Landscape Composition and Forest Structure Shape Phyllostomid Bat Assemblages in the Atlantic Forest Remnants
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Area
2.2. Landscape Variables
2.3. Forest Structure
2.4. Bat Sampling
2.5. Data Analysis
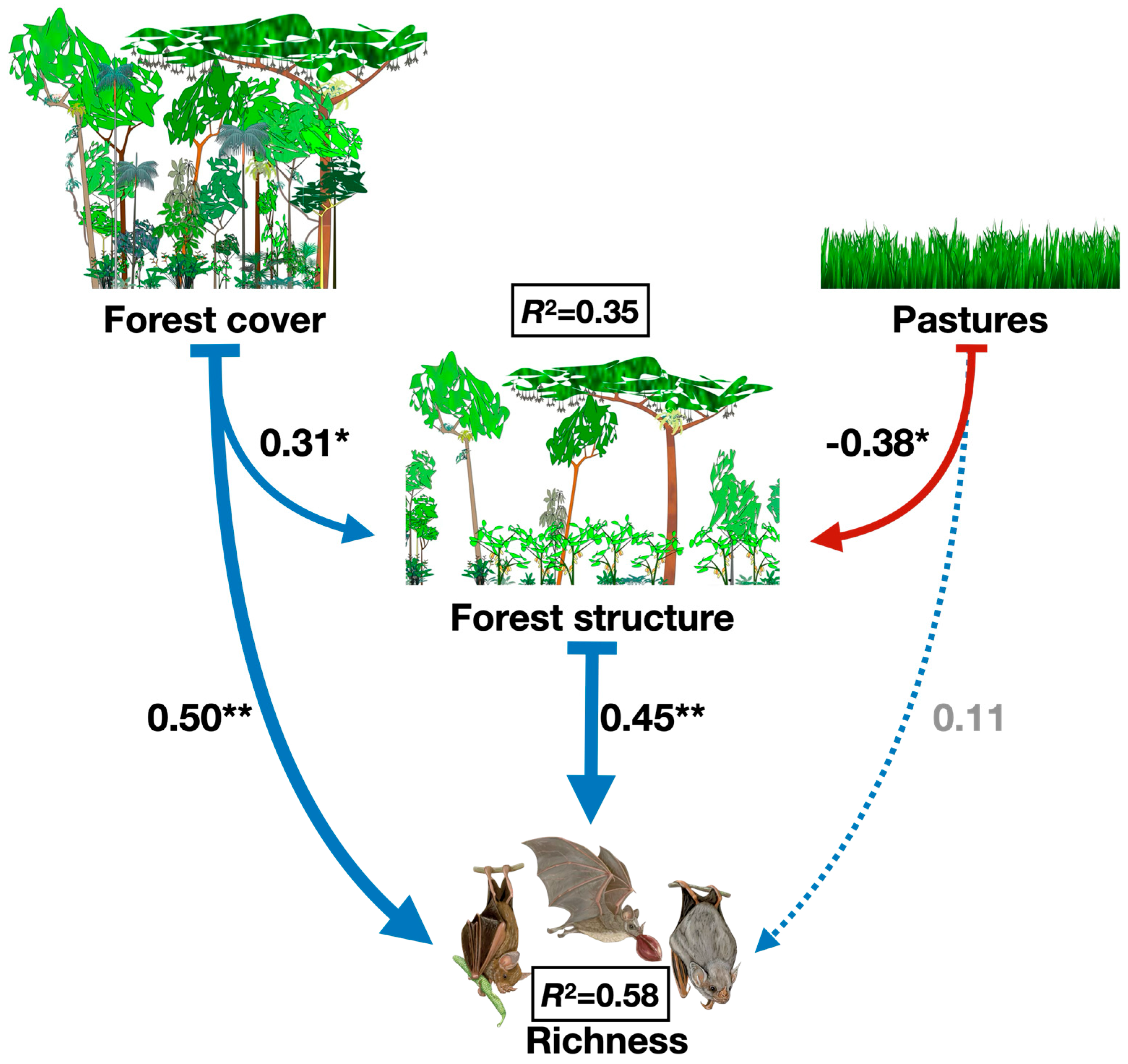
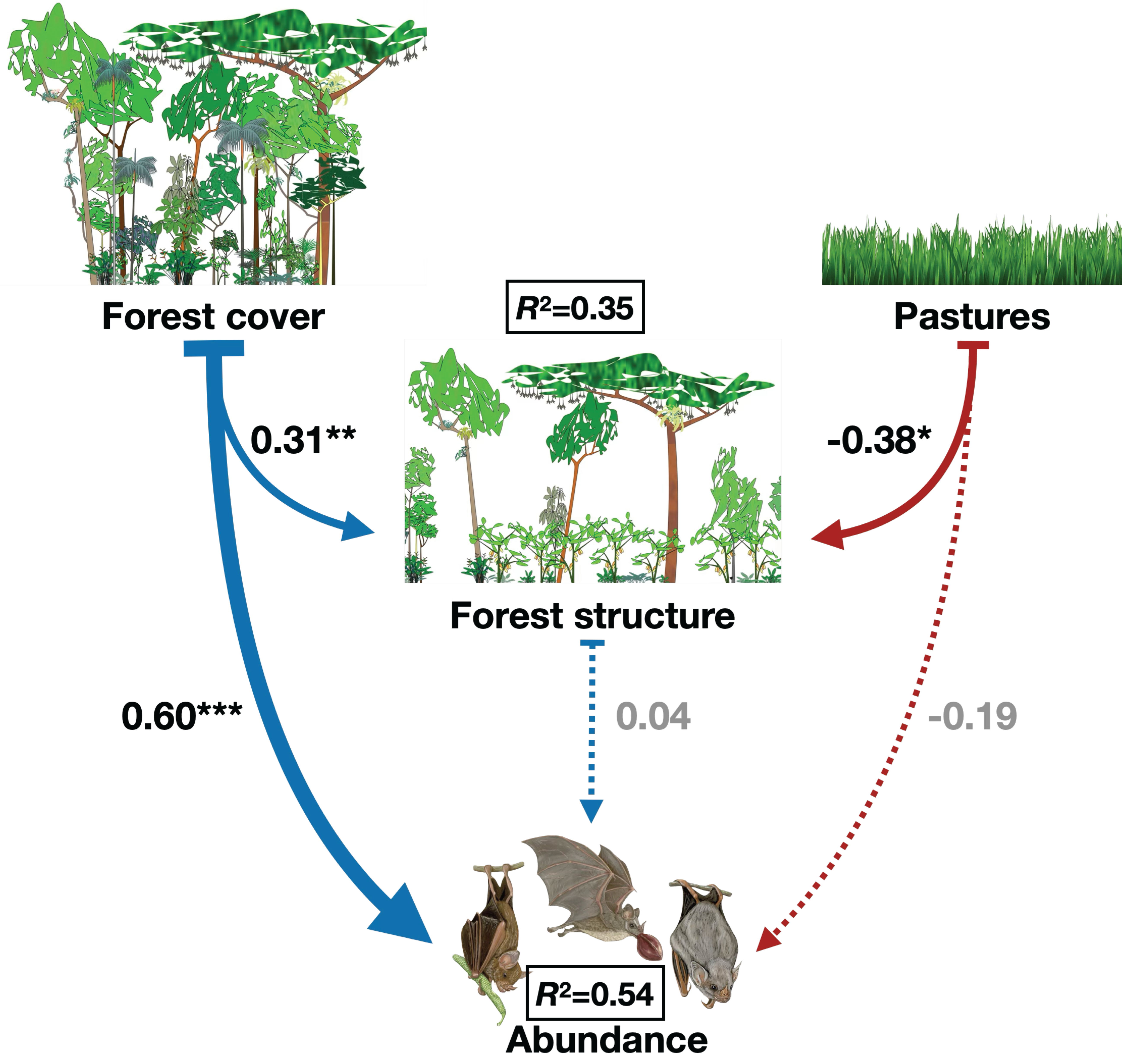
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1
References
- Maxwell, S.L.; Fuller, R.A.; Brooks, T.M.; Watson, J.E. Biodiversity: The ravages of guns, nets and bulldozers. Nature 2016, 536, 143–145. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.; Choimes, A.; Collen, B. Global land-use impacts on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Sala, O.E.; Chapin, F.S., 3rd; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Chazdon, R.L.; Lindenmayer, D.; Guariguata, M.R.; Crouzeilles, R.; Benayas, J.M.R.; Chavero, E.L. Fostering natural forest regeneration on former agricultural land through economic and policy interventions. Environ. Res. Lett. 2020, 15, 043002. [Google Scholar] [CrossRef]
- Tscharntke, T.; Clough, Y.; Wanger, T.C.; Jackson, L.; Motzke, I.; Perfecto, I.; Vandermeer, J.; Whitbread, A. Global food security, biodiversity conservation and the future of agricultural intensification. Biol. Conserv. 2012, 151, 53–59. [Google Scholar] [CrossRef]
- Bhagwat, S.A.; Willis, K.J.; Birks, H.J.B.; Whittaker, R.J. Agroforestry: A refuge for tropical biodiversity? Trends Ecol. Evol. 2008, 23, 261–267. [Google Scholar] [CrossRef]
- Schroth, G.; Jeusset, A.; Gomes, A.d.S.; Florence, C.T.; Coelho, N.A.P.; Faria, D.; Läderach, P. Climate friendliness of cocoa agroforests is compatible with productivity increase. Mitig. Adapt. Strateg. Glob. Change 2016, 21, 67–80. [Google Scholar] [CrossRef]
- Fahrig, L. Rethinking patch size and isolation effects: The habitat amount hypothesis. J. Biogeogr. 2013, 40, 1649–1663. [Google Scholar] [CrossRef]
- Medellín, R.A.; Equihua, M.; Amin, M.A. Bat diversity and abundance as indicators of disturbance in Neotropical rainforests. Conserv. Biol. 2000, 14, 1666–1675. [Google Scholar] [CrossRef]
- Marjakangas, E.L.; Abrego, N.; Grøtan, V.; de Lima, R.A.; Bello, C.; Bovendorp, R.S.; Culot, L.; Hasui, É.; Lima, F.; Muylaert, R.L.; et al. Fragmented tropical forests lose mutualistic plant–animal interactions. Divers. Distrib. 2020, 26, 154–168. [Google Scholar] [CrossRef]
- Faria, D. Phyllostomid bats of a fragmented landscape in the north-eastern Atlantic forest, Brazil. J. Trop. Ecol. 2006, 22, 531–542. [Google Scholar] [CrossRef]
- Laurance, W.F.; Lovejoy, T.E.; Vasconcelos, H.L.; Bruna, E.M.; Didham, R.K.; Stouffer, P.C.; Gascon, C.; Bierregaard, R.O.; Laurance, S.G.; Sampaio, E. Ecosystem decay of Amazonian forest fragments: A 22-year investigation. Conserv. Biol. 2002, 16, 605–618. [Google Scholar] [CrossRef]
- Bovendorp, R.S.; Brum, F.T.; McCleery, R.A.; Baiser, B.; Loyola, R.; Cianciaruso, M.V.; Galetti, M. Defaunation and fragmentation erode small mammal diversity dimensions in tropical forests. Ecography 2018, 42, 23–35. [Google Scholar] [CrossRef]
- Banks-Leite, C.; Pardini, R.; Tambosi, L.R.; Pearse, W.D.; Bueno, A.A.; Bruscagin, R.T.; Condez, T.H.; Dixo, M.; Igari, A.T.; Martensen, A.C.; et al. Using ecological thresholds to evaluate the costs and benefits of set-asides in a biodiversity hotspot. Science 2014, 345, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Morante-Filho, J.C.; Arroyo-Rodríguez, V.; Faria, D. Patterns and predictors of β-diversity in the fragmented Brazilian Atlantic forest: A multiscale analysis of forest specialist and generalist birds. J. Anim. Ecol. 2016, 85, 240–250. [Google Scholar] [CrossRef]
- Kunz, T.H.; de Torrez, E.B.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. In Year in Ecology and Conservation Biology; Ostfeld, R.S., Schlesinger, W.H., Eds.; Annals of the New York Academy of Sciences; Wiley-Blackwell: Oxford, UK, 2011; Volume 1223, pp. 1–38. [Google Scholar]
- Fleming, T.H.; Martino, A.M.; Dávalos, L.; Mello, M. Population Biology; The University of Chicago Press: Chicago, IL, USA, 2020. [Google Scholar]
- Bredt, A.; Uieda, W.; Pedro, W.A. Plantas e Morcegos: Na Recuperação de Áreas Degradadas e na Paisagem Urbana; Rede de sementes do Cerrado: Brazil, Spain, 2012. [Google Scholar]
- Cleveland, C.J.; Betke, M.; Federico, P.; Frank, J.D.; Hallam, T.G.; Horn, J.; López, J.D., Jr.; McCracken, G.F.; Medellín, R.A.; Moreno-Valdez, A. Economic value of the pest control service provided by Brazilian free-tailed bats in south-central Texas. Front. Ecol. Environ. 2006, 4, 238–243. [Google Scholar] [CrossRef]
- Cassano, C.R.; Silva, R.M.; Mariano-Neto, E.; Schroth, G.; Faria, D. Bat and bird exclusion but not shade cover influence arthropod abundance and cocoa leaf consumption in agroforestry landscape in northeast Brazil. Agric. Ecosyst. Environ. 2016, 232, 247–253. [Google Scholar] [CrossRef]
- Meyer, C.F.; Kalko, E.K. Assemblage-level responses of phyllostomid bats to tropical forest fragmentation: Land-bridge islands as a model system. J. Biogeogr. 2008, 35, 1711–1726. [Google Scholar] [CrossRef]
- Arroyo-Rodríguez, V.; Rojas, C.; Saldaña-Vázquez, R.A.; Stoner, K.E. Landscape composition is more important than landscape configuration for phyllostomid bat assemblages in a fragmented biodiversity hotspot. Biol. Conserv. 2016, 198, 84–92. [Google Scholar] [CrossRef]
- Rezende, C.L.; Scarano, F.R.; Assad, E.D.; Joly, C.A.; Metzger, J.P.; Strassburg, B.B.N.; Tabarelli, M.; Fonseca, G.A.; Mittermeier, R.A. From hotspot to hopespot: An opportunity for the Brazilian Atlantic Forest. Perspect. Ecol. Conserv. 2018, 16, 208–214. [Google Scholar] [CrossRef]
- Vancine, M.H.; Muylaert, R.L.; Niebuhr, B.B.; Oshima, J.E.D.F.; Tonetti, V.; Bernardo, R.; De Angelo, C.; Rosa, M.R.; Grohmann, C.H.; Ribeiro, M.C. The Atlantic Forest of South America: Spatiotemporal dynamics of the vegetation and implications for conservation. Biol. Conserv. 2024, 291, 110499. [Google Scholar] [CrossRef]
- Benchimol, M.; Talora, D.C.; Mariano-Neto, E.; Oliveira, T.L.; Leal, A.; Mielke, M.S.; Faria, D. Losing our palms: The influence of landscape-scale deforestation on Arecaceae diversity in the Atlantic forest. For. Ecol. Manag. 2017, 384, 314–322. [Google Scholar] [CrossRef]
- Faria, D.; Paciencia, M.L.B.; Dixo, M.; Laps, R.R.; Baumgarten, J. Ferns, frogs, lizards, birds and bats in forest fragments and shade cacao plantations in two contrasting landscapes in the Atlantic forest, Brazil. Biodivers. Conserv. 2007, 16, 2335–2357. [Google Scholar] [CrossRef]
- Melo, R.S.; Alexandrino, E.R.; de Paula, F.R.; Boscolo, D.; de Barros Ferraz, S.F. Promoting bird functional diversity on landscapes with a matrix of planted Eucalyptus spp. in the Atlantic Forest. Environ. Manag. 2024, 73, 395–407. [Google Scholar] [CrossRef]
- García-Morales, R.; Badano, E.I.; Moreno, C.E. Response of Neotropical bat assemblages to human land use. Conserv. Biol. 2013, 27, 1096–1106. [Google Scholar] [CrossRef]
- Estrada, A.; Coates-Estrada, R. Bats in continuous forest, forest fragments and in an agricultural mosaic habitat-island at Los Tuxtlas, Mexico. Biol. Conserv. 2002, 103, 237–245. [Google Scholar] [CrossRef]
- Quesada, M.; Stoner, K.E.; Rosas-Guerrero, V.; Palacios-Guevara, C.; Lobo, J.A. Effects of habitat disruption on the activity of nectarivorous bats (Chiroptera: Phyllostomidae) in a dry tropical forest: Implications for the reproductive success of the neotropical tree Ceiba grandiflora. Oecologia 2003, 135, 400–406. [Google Scholar] [CrossRef]
- Falcão, F.; Dodonov, P.; Caselli, C.B.; dos Santos, J.S.; Faria, D. Landscape structure shapes activity levels and composition of aerial insectivorous bats at different spatial scales. Biodivers. Conserv. 2021, 30, 2545–2564. [Google Scholar] [CrossRef]
- Orihuela, R.L.; Peres, C.A.; Mendes, G.; Jarenkow, J.A.; Tabarelli, M. Markedly Divergent Tree Assemblage Responses to Tropical Forest Loss and Fragmentation across a Strong Seasonality Gradient. PLoS ONE 2015, 10, e0136018. [Google Scholar] [CrossRef]
- Avila-Cabadilla, L.D.; Sanchez-Azofeifa, G.A.; Stoner, K.E.; Alvarez-Anorve, M.Y.; Quesada, M.; Portillo-Quintero, C.A. Local and landscape factors determining occurrence of phyllostomid bats in tropical secondary forests. PLoS ONE 2012, 7, e35228. [Google Scholar] [CrossRef]
- Li, H.; Petric, R.; Alazzawi, Z.; Kauzlarich, J.; Mahmoud, R.H.; McFadden, R.; Perslow, N.; Rodriguez Flores, A.; Soufi, H.; Morales, K. Four years continuous monitoring reveals different effects of urban constructed wetlands on bats. Land 2021, 10, 1087. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.T.; Fontes, M.A.L. Patterns of floristic differentiation among Atlantic forests in southeastern Brazil and the influence of climate. Biotropica 2000, 32, 793–810. [Google Scholar] [CrossRef]
- Thomas, W.W.; Carvalho, A.M.D.; Amorim, A.M.; Garrison, J.; Arbela’ez, A.L. Plant endemism in two forests in southern Bahia, Brazil. Biodivers. Conserv. 1998, 7, 311–322. [Google Scholar] [CrossRef]
- Mori, S.A.; Boom, B.M.; Decarvalho, A.M.; Dossantos, T.S. Southern Bahian Moist Forests. Bot. Rev. 1983, 49, 155–232. [Google Scholar] [CrossRef]
- QGIS, D.T. QGIS, Geographic Information System; Open Source Geospatial Foundation: San Michele all’Adige, Italy, 2017. [Google Scholar]
- Eigenbrod, F.; Hecnar, S.J.; Fahrig, L. Sub-optimal study design has major impacts on landscape-scale inference. Biol. Conserv. 2011, 144, 298–305. [Google Scholar] [CrossRef]
- Huais, P.Y. multifit: An R function for multi-scale analysis in landscape ecology. Landsc. Ecol. 2018, 33, 1023–1028. [Google Scholar] [CrossRef]
- Malcolm, J.R. Forest structure and the abundance and diversity of neotropical small mammals. In Forest Canopies; Lowman, M.D., Nadkarni, N.M., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 179–197. [Google Scholar]
- Muylaert, R.L.; Stevens, R.D.; Ribeiro, M.C. Threshold effect of habitat loss on bat richness in cerrado-forest landscapes. Ecol. Appl. 2016, 26, 1854–1867. [Google Scholar] [CrossRef]
- Bergallo, H.G.; Esberard, C.E.L.; Mello, M.A.R.; Lins, V.; Mangolin, R.; Melo, G.G.S.; Baptista, M. Bat species richness in Atlantic forest: What is the minimum sampling effort? Biotropica 2003, 35, 278–288. [Google Scholar]
- Vizotto, L.; Taddei, V.A. Chave para Determinação de Quirópteros Brasileiros; ScienceOpen, Inc.: Lexington, MA, USA, 1973. [Google Scholar]
- Simmons, N.B.; Voss, R.S. The Mammals of Paracou, French Guiana, a Neotropical Lowland Rainforest Fauna; American Museum of Natural History: New York, NY, USA, 1998. [Google Scholar]
- Gardner, A. Mammals of South America, Volume 1: Marsupials, Xenarthrans, Shrews, and Bats; University of Chicago Press: Chicago, IL, USA, 2009; Volume 1, pp. 1–690. [Google Scholar]
- Simmons, N.B. Order chiroptera. Mammal Species World 2005, 1, 337. [Google Scholar]
- Nogueira, M.R.; Lima, I.P.D.; Moratelli, R.; Tavares, V.D.C.; Gregorin, R.; Lúcio, P. Checklist of Brazilian bats, with comments on original records. Check List. 2014, 10, 808–821. [Google Scholar] [CrossRef]
- Dale, M.R.; Fortin, M.-J. Spatial Analysis: A Guide for Ecologists; University Press: Cambridge, UK, 2014. [Google Scholar]
- Bolker, B.; Skaug, H.; Magnusson, A.; Nielsen, A. Getting Started with the glmmADMB Package. 2012. Available online: https://glmmadmb.r-forge.r-project.org/glmmADMB.pdf (accessed on 1 December 2024).
- Muggeo, V. Segmented: An R package to fit regression models with broken-line relationships. R. News 2008, 8, 20–25. Available online: https://journal.r-project.org/articles/RN-2008-004/RN-2008-004.pdf (accessed on 1 December 2024).
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: http://www.R-project.org/ (accessed on 1 December 2024).
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’hara, R.; Solymos, P.; Stevens, M.; Szoecs, E. Vegan: Community Ecology Package, version 2.6-4; R package: Madison, WI, USA, 2022. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. nlme: Linear and Nonlinear Mixed Effects Models, version 3; R package: Madison, WI, USA, 2017; pp. 1–131. Available online: https://CRAN.R–project.org/package=nlme (accessed on 1 December 2024).
- Sanchez, G. PLS path modeling with R. Trowchez Ed. 2013, 383, 551. [Google Scholar]
- Wickham, H.; Bryan, J. R Packages; O’Reilly Media, Inc.: Sevastopol, CA, USA, 2023. [Google Scholar]

| Species | Trophic Guilds | N | (%) |
|---|---|---|---|
| Family Phyllostomidae | |||
| Subfamily Desmodontinae | |||
| Desmodus rotundus (É. Geoffroy, 1810) | H | 4 | 0.67 |
| Subfamily Glossophaginae | |||
| Glossophaga soricina (Pallas, 1766) | N | 7 | 1.17 |
| Lonchophyla sp. | N | 1 | 0.17 |
| Subfamily Phyllostominae | |||
| Chrotopterus auritus (Peters, 1856) | C | 3 | 0.50 |
| Micronycteris megalotis (Gray, 1842) | Ic | 1 | 0.17 |
| Micronycteris sp. | Ic | 1 | 0.17 |
| Phylloderma stenops (Peters 1865) | O | 3 | 0.50 |
| Phyllostomus discolor (Wagner, 1843) | O | 3 | 0.50 |
| Gardenericterys crenulatum (É. Geoffroy, 1803) | Ic | 1 | 0.17 |
| Lophostoma brasiliensis (Peters, 1866) | Ic | 2 | 0.34 |
| Subfamily Carolliinae | |||
| Carollia brevicauda (Linnaeus, 1758) | F | 12 | 2.01 |
| Carollia perspicillata (Linnaeus, 1758) | F | 268 | 44.97 |
| Subfamily Rhinophyllinae | |||
| Rhinophylla fischerae (Carter, 1966) | F | 3 | 0.50 |
| Rhinophylla pumilio (Peters, 1865) | F | 142 | 23.83 |
| Subfamily Stenodermatinae | |||
| Artibeus lituratus (Olfers, 1818) | F | 33 | 5.54 |
| Artibeus planirostris (Spix, 1823) | F | 10 | 1.68 |
| Artibeus obscurus (Schinz, 1821) | F | 57 | 9.56 |
| Dermanura cinerea (Gervais, 1856) | F | 40 | 6.71 |
| Platyrrhinus lineatus (É. Geoffroy, 1810) | F | 1 | 0.17 |
| Sturnira lilium (É. Geoffroy, 1810) | F | 4 | 0.67 |
| TOTAL: | 596 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bovendorp, R.; Mariano-Neto, E.; Queiroz, A.; Faria, D. Landscape Composition and Forest Structure Shape Phyllostomid Bat Assemblages in the Atlantic Forest Remnants. Animals 2025, 15, 2082. https://doi.org/10.3390/ani15142082
Bovendorp R, Mariano-Neto E, Queiroz A, Faria D. Landscape Composition and Forest Structure Shape Phyllostomid Bat Assemblages in the Atlantic Forest Remnants. Animals. 2025; 15(14):2082. https://doi.org/10.3390/ani15142082
Chicago/Turabian StyleBovendorp, Ricardo, Eduardo Mariano-Neto, Albérico Queiroz, and Deborah Faria. 2025. "Landscape Composition and Forest Structure Shape Phyllostomid Bat Assemblages in the Atlantic Forest Remnants" Animals 15, no. 14: 2082. https://doi.org/10.3390/ani15142082
APA StyleBovendorp, R., Mariano-Neto, E., Queiroz, A., & Faria, D. (2025). Landscape Composition and Forest Structure Shape Phyllostomid Bat Assemblages in the Atlantic Forest Remnants. Animals, 15(14), 2082. https://doi.org/10.3390/ani15142082







