Regulatory of Oleuropein on the In Vitro Maturation of Oocytes and the Development of Parthenogenetic Embryos in Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oocyte Collection and In Vitro Maturation
2.3. Parthenogenetic Activation and Embryo Culture
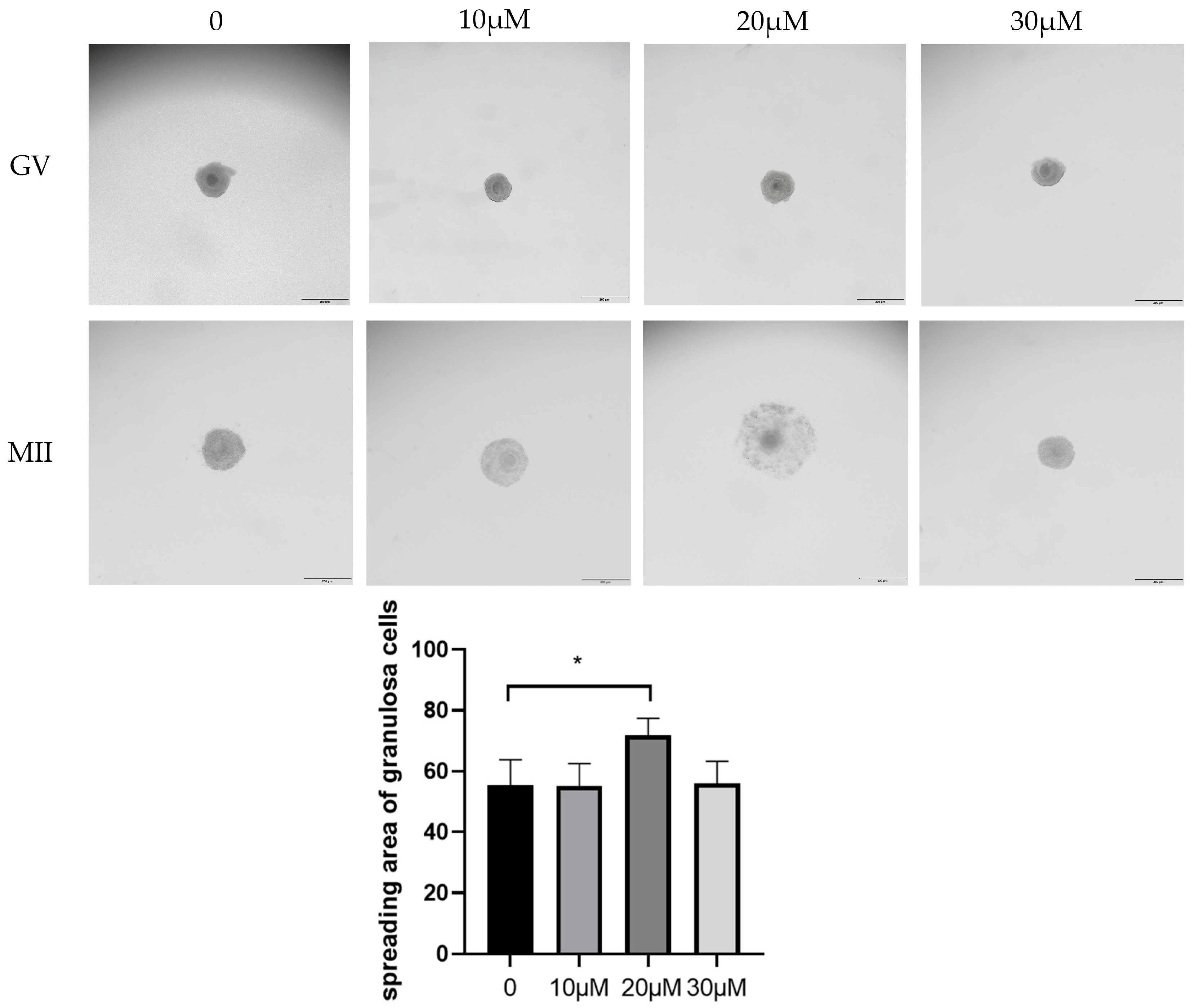
2.4. Granule Cell Expansion Assessment and Calculation of Maturation Rate, Cleavage Rate, and Blastocyst Rate
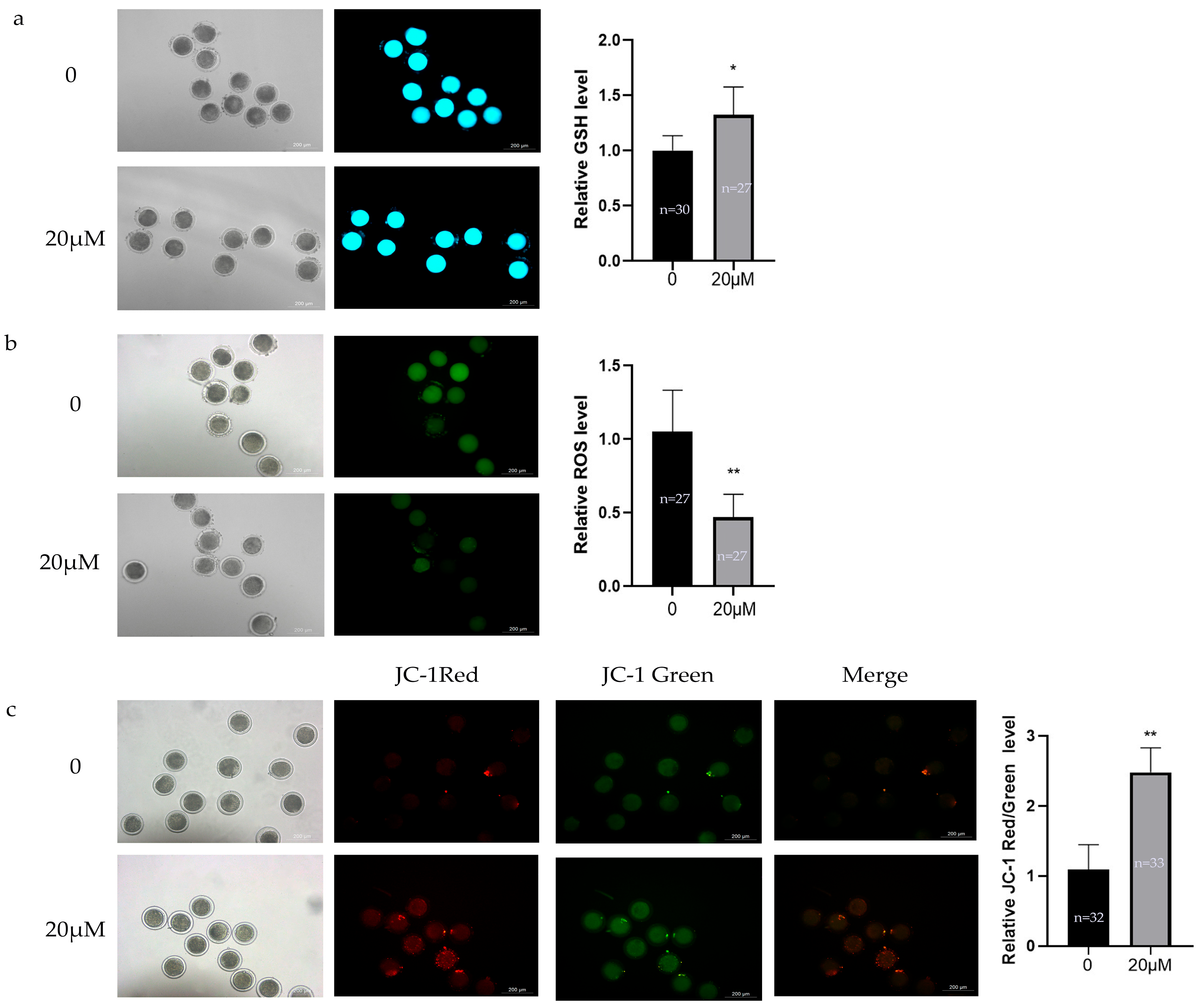
2.5. ROS and GSH Detection
2.6. Mitochondrial Membrane Potential Detection
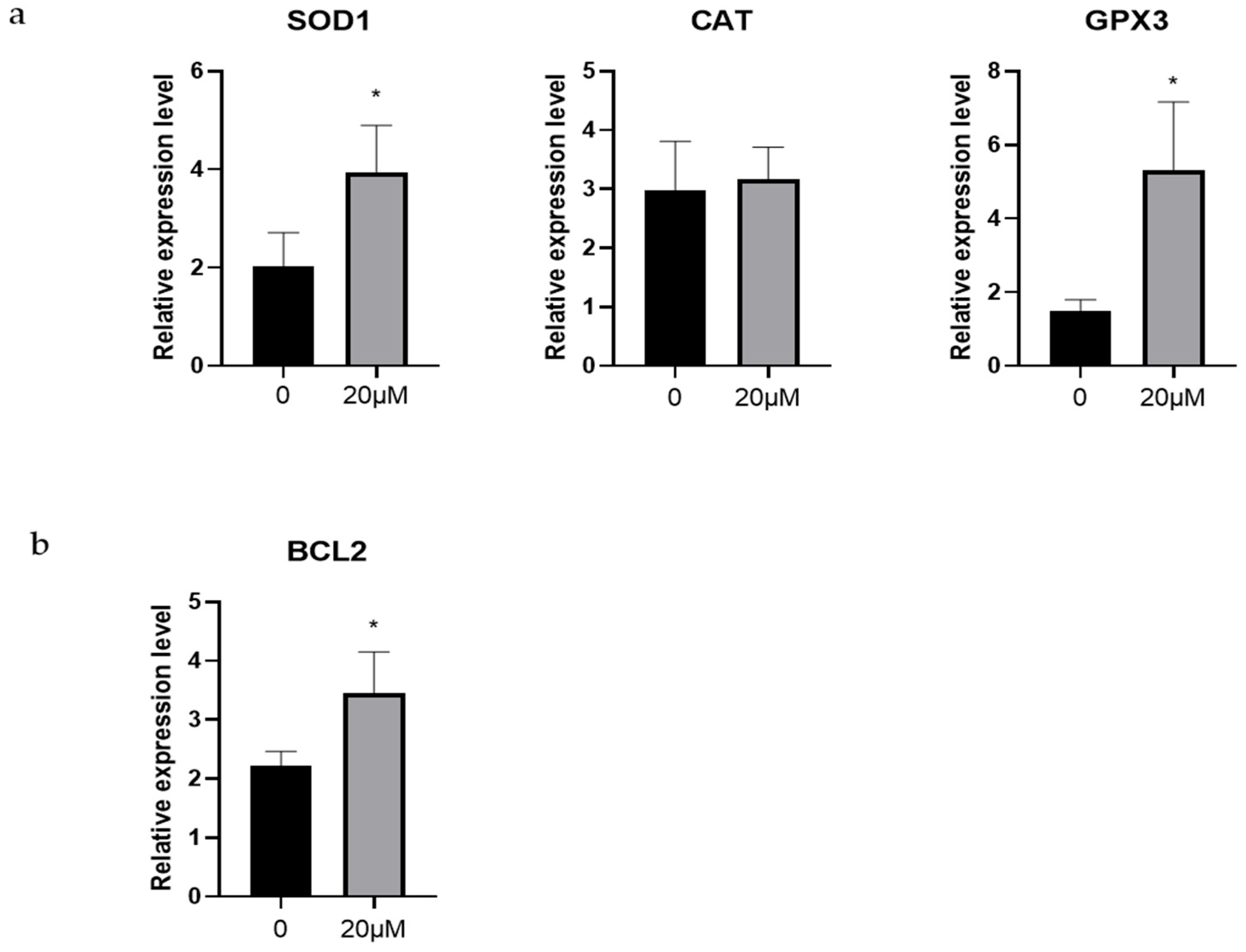
2.7. Quantitative RT-PCR
2.8. Statistical Analysis
3. Results
3.1. Effects of Different OLE Concentrations on In Vitro Maturation of Sheep Oocytes
3.2. Effects of OLE on Oxidative Stress Levels in Sheep Oocytes
3.3. Effects of OLE on Antioxidant-Related and Antiapoptosis-Related Genes in Sheep Oocytes
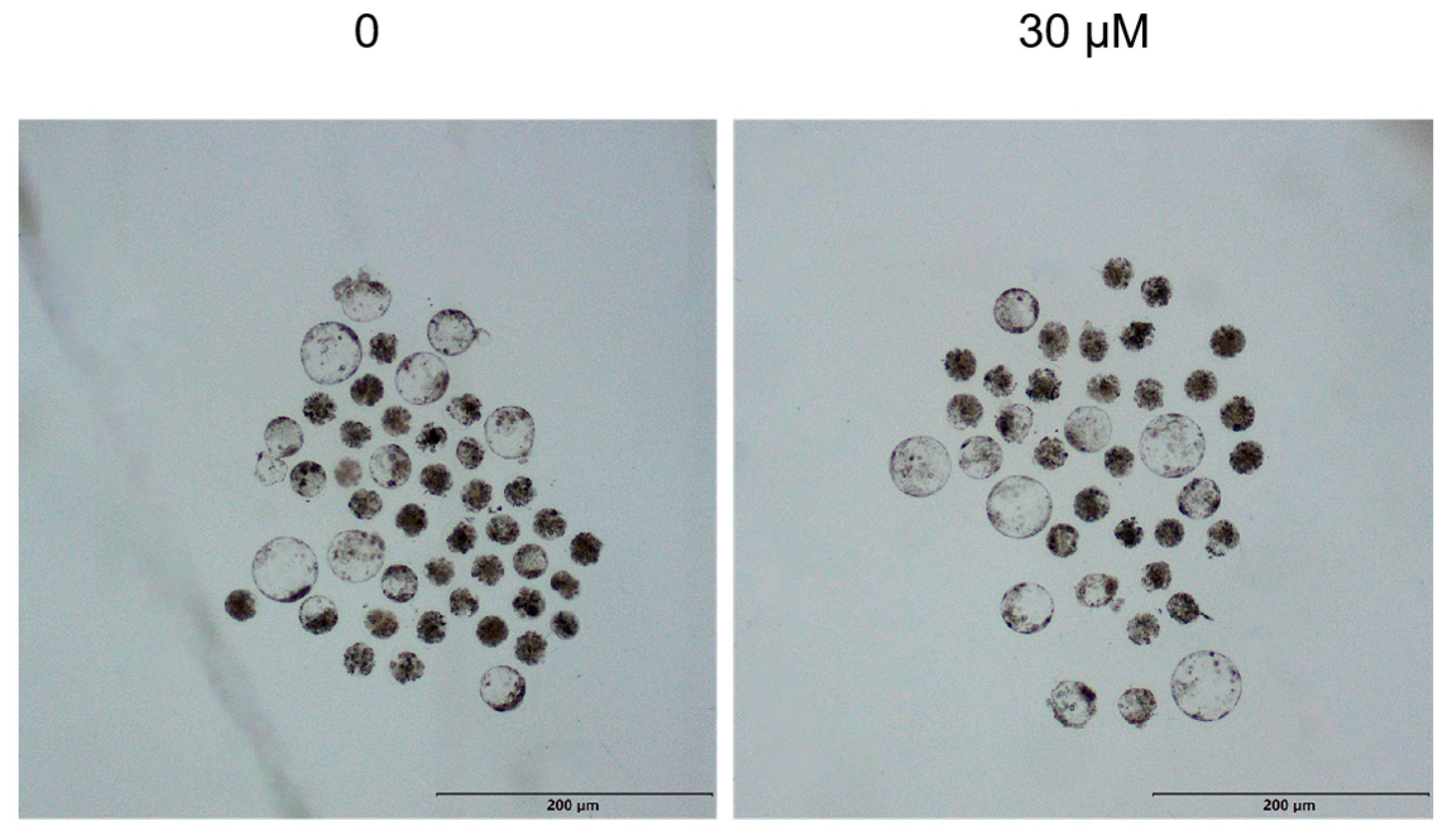
3.4. Effects of Different OLE Concentrations on Early Embryonic Development
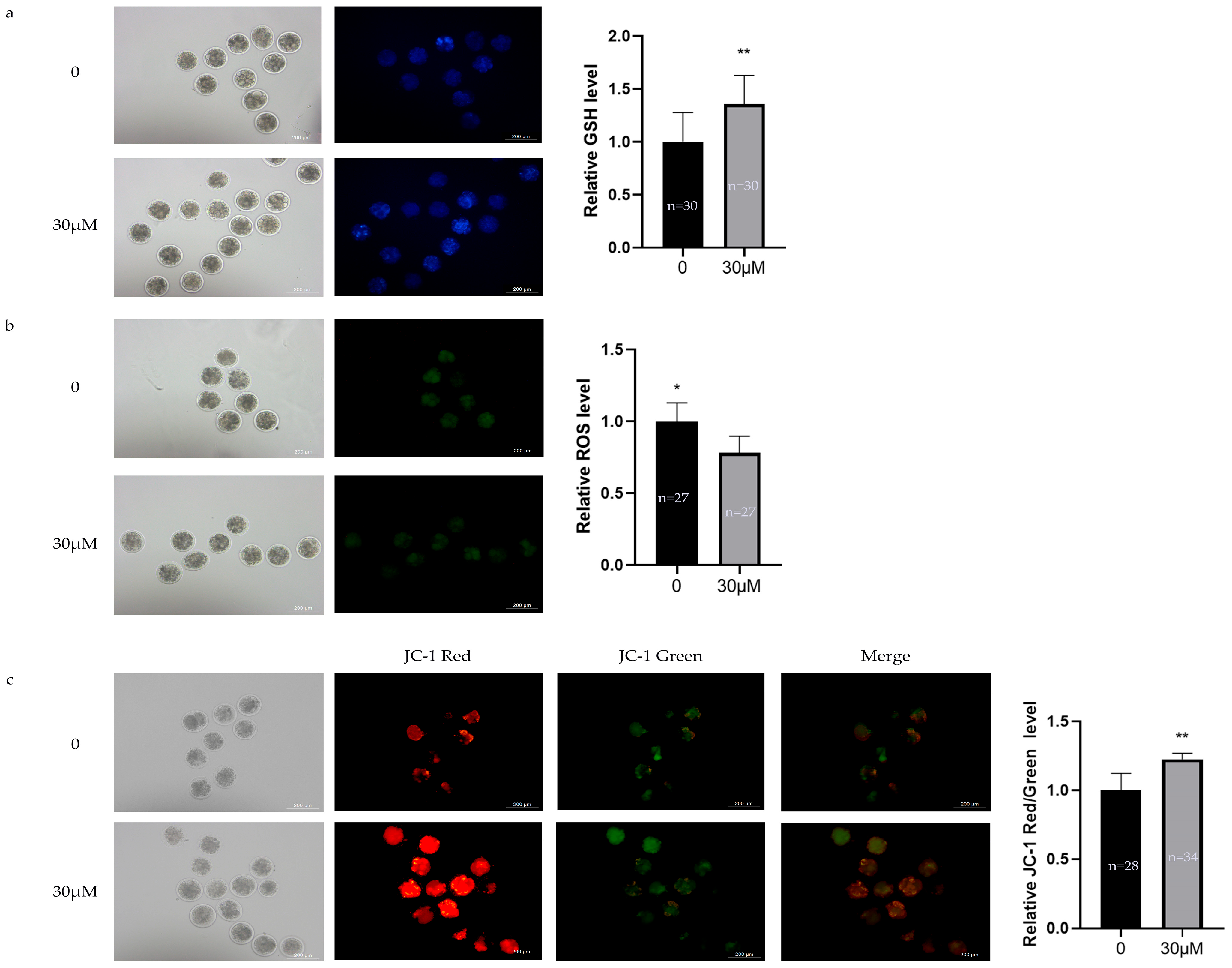
3.5. Effects of OLE on Oxidative Stress Levels in Sheep Early Embryos
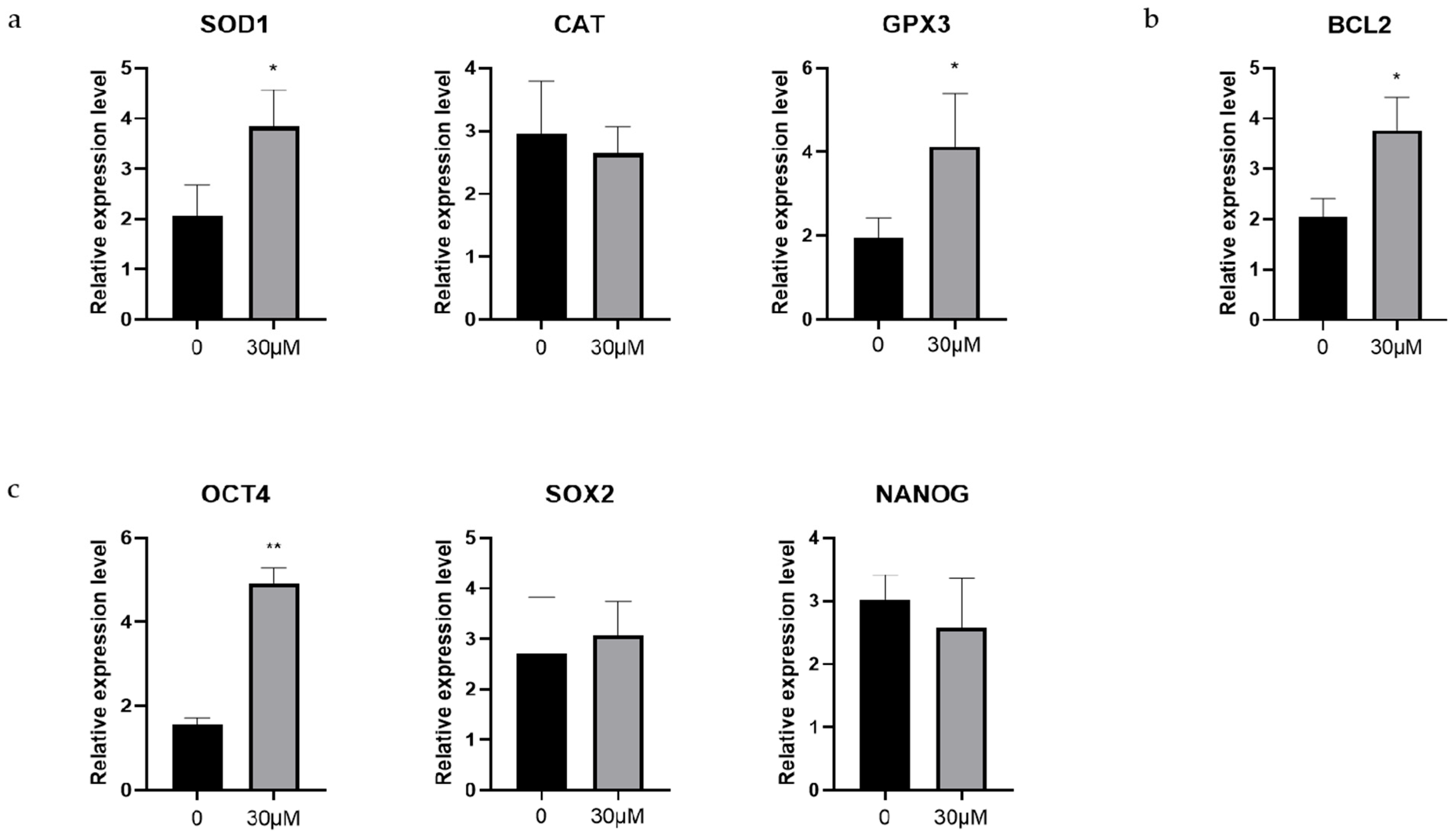
3.6. Effects of OLE on Antioxidant-Related, Antiapoptosis-Related and Early Embryonic Development-Related Genes in Sheep Early Embryos
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, Y.; Wu, R.; Lee, J.M.; Chan, L.H.M.; Chan, K.Y.J. Microfluidic In-Vitro Fertilization Technologies: Transforming the Future of Human Reproduction. TrAC Trends Anal. Chem. 2023, 160, 116959. [Google Scholar] [CrossRef]
- Cognié, Y.; Baril, G.; Poulin, N.; Mermillod, P. Current Status of Embryo Technologies in Sheep and Goat. Theriogenology 2003, 59, 171–188. [Google Scholar] [CrossRef]
- Wale, P.L.; Gardner, D.K. The Effects of Chemical and Physical Factors on Mammalian Embryo Culture and Their Importance for the Practice of Assisted Human Reproduction. Hum. Reprod. Update 2015, 22, 2–22. [Google Scholar] [CrossRef]
- Sirard, M.-A. 40 Years of Bovine IVF in the New Genomic Selection Context. Reproduction 2018, 156, R1–R7. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heras, S.; Paramio, M.-T. Impact of Oxidative Stress on Oocyte Competence for in Vitro Embryo Production Programs. Res. Vet. Sci. 2020, 132, 342–350. [Google Scholar] [CrossRef]
- Cui, Y.; Gao, H.; Han, S.; Yuan, R.; He, J.; Zhuo, Y.; Feng, Y.-L.; Tang, M.; Feng, J.; Yang, S. Oleuropein Attenuates Lipopolysaccharide-Induced Acute Kidney Injury In Vitro and In Vivo by Regulating Toll-Like Receptor 4 Dimerization. Front. Pharmacol. 2021, 12, 617314. [Google Scholar] [CrossRef]
- Leto, G.; Flandina, C.; Crescimanno, M.; Giammanco, M.; Sepporta, M.V. Effects of Oleuropein on Tumor Cell Growth and Bone Remodelling: Potential Clinical Implications for the Prevention and Treatment of Malignant Bone Diseases. Life Sci. 2021, 264, 118694. [Google Scholar] [CrossRef] [PubMed]
- Santis, S.D.; Liso, M.; Verna, G.; Curci, F.; Milani, G.; Faienza, M.F.; Franchini, C.; Moschetta, A.; Chieppa, M.; Clodoveo, M.L.; et al. Extra Virgin Olive Oil Extracts Modulate the Inflammatory Ability of Murine Dendritic Cells Based on Their Polyphenols Pattern: Correlation between Chemical Composition and Biological Function. Antioxidants 2021, 10, 1016. [Google Scholar] [CrossRef]
- Micheli, L.; Bertini, L.; Bonato, A.; Villanova, N.; Caruso, C.; Caruso, M.; Bernini, R.; Tirone, F. Role of Hydroxytyrosol and Oleuropein in the Prevention of Aging and Related Disorders: Focus on Neurodegeneration, Skeletal Muscle Dysfunction and Gut Microbiota. Nutrients 2023, 15, 1767. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, Y.; Park, T. Hepatoprotective Effect of Oleuropein in Mice: Mechanisms Uncovered by Gene Expression Profiling. Biotechnol. J. 2010, 5, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Kerimi, A.; Nyambe-Silavwe, H.; Pyner, A.; Oladele, E.; Gauer, J.S.; Stevens, Y.; Williamson, G. Nutritional Implications of Olives and Sugar: Attenuation of Post-Prandial Glucose Spikes in Healthy Volunteers by Inhibition of Sucrose Hydrolysis and Glucose Transport by Oleuropein. Eur. J. Nutr. 2018, 58, 1315–1330. [Google Scholar] [CrossRef]
- Hermans, M.P.; Lempereur, P.; Salembier, J.-P.; Maes, N.; Albert, A.; Jansen, O.; Pincemail, J. Supplementation Effect of a Combination of Olive (Olea europea L.) Leaf and Fruit Extracts in the Clinical Management of Hypertension and Metabolic Syndrome. Antioxidants 2020, 9, 872. [Google Scholar] [CrossRef]
- Merola, N.; Castillo, J.; Benavente-García, O.; Ros, G.; Nieto, G. The Effect of Consumption of Citrus Fruit and Olive Leaf Extract on Lipid Metabolism. Nutrients 2017, 9, 1062. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Ghorbel, H.; Feki, I.; Bouallagui, Z.; Guermazi, F.; Ayadi, L.; Sayadi, S. Oleuropein and Hydroxytyrosol Protect Rats’ Pups against Bisphenol A Induced Hypothyroidism. Biomed. Pharmacother. 2018, 103, 1115–1126. [Google Scholar] [CrossRef]
- He, J.; Huang, L.; Sun, K.; Li, J.; Han, S.; Gao, X.; Wang, Q.-Q.; Yang, S.; Sun, W.; Gao, H. Oleuropein Alleviates Myocardial Ischemia–Reperfusion Injury by Suppressing Oxidative Stress and Excessive Autophagy via TLR4/MAPK Signaling Pathway. Chin. Med. 2024, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, C.; Zhang, Y.; Li, Z.; Pi, W.; Hu, G. Regulation of PPARγ in the Development of Early Sheep Embryos in Vitro. Theriogenology 2025, 234, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Y.; Saredy, J.; Wang, X.; IV, C.D.; Shao, Y.; Saaoud, F.; Xu, K.; Liu, M.; Yang, W.Y.; et al. ROS Systems Are a New Integrated Network for Sensing Homeostasis and Alarming Stresses in Organelle Metabolic Processes. Redox Biol. 2020, 37, 101696. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Antognelli, C.; Frosini, R.; Santolla, M.F.; Peirce, M.J.; Talesa, V.N. Oleuropein-Induced Apoptosis Is Mediated by Mitochondrial Glyoxalase 2 in NSCLC A549 Cells: A Mechanistic Inside and a Possible Novel Nonenzymatic Role for an Ancient Enzyme. Oxid. Med. Cell. Longev. 2019, 2019, 8576961. [Google Scholar] [CrossRef]
- Gherardi, G.; Weiser, A.; Bermont, F.; Migliavacca, E.; Brinon, B.; Jacot, G.E.; Hermant, A.; Sturlese, M.; Nogara, L.; Vascon, F.; et al. Mitochondrial Calcium Uptake Declines during Aging and Is Directly Activated by Oleuropein to Boost Energy Metabolism and Skeletal Muscle Performance. Cell Metab. 2025, 37, 477–495.e11. [Google Scholar] [CrossRef]
- Devi, S.; Negi, S.; Tandel, N.; Dalai, S.K.; Tyagi, R.K. Oleuropein: A Viable Therapeutic Option for Malaria and Cancer. Drug Discov. Today 2025, 30, 104254. [Google Scholar] [CrossRef]
- Blerkom, J.V. Mitochondrial Function in the Human Oocyte and Embryo and Their Role in Developmental Competence. Mitochondrion 2011, 11, 797–813. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Kang, Z.; Qiao, N.; Wang, C.; Tang, Z. Cu-Induced Mitochondrial Dysfunction Is Mediated by Abnormal Mitochondrial Fission through Oxidative Stress in Primary Chicken Embryo Hepatocytes. J. Trace Elem. Med. Biol. 2021, 65, 126721. [Google Scholar] [CrossRef]
- Kirillova, A.; Smitz, J.E.J.; Sukhikh, G.T.; Mazunin, I. The Role of Mitochondria in Oocyte Maturation. Cells 2021, 10, 2484. [Google Scholar] [CrossRef] [PubMed]
- Kadenbach, B.; Ramzan, R.; Vogt, S. High Efficiency versus Maximal Performance—The Cause of Oxidative Stress in Eukaryotes: A Hypothesis. Mitochondrion 2013, 13, 1–6. [Google Scholar] [CrossRef]
- Spikings, E.C.; Alderson, J.; John, J.C.S. Regulated Mitochondrial DNA Replication during Oocyte Maturation Is Essential for Successful Porcine Embryonic Development. Biol. Reprod. 2007, 76, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Tollner, T.L.; Hu, Z.; Dai, M.; Li, X.; Guan, H.; Shan, D.; Zhang, X.; Lv, J.; Huang, C.; et al. The Importance of Mitochondrial Metabolic Activity and Mitochondrial DNA Replication during Oocyte Maturation in Vitro on Oocyte Quality and Subsequent Embryo Developmental Competence. Mol. Reprod. Dev. 2012, 79, 392–401. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Ji, Q.; Cong, P.; Zhao, H.; Song, Z.; Zhao, G.; Gao, J.; Nie, Y.; Chen, Y. Exogenous Expression of OCT4 Facilitates Oocyte-Mediated Reprogramming in Cloned Porcine Embryos. Mol. Reprod. Dev. 2014, 81, 820–832. [Google Scholar] [CrossRef]
- Hisey, E.; Ross, P.J.; Meyers, S.A. A Review of OCT4 Functions and Applications to Equine Embryos. J. Equine Vet. Sci. 2021, 98, 103364. [Google Scholar] [CrossRef] [PubMed]
- Abdulhasan, M.K.; Li, Q.; Dai, J.; Abu-Soud, H.M.; Puscheck, E.E.; Rappolee, D.A. CoQ10 Increases Mitochondrial Mass and Polarization, ATP and Oct4 Potency Levels, and Bovine Oocyte MII during IVM While Decreasing AMPK Activity and Oocyte Death. J. Assist. Reprod. Genet. 2017, 34, 1595–1607. [Google Scholar] [CrossRef]
- Zuccotti, M.; Merico, V.; Belli, M.; Mulas, F.; Sacchi, L.; Zupan, B.; Redi, C.A.; Prigione, A.; Adjaye, J.; Bellazzi, R.; et al. OCT4 and the Acquisition of Oocyte Developmental Competence during Folliculogenesis. Int. J. Dev. Biol. 2012, 56, 853–858. [Google Scholar] [CrossRef]
- Pan, H.; Schultz, R.M. Sox2 Modulates Reprogramming of Gene Expression in Two-Cell Mouse Embryos. Biol. Reprod. 2011, 85, 409–416. [Google Scholar] [CrossRef]
- He, M.; Jiao, S.; Zhang, R.; Ye, D.; Wang, H.; Sun, Y. Translational Control by Maternal Nanog Promotes Oogenesis and Early Embryonic Development. Development 2022, 149, dev201213. [Google Scholar] [CrossRef] [PubMed]
- Navarro, P.; Festuccia, N.; Colby, D.; Gagliardi, A.; Mullin, N.P.; Zhang, W.; Karwacki-Neisius, V.; Osorno, R.; Kelly, D.; Robertson, M.; et al. OCT4/SOX2-Independent Nanog Autorepression Modulates Heterogeneous Nanog Gene Expression in Mouse ES Cells. EMBO J. 2012, 31, 4547–4562. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | GenBank Accession | Sequence (5′-3′) | Length (bp) |
|---|---|---|---|
| CAT | XM_004016396 | F: CCAGCGACCAGATGAAAC R: CGGTCAAAGTGAGCCATT | 175 |
| GPX3 | XM_015096153 | F: AGGAGAAGTCGAAGATGGACTG R: TCAGTAGCTGGCCACGTTGA | 156 |
| SOD1 | NM_001145185 | F: AGGGAGATAAAGTCGTCGTA R: ACAGAGGATTAAAGTGAGGG | 129 |
| BAX | XM_027978594.2 | F: CATGGGCTGGACATTGGACT R: CCAGATGGTGAGTGAGGCAG | 157 |
| BCL2 | XM_027960877.2 | F: TGGCCTTCTTTGAGTTCGGA R: CGGTTCAGGTACTCGGTCAT | 106 |
| β-Actin | XM_004013078.5 | F: AGATTATCGCTCCTCCCG R: CTCATCATACTCCTGCTTGCT | 110 |
| Group | Oocytes | PB1 Extrusion Rate/% |
|---|---|---|
| 0 | 99 | 54.55 ± 4.83 b |
| 10 μM/L | 103 | 55.34 ± 4.37 b |
| 20 μM/L | 105 | 71.73 ± 3.26 a |
| 30 μM/L | 98 | 56.12 ± 4.19 b |
| Group | Oocytes | Cleavage Rate/% | Blastocyst Rate/% |
|---|---|---|---|
| 0 | 103 | 69.57% | 42.72 ± 4.37 a |
| 20 μM/L | 105 | 70.00% | 44.76 ± 3.42 a |
| 30 μM/L | 112 | 69.23% | 49.11 ± 3.07 b |
| 40 μM/L | 101 | 70.37% | 38.61 ± 3.36 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhao, W.; Ma, Z.; Li, Z.; Liu, Z.; Wan, P.; Hu, G. Regulatory of Oleuropein on the In Vitro Maturation of Oocytes and the Development of Parthenogenetic Embryos in Sheep. Animals 2025, 15, 3011. https://doi.org/10.3390/ani15203011
Zhang Y, Zhao W, Ma Z, Li Z, Liu Z, Wan P, Hu G. Regulatory of Oleuropein on the In Vitro Maturation of Oocytes and the Development of Parthenogenetic Embryos in Sheep. Animals. 2025; 15(20):3011. https://doi.org/10.3390/ani15203011
Chicago/Turabian StyleZhang, Yue, Wenjuan Zhao, Zihao Ma, Zhenghang Li, Zhijiao Liu, Pengcheng Wan, and Guangdong Hu. 2025. "Regulatory of Oleuropein on the In Vitro Maturation of Oocytes and the Development of Parthenogenetic Embryos in Sheep" Animals 15, no. 20: 3011. https://doi.org/10.3390/ani15203011
APA StyleZhang, Y., Zhao, W., Ma, Z., Li, Z., Liu, Z., Wan, P., & Hu, G. (2025). Regulatory of Oleuropein on the In Vitro Maturation of Oocytes and the Development of Parthenogenetic Embryos in Sheep. Animals, 15(20), 3011. https://doi.org/10.3390/ani15203011





