacova, V.; Kubickova, A.; Abdelsalam, E.E.; Bartonička, T.; Zajíčková, R.; et al. Low seasonal variation in greater mouse-eared bat (Myotis myotis) blood parameters. PLoS ONE 2020, 15, e0234784. [Google Scholar] [CrossRef]
- Węgiel, A.; Grzywiński, W.; Kosicki, J.Z.; Tryjanowski, P.; Nowak, J.; Węgiel, J. Long-term population trends of the lesser horseshoe bat Rhinolophus hipposideros and the greater mouse-eared bat Myotis myotis in Poland. Eur. Zool. J. 2021, 88, 1189–1200. [Google Scholar] [CrossRef]
- Wojtaszyn, G.; Dzięgielewska, M.; Ignaszak, K.; Rutkowski, T.; Bernard, R.; Heise, G.; Blohm, T.; Schmidt, A.; Oldenburg, W.; Ittermann, L.; et al. Migration of the greater mouse-eared bat (Myotis myotis) between Poland and Germany. Nyctalus (N.F.) 2021, 19, 356–365. [Google Scholar]
- Langwig, K.E.; White, J.P.; Parise, K.L.; Kaarakka, H.M.; Redell, J.A.; DePue, J.E.; Scullon, W.H.; Foster, J.T.; Kilpatrick, A.M.; Hoyt, J.R. Mobility and infectiousness in the spatial spread of an emerging fungal pathogen. J. Anim. Ecol. 2021, 90, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Herrera, K.; Villalobos, F.; Guillén-Servent, A.; Solari, S.; Rojas-Soto, O. Seasonal distribution analysis of five lasiurine bat species: Clues to migration patterns and behavior. Mammalia 2023, 87, 499–510. [Google Scholar] [CrossRef]
- Seidel, J.; Valenzano, D.R. The role of the gut microbiome during host ageing. F1000Research 2018, 7, F1000 Faculty. [Google Scholar] [CrossRef]
- Ameri, S.; Nasrollahi, S.A.; Samadi, A.; Amiri, F.; Ahmadvand, S.; Yadangi, S.; Fattahi, M.; Ehsani, M.; Firooz, A. Assessment of skin microbiota and biometric parameters: A comprehensive comparison of four types of hand cleansers. Iran. J. Dermatol. 2021, 24, 306–314. [Google Scholar]
- Cheng, T.L.; Reichard, J.D.; Coleman, J.T.H.; Weller, T.J.; Thogmartin, W.E.; Reichert, B.E.; Bennett, A.B.; Broders, H.G.; Campbell, J.; Etchison, K.; et al. The scope and severity of white-nose syndrome on hibernating bats in North America. Conserv. Biol. 2021, 35, 1586–1597. [Google Scholar] [CrossRef]
- Ogórek, R.; Kurczaba, K.; Cal, M.; Apoznański, G.; Kokurewicz, T. A culture-based ID of micromycetes on the wing membranes of Greater Mouse-Eared Bats (Myotis myotis) from the “Nietoperek” site (Poland). Animals 2020, 10, 1337. [Google Scholar] [CrossRef]
- Kokurewicz, T.; Ogórek, R.; Pusz, W.; Matkowski, K. Bats increase the number of cultivable airborne fungi in the “Nietoperek” bat reserve in Western Poland. Microb. Ecol. 2016, 72, 36–48. [Google Scholar] [CrossRef]
- Pikula, J.; Amelon, S.K.; Bandouchova, H.; Bartoničk, T.; Berkova, H.; Brichta, J.; Hooper, S.; Kokurewicz, T.; Kolarik, M.; Köllner, B.; et al. White-nose syndrome pathology grading in Nearctic and Palearctic bats. PLoS ONE 2017, 12, e0180435. [Google Scholar] [CrossRef]
- Bandouchova, H.; Bartonicka, T.; Berkova, H.; Brichta, J.; Cerny, J.; Kovacova, V.; Kolarik, M.; Köllner, B.; Kulich, P.; Martínková, N.; et al. Pseudogymnoascus destructans: Evidence of virulent skin invasion for bats under natural conditions, Europe. Transbound. Emerg. Dis. 2015, 62, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.O.B.; Bezerra, J.D.P.; Oliveira, T.G.L.; Barbier, E.; Bernard, E.; Machado, A.R.; Souza-Motta, C.M. Living in the dark: Bat caves as hotspots of fungal diversity. PLoS ONE 2020, 15, e0243494. [Google Scholar] [CrossRef] [PubMed]
- Borzęcka, J.; Piecuch, T.; Kokurewicz, T.; Lavoie, K.H.; Ogórek, R. Greater mouse-eared bats (Myotis myotis) hibernating in the “Nietoperek” bat reserve (Poland) as a vector of airborne culturable fungi. Biology 2021, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, J.D.; Dimitriadis, G.; Codd, J.R.; Nudds, R.L. A potential role for bat tail membranes in flight control. PLoS ONE 2011, 6, e18214. [Google Scholar] [CrossRef]
- Muszer, J.; Muszer, A. Evaluation of the geotouristic attractions from the Wojcieszów area. Geotourism 2017, 1–2, 48–49. [Google Scholar]
- Migoń, P.; Łach, J. Geomorphological evidence of neotectonics in the Kaczawa sector of the Sudetic Marginal Fault, southwestern Poland. Geol. Sudet. 1998, 31, 307–316. [Google Scholar]
- Furmankiewicz, J.; Kmiecik, P.; Kmiecik, A.; Jabłoński, J.; Jabłońska, J.; Mikołajczyk, E.; Duma, K.; Furmankiewicz, M.; Horáček, D.; Jóža, M. The largest bat hibernacula in Lower Silesia (SW Poland). In Proceedings of the 6. Conference “Bats of the Sudety Mts”; Zinke, O., Ed.; Veröffentlichungen des Museums der Westlausitz: Kamenz, Germany, 2016; pp. 17–38. [Google Scholar]
- Dietz, C.; von Helversen, O. Illustrated Identification Key to the Bats of Europe. Tech. Rep. 2004, 1, 72. [Google Scholar]
- Brunet-Rossinni, A.K.; Wilkinson, G.S. Methods for age estimation and the study of senescence in bats. In Ecological and Behavioral Methods for the Study of Bats; Kunz, T.H., Parsons, S., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2009. [Google Scholar]
- Spychała, K.; Kłosińska, K.; Salwińska, W.; Ogórek, R. Diversity of Soil-Borne Fungi Isolated from Places Frequently Visited by People in the City of Wrocław (Poland). Appl. Sci. 2024, 14, 2782. [Google Scholar] [CrossRef]
- Zare, R.; Gams, W. A revision of Verticillium section Prostrata. IV. The genera Lecanicillium and Simplicillium. Nova Hedwig. 2001, 73, 1–50. [Google Scholar] [CrossRef]
- Gräfenhan, T.; Schroers, H.J.; Nirenberg, H.I.; Seifert, K.A. An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Stud. Mycol. 2011, 68, 79–113. [Google Scholar] [CrossRef] [PubMed]
- Minnis, A.M.; Lindner, D.L. Phylogenetic evaluation of Geomyces and allies reveals no close relatives of Pseudogymnoascus destructans, comb. nov., in bat hibernacula of eastern North America. Fungal Biol. 2013, 117, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Visagie, C.M.; Hirooka, Y.; Tanney, J.B.; Whitfield, E.; Mwange, K.; Meijer, M.; Amend, A.S.; Seifert, K.A.; Samson, R.A. Aspergillus, Penicillium, and Talaromyces isolated from house dust samples collected around the world. Stud. Mycol. 2014, 78, 63–139. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zheng, R.Y. New taxa of Ambomucor (Mucorales, Mucoromycotina) from China. Mycotaxon 2015, 30, 165–171. [Google Scholar] [CrossRef]
- Liu, X.Z.; Wang, Q.M.; Göker, M. Towards an integrated phylogenetic classification of the Tremellomycetes. Stud. Mycol. 2015, 81, 85–147. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Guarro, J.; Cano-Lira, J.F.; Sutton, D.A.; Wiederhold, N.P.; de Hoog, G.S.; Abbott, S.P.; Decock, C.; Sigler, L.; Gené, J. Phylogeny and taxonomic revision of Microascaceae with emphasis on synnematous fungi. Stud. Mycol. 2016, 83, 193–233. [Google Scholar] [CrossRef]
- Vandepol, N.; Liber, J.; Desiró, A.; Na, H.; Kennedy, M.; Barry, K.; Grigoriev, I.V.; Miller, A.N.; O’Donnell, K.; Stajich, J.E.; et al. Resolving the Mortierellaceae phylogeny through synthesis of multi-gene phylogenetics and phylogenomics. Fungal Divers. 2020, 104, 267–289. [Google Scholar] [CrossRef]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Sninsky, J., White, T., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Ogórek, R.; Dyląg, M.; Kozak, B. Dark stains on rock surfaces in Driny Cave (Little Carpathian Mountains, Slovakia). Extremophiles 2016, 20, 641–652. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, X.-L.; Zhang, Y.Q.; Liu, H.-Y.; Yu, L.-Y. Diversity and distribution of lichen-associated fungi in the Ny-Ålesund Region (Svalbard, High Arctic) as revealed by 454 pyrosequencing. Sci. Rep. 2015, 5, 14850. [Google Scholar] [CrossRef]
- Spellerberg, I.F.; Fedor, P.J. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon-Wiener’ index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- Verant, M.L.; Boyles, J.G.; Waldrep, W., Jr.; Wibbelt, G.; Blehert, D.S. Temperature-dependent growth of Geomyces destructans, the fungus that causes bat white-nose syndrome. PLoS ONE 2012, 7, e46280. [Google Scholar] [CrossRef]
- Ballmann, A.E.; Torkelson, M.R.; Bohuski, E.A.; Russell, R.E.; Blehert, D.S. Dispersal hazards of Pseudogymnoascus destructans by bats and uman activity at hibernacula In summer. J. Wildl. Dis. 2017, 53, 725–735. [Google Scholar] [CrossRef]
- Zukal, J.; Bandouchova, H.; Brichta, J.; Cmokova, A.; Jaron, K.S.; Kolarik, M.; Kovacova, V.; Kubátová, A.; Nováková, A.; Orlov, O.; et al. White-nose syndrome without borders: Pseudogymnoascus destructans infection tolerated in Europe and Palearctic Asia but not in North America. Sci. Rep. 2016, 6, 19829. [Google Scholar]
- Zukal, J.; Berková, H.; Řehák, Z. Activity and shelter selection by Myotis myotis and Rhinolophus hipposideros hibernating in the Kateřinská cave (Czech Republic). Mamm. Biol. 2005, 70, 271–281. [Google Scholar] [CrossRef]
- Zukal, J.; Bandouchova, H.; Bartonicka, T.; Berkova, H.; Brack, V.; Brichta, J.; Dolinay, M.; Jaron, K.S.; Kovacova, V.; Kovarik, M.; et al. White-nose syndrome fungus: A generalist pathogen of hibernating bats. PLoS ONE 2014, 9, e97224. [Google Scholar] [CrossRef]
- Fofanov, V.Y.; Furstenau, T.N.; Sanchez, D.; Hepp, C.M.; Cocking, J.; Sobek, C.; Pagel, N.; Walker, F.; Chambers, C.L. Guano exposed: Impact of aerobic conditions on bat fecal microbiota. Ecol. Evol. 2018, 8, 5563–5574. [Google Scholar] [CrossRef] [PubMed]
- Ogórek, R.; Dyląg, M.; Kozak, B.; Višňovská, Z.; Tancinováet, D.; Lejman, A. Fungi isolated and quantified from bat guano and air in Harmanecka and Driny Caves (Slovakia). J. Cave Karst Stud. 2016, 78, 41–49. [Google Scholar] [CrossRef]
- Ben–Hamo, M.; Munoz-Garcia, A.; Larrain, P.; Pinshow, B.; Korine, C.; Williams, J.B. The cutaneous lipid composition of batwing and tail membranes: A case of convergent evolution with birds. Proc. R. Soc. 2016, B283, 20160636. [Google Scholar]
- Arlettaz, R. Feeding behaviour and foraging strategy of free-living mouse-eared bats, Myotis myotis and Myotis blythii. Anim. Behav. 1996, 51, 1–11. [Google Scholar] [CrossRef]
- Lunde, L.F.; Boddy, L.; Sverdrup-Thygeson, A.; Jacobsen, R.M.; Kauserud, H.; Birkemoe, T. Beetles provide directed dispersal of viable spores of a keystone wood decay fungus. Fungal Ecol. 2023, 63, 101232. [Google Scholar] [CrossRef]
- Binder, U.; Lass-Flörl, C. New insights into invasive aspergillosis—From the pathogen to the disease. Curr. Pharm. Des. 2013, 19, 3679–3688. [Google Scholar] [CrossRef]
- Montazeri, A.; Zandi, H.; Teymouri, F.; Soltanianzadeh, Z.; Jambarsang, S.; Mokhtari, M. Microbiological analysis of bacterial and fungal bioaerosols from a burn hospital in Yazd, Iran, during 2019. J. Environ. Health Sci. Eng. 2020, 18, 1121–1130. [Google Scholar] [CrossRef]
- Bräse, S.; Encinas, A.; Keck, J.; Nising, C.F. Chemistry and Biology of Mycotoxins and Related Fungal Metabolites. Chem. Rev. 2009, 109, 3903–3990. [Google Scholar] [CrossRef]
- Lopez-Diaz, T.M.; Flannigan, B. Production of patulin and cytochalasin E by Aspergillus clavatus during malting of barley and wheat. Int. J. Food Microbiol. 1997, 35, 129–136. [Google Scholar] [CrossRef]
- Chen, Z.; Ao, J.; Yang, W.; Jiao, L.; Zheng, T.; Chen, X. Purification and characterization of a novel antifungal protein secreted by Penicillium chrysogenum from an Arctic sediment. Appl. Microbiol. Biotechnol. 2013, 97, 10381–10390. [Google Scholar] [CrossRef] [PubMed]
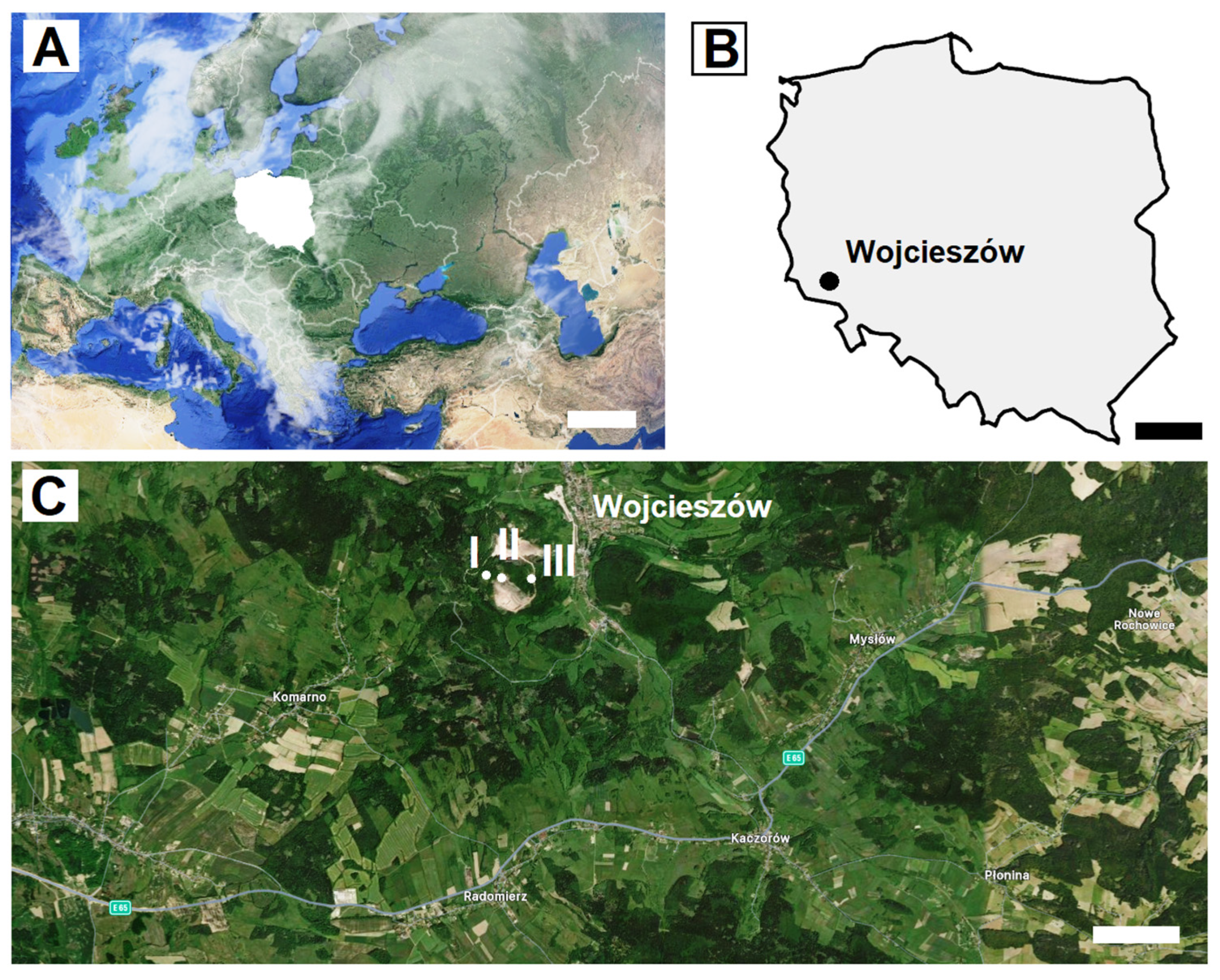


| Bat | Sampling | Sex | Forearm Length (mm) | Weight (g) | Age | |||
|---|---|---|---|---|---|---|---|---|
| No. | Ring No. | Location | Date | Hour | ||||
| 1 | C20744PL | Szczelina Wojcieszowska cave | 07.09.2021 | 2:30 | male | 61.3 | 25.0 | adult |
| 2 | C20743PL | 07.09.2021 | 1:50 | male | 55.0 | 24.5 | subadult | |
| 3 | C20782PL | 07.09.2021 | 22:45 | female | 61.4 | 29.5 | adult | |
| 4 | C20745PL | 07.09.2021 | 1:50 | male | 62.0 | 25.0 | adult | |
| 5 | C20741PL | 07.09.2021 | 2:30 | male | 61.2 | 25.0 | subadult | |
| 6 | C20742PL | 07.09.2021 | 2:30 | male | 61.8 | 23.0 | subadult | |
| 7 | C20781PL | 15.09.2021 | 22:15 | male | 52.9 | 26.5 | adult | |
| 8 | –1 | Nowa cave | 07.09.2021 | 23:00 | female | 61.5 | 23.0 | adult |
| 9 | – | 07.09.2021 | 00:20 | male | 61.0 | 24.5 | subadult | |
| 10 | – | 07.09.2021 | 23:30 | female | 61.1 | 26.0 | subadult | |
| 11 | C20710PL | Północna Duża cave | 07.09.2021 | 22:50 | male | 60.9 | 22.7 | adult |
| 12 | C20929PL | 07.09.2021 | 1:10 | male | 60.1 | 29.5 | adult | |
| 13 | – | 29.09.2021 | 21:30 | male | 58.2 | 25.5 | subadult | |
| 14 | – | 29.09.2021 | 20:55 | female | 63.0 | 36.5 | adult | |
| 15 | C20953PL | 20.10.2021 | 19:50 | female | 63.4 | 32.0 | adult | |
| Isolate Number | Identified Fungi | GenBank Accession No. | The Sequence Length (bp) | Identity (%) | Accession |
|---|---|---|---|---|---|
| UWR_438 | Absidia cylindrospora | PV016708 | 513 | 99.81 | MN817778.1 |
| UWR_439 | Alternaria alternata | PV016709 | 518 | 100 | MN907440.1 |
| UWR_440 | Alternaria infectoria | PV016710 | 522 | 100 | MN534845.1 |
| UWR_441 | Alternaria lini | PV016711 | 517 | 100 | OL687532.1 |
| UWR_442 | Apiospora arundinis | PV016712 | 506 | 100 | KF144885.1 |
| UWR_443 | Arthrinium phaeospermum | PV016713 | 413 | 100 | OW984648.1 |
| UWR_444 | Aspergillus clavatus | PV016714 | 488 | 100 | MK271292.1 |
| UWR_445 | Aspergillus flavus | PV016715 | 533 | 100 | MT447477.1 |
| UWR_446 | Aspergillus fumigatus | PV016716 | 527 | 100 | MT558940.1 |
| UWR_447 | Aspergillus jensenii | PV016717 | 507 | 100 | MT582748.1 |
| UWR_448 | Aspergillus pseudoglaucus | PV016718 | 482 | 100 | MT582752.1 |
| UWR_449 | Aureobasidium melanogenum | PV016719 | 516 | 100 | MH855849.1 |
| UWR_450 | Aureobasidium pullulans | PV016720 | 514 | 100 | MT035961.1 |
| UWR_451 | Chaetomium globosum | PV016721 | 473 | 100 | MN654349.1 |
| UWR_452 | Cladosporium allicinum | PV016722 | 463 | 100 | OK445643.1 |
| UWR_453 | Cladosporium cladosporioides | PV016723 | 493 | 100 | MT781987.1 |
| UWR_454 | Cladosporium halotolerans | PV016724 | 486 | 100 | MN826823.1 |
| UWR_455 | Coniochaeta ligniaria | PV016725 | 461 | 100 | MT920581.1 |
| UWR_456 | Coprinopsis gonophylla | PV016726 | 570 | 100 | MW560230.1 |
| UWR_457 | Didymella pomorum | PV016727 | 466 | 100 | KU554583.1 |
| UWR_458 | Epicoccum nigrum | PV016728 | 495 | 100 | KP794171.1 |
| UWR_459 | Fusarium solani | PV016729 | 430 | 100 | OP482353.1 |
| UWR_460 | Naganishia albida | PV016730 | 525 | 100 | OM021981.1 |
| UWR_461 | Penicillium bialowiezense | PV016731 | 500 | 100 | MT582764.1 |
| UWR_462 | Penicillium citreonigrum | PV016732 | 364 | 99.18 | EF198645.1 |
| UWR_463 | Penicillium coprophilum | PV016733 | 484 | 100 | MT410465.1 |
| UWR_464 | Penicillium polonicum | PV016734 | 509 | 100 | MT582786.1 |
| UWR_465 | Penicillium rubens | PV016735 | 476 | 100 | MT079294.1 |
| UWR_466 | Pseudogymnoascus pannorum | PV016736 | 459 | 99.78 | MW019476.1 |
| UWR_467 | Rhizopus arrhizus | PV016737 | 544 | 100 | MT590596.1 |
| UWR_468 | Sordaria fimicola | PV016738 | 510 | 100 | MN341414.1 |
| UWR_469 | Talaromyces flavus | PV016739 | 437 | 100 | MT074667.1 |
| UWR_470 | Talaromyces purpureogenus | PV016740 | 509 | 100 | MN206956.1 |
| UWR_471 | Thamnidium elegans | PV016741 | 561 | 100 | JN206059.1 |
| UWR_472 | Trichocladium crispatum | PV016742 | 495 | 100 | OP699917.1 |
| UWR_473 | Trichoderma atroviride | PV016743 | 532 | 100 | OP539101.1 |
| UWR_474 | Trichoderma harzianum | PV016744 | 394 | 100 | MT584872.1 |
| UWR_475 | Trichoderma sinuosum | PV016745 | 374 | 99.73 | JQ272463.1 |
| UWR_476 | Umbelopsis isabellina | PV016746 | 548 | 100 | MZ078794.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borzęcka, J.; Suchodolski, J.; Cal-Smok, M.; Furmankiewicz, J.; Ogórek, R. Insight into the Skin Mycobiota of Myotis myotis: How Age, Sex, and Biometric Traits Correlate with Fungal Diversity. Animals 2025, 15, 3020. https://doi.org/10.3390/ani15203020
Borzęcka J, Suchodolski J, Cal-Smok M, Furmankiewicz J, Ogórek R. Insight into the Skin Mycobiota of Myotis myotis: How Age, Sex, and Biometric Traits Correlate with Fungal Diversity. Animals. 2025; 15(20):3020. https://doi.org/10.3390/ani15203020
Chicago/Turabian StyleBorzęcka, Justyna, Jakub Suchodolski, Magdalena Cal-Smok, Joanna Furmankiewicz, and Rafał Ogórek. 2025. "Insight into the Skin Mycobiota of Myotis myotis: How Age, Sex, and Biometric Traits Correlate with Fungal Diversity" Animals 15, no. 20: 3020. https://doi.org/10.3390/ani15203020
APA StyleBorzęcka, J., Suchodolski, J., Cal-Smok, M., Furmankiewicz, J., & Ogórek, R. (2025). Insight into the Skin Mycobiota of Myotis myotis: How Age, Sex, and Biometric Traits Correlate with Fungal Diversity. Animals, 15(20), 3020. https://doi.org/10.3390/ani15203020







