Effect of N-Carbamylglutamate Supplementation in Late Pregnancy on Nutrient-Restricted Twin-Bearing Ewes on the Pre-Lambing Maternal Metabolome, Colostrum Quality and Lamb Birth Weight
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Experimental Procedure
2.3. Assessment of Maternal Blood Metabolites
Sample Preparation for Metabolite Extraction
2.4. Colostrum Analysis
2.5. Statistical Analysis
3. Results
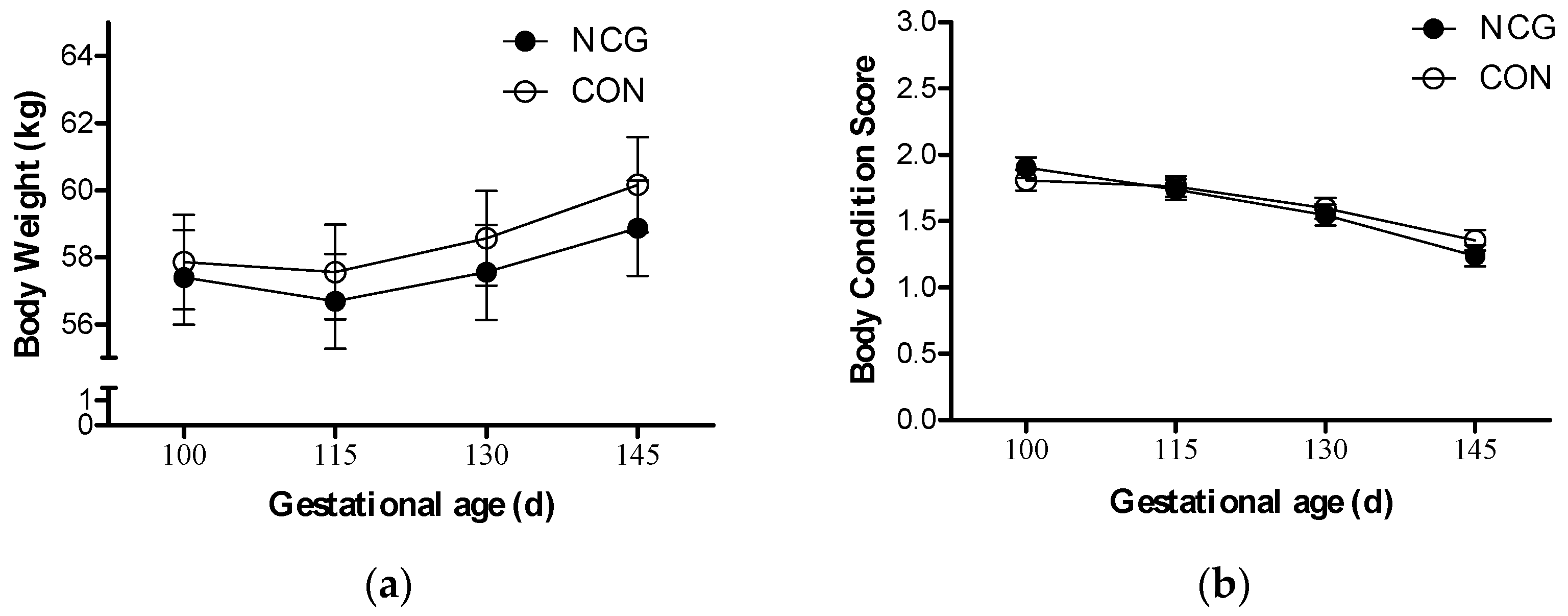
3.1. Maternal Body Weight and BCS
3.2. Lamb Body Weight and Biometric Variables
3.3. Maternal Blood Metabolites
3.4. Colostrum Composition and IgG Concentration
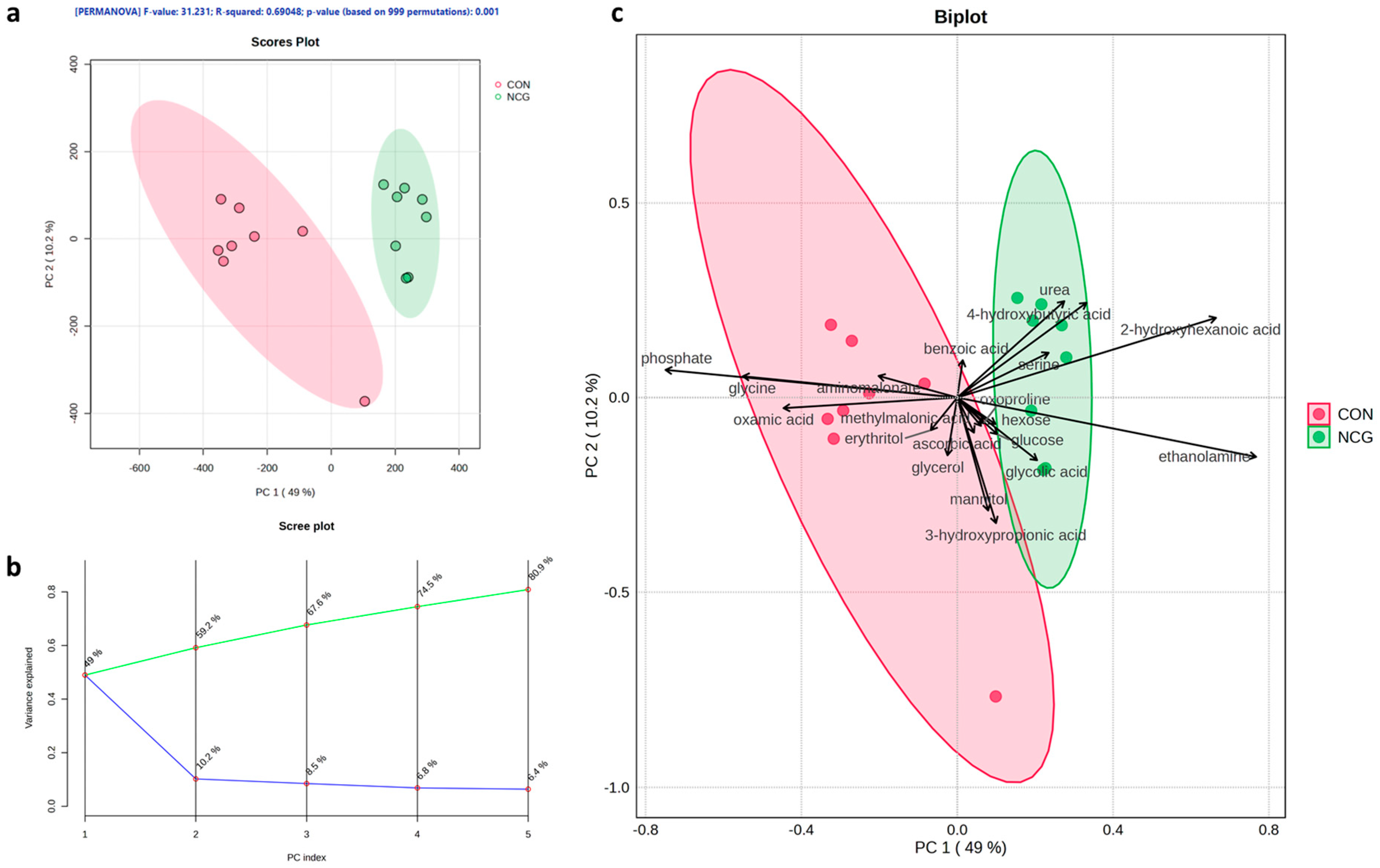
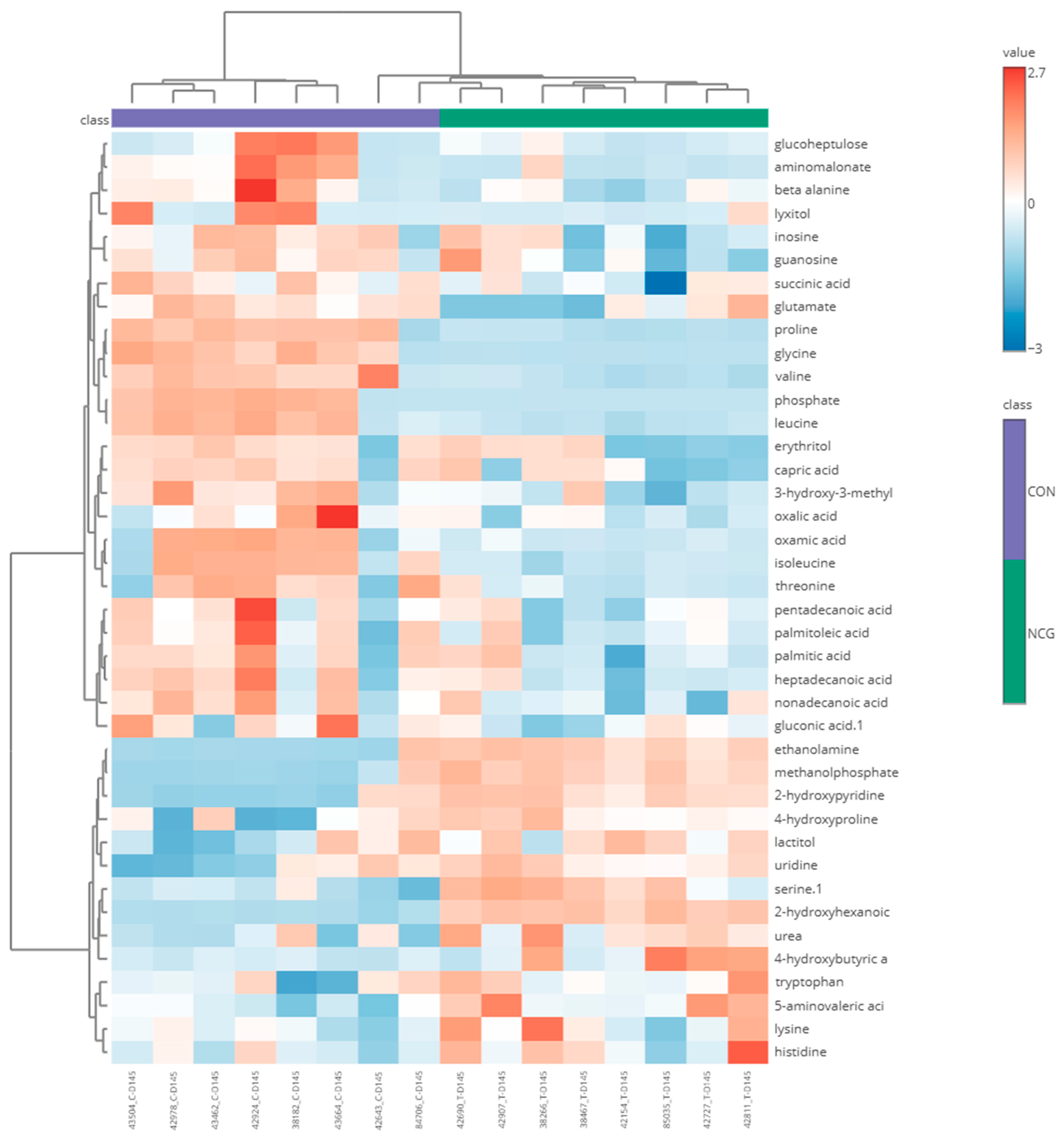
3.5. Maternal Serum Metabolome at 145 dg
3.5.1. Principal Component Analysis (PCA)
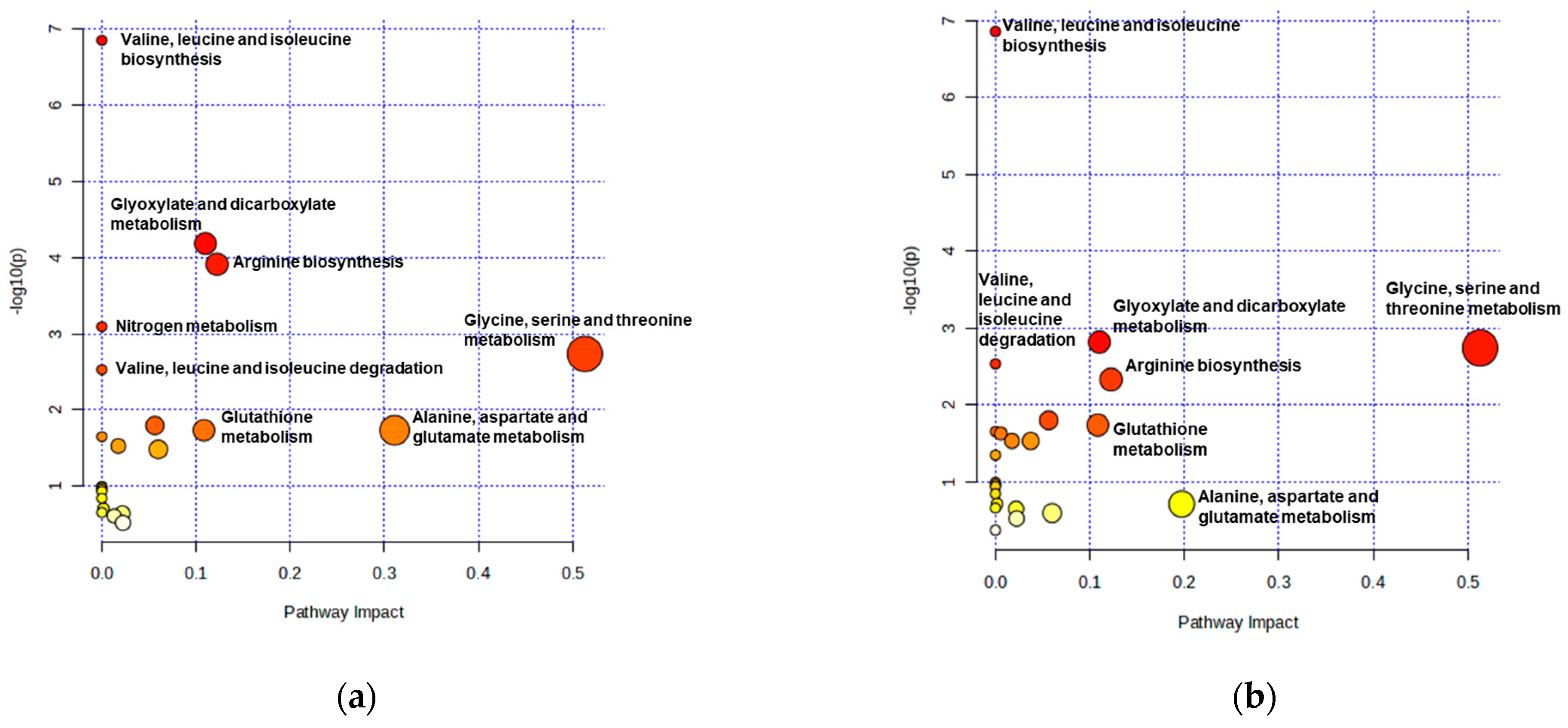
3.5.2. Pathway Topology Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NCG | N-carbamylglutamate |
| BW | Body weight |
| NRC | National Research Council |
| Arg | Arginine |
| dg | Days gestation |
| BCS | Body Condition Score |
References
- Peri, P.L.; Rosas, Y.M.; Rivera, E.; Pastur, G.M. Lamb and Wool Provisioning Ecosystem Services in Southern Patagonia. Sustainability 2021, 13, 8544. [Google Scholar] [CrossRef]
- Covacevich, N.; Ruz, E. Praderas En La Zona Austral: XII Región (Magallanes). In Praderas Para Chile, 2nd ed.; Instituto de Investigaciones Agropecuarias: Chillán, Chile, 1996; pp. 639–655. [Google Scholar]
- Parraguez, V.H.; Sales, F.; Peralta, O.; De los Reyes, M.; Gonzalez-Bulnes, A. Oxidative Stress and Fetal Growth Restriction Set Up Earlier in Undernourished Sheep Twin Pregnancies: Prevention with Antioxidant and Nutritional Supplementation. Antioxidants 2022, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Sales, F.; Peralta, O.; Narbona, E.; McCoard, S.; De los Reyes, M.; González-Bulnes, A.; Parraguez, V. Hypoxia and Oxidative Stress Are Associated with Reduced Fetal Growth in Twin and Undernourished Sheep Pregnancies. Animals 2018, 8, 217. [Google Scholar] [CrossRef]
- Sales, F.; Peralta, O.; Narbona, E.; McCoard, S.; Lira, R.; De Los Reyes, M.; González-Bulnes, A.; Parraguez, V. Maternal Supplementation with Antioxidant Vitamins in Sheep Results in Increased Transfer to the Fetus and Improvement of Fetal Antioxidant Status and Development. Antioxidants 2019, 8, 59. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids. In National Research Council, Committee on the Nutrient Requirements of Small Ruminants, Board on Agriculture, Division on Earth, and Life Studies NRC; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Scales, G.H.; Burton, R.N.; Moss, R.A. Lamb Mortality, Birth Weight and Nutrition in Late Pregnancy. N. Z. J. Agric. Res. 1986, 29, 75–82. [Google Scholar] [CrossRef][Green Version]
- Kleemann, D.O.; Walker, S.K. Fertility in South Australian Commercial Merino Flocks: Sources of Reproductive Wastage. Theriogenology 2005, 63, 2075–2088. [Google Scholar] [CrossRef]
- Hinch, G.N.; Brien, F. Lamb Survival in Australian Flocks: A Review. Anim. Prod. Sci. 2014, 54, 656–666. [Google Scholar] [CrossRef]
- Dwyer, C.M.; Conington, J.; Corbiere, F.; Holmoy, I.H.; Muri, K.; Nowak, R.; Rooke, J.; Vipond, J.; Gautier, J.M. Invited Review: Improving Neonatal Survival in Small Ruminants: Science into Practice. Animal 2016, 10, 449–459. [Google Scholar] [CrossRef]
- Geenty, K.G.; Brien, F.D.; Hinch, G.N.; Dobos, R.C.; Refshauge, G.; Mccaskill, M.; Ball, A.J.; Behrendt, R.; Gore, K.P.; Savage, D.B.; et al. Reproductive Performance in the Sheep CRC Information Nucleus Using Artificial Insemination across Different Sheep-Production Environments in Southern Australia. Anim. Prod. Sci. 2014, 54, 715–726. [Google Scholar] [CrossRef]
- Campion, F.P.; Crosby, T.F.; Creighton, P.; Fahey, A.G.; Boland, T.M. An Investigation into the Factors Associated with Ewe Colostrum Production. Small Rumin. Res. 2019, 178, 55–62. [Google Scholar] [CrossRef]
- McCoard, S.A.; Sales, F.A.; Sciascia, Q.L. Invited Review: Impact of Specific Nutrient Interventions during Mid-to-Late Gestation on Physiological Traits Important for Survival of Multiple-Born Lambs. Animal 2017, 11, 1727–1736. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M., Jr. Arginine Metabolism: Nitric Oxide and Beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Meininger, C.J.; Spencer, T.E. Maternal Nutrition and Fetal Development. J. Nutr. 2004, 134, 2169–2172. [Google Scholar] [CrossRef]
- Lassala, A.; Bazer, F.W.; Cudd, T.A.; Datta, S.; Keisler, D.H.; Satterfield, M.C.; Spencer, T.E.; Wu, G. Parenteral Administration of L-Arginine Prevents Fetal Growth Restriction in Undernourished Ewes. J. Nutr. 2010, 140, 1242–1248. [Google Scholar] [CrossRef]
- Lassala, A.; Bazer, F.W.; Cudd, T.A.; Datta, S.; Keisler, D.H.; Satterfield, M.C.; Spencer, T.E.; Wu, G. Parenteral Administration of L-Arginine Enhances Fetal Survival and Growth in Sheep Carrying Multiple Fetuses. J. Nutr. 2011, 141, 849–855. [Google Scholar] [CrossRef]
- Sciascia, Q.L.; van der Linden, D.S.; Sales, F.A.; Wards, N.J.; Blair, H.T.; Pacheco, D.; Oliver, M.H.; McCoard, S.A. Parenteral Administration of L-Arginine to Twin-Bearing Romney Ewes during Late Pregnancy Is Associated with Reduced Milk Somatic Cell Count during Early Lactation. J. Dairy Sci. 2019, 102, 3071–3081. [Google Scholar] [CrossRef] [PubMed]
- Krogh, U.; Oksbjerg, N.; Purup, S.; Ramaekers, P.; Theil, P.K. Colostrum and Milk Production in Multiparous Sows Fed Supplementary Arginine during Gestation and Lactation. J. Anim. Sci. 2016, 94, 22–25. [Google Scholar] [CrossRef]
- Nuntapaitoon, M.; Muns, R.; Theil, P.K.; Tummaruk, P. L-Arginine Supplementation in Sow Diet during Late Gestation Decrease Stillborn Piglet, Increase Piglet Birth Weight and Increase Immunoglobulin G Concentration in Colostrum. Theriogenology 2018, 121, 27–34. [Google Scholar] [CrossRef]
- Chacher, B.; Wang, D.-M.; Liu, H.-Y.; Liu, J.-X. Degradation of L-Arginine and N-Carbamoyl Glutamate and Their Effect on Rumen Fermentation In Vitro. Ital. J. Anim. Sci. 2012, 11, e68. [Google Scholar] [CrossRef]
- McCarthy, N.; Weaver, A.C.; Agenbag, B.; Flinn, T.; Brougham, B.J.; Swinbourne, A.M.; Kelly, J.M.; Kleemann, D.O.; Gatford, K.L.; van Wettere, W.H.E.J. Maternal Lysine, Methionine and Choline Supplementation in Twin-Bearing Merino Ewes during Mid-to-Late Gestation Does Not Alter Pregnancy Outcomes or Progeny Growth and Survival. Livest. Sci. 2021, 251, 104620. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Satterfield, M.C.; Gilbreath, K.R.; Posey, E.A.; Sun, Y. L-Arginine Nutrition and Metabolism in Ruminants. In Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2022; Volume 1354. [Google Scholar]
- McCoard, S.A.; Pacheco, D. The Significance of N-Carbamoylglutamate in Ruminant Production. J. Anim. Sci. Biotechnol. 2023, 14, 48. [Google Scholar] [CrossRef]
- Ma, Y.; Zeng, Z.; Kong, L.; Chen, Y.; He, P. Determination of N-Carbamylglutamate in Feeds and Animal Products by High Performance Liquid Chromatography Tandem Mass Spectrometry. Molecules 2019, 24, 3172. [Google Scholar] [CrossRef]
- Oba, M.; Baldwin, R.L.; Owens, S.L.; Bequette, B.J. Metabolic Fates of Ammonia-N in Ruminal Epithelial and Duodenal Mucosal Cells Isolated from Growing Sheep. J. Dairy Sci. 2005, 88, 3963–3970. [Google Scholar] [CrossRef] [PubMed]
- Castillo, L.; Chapman, T.E.; Yu, Y.M.; Ajami, A.; Burke, J.F.; Young, V.R. Dietary Arginine Uptake by the Splanchnic Region in Adult Humans. Am. J. Physiol.-Endocrinol. Metab. 1993, 265, E532–E539. [Google Scholar] [CrossRef]
- Wu, G.; Knabe, D.; Kim, S. Arginine Nutrition in Neonatal Pigs. J. Nutr. 2004, 134, 2783S–2790S. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, H.; Wang, Z.; Fan, Y.; Guo, Y.; Wang, F. Dietary Rumen-Protected Arginine and N-Carbamylglutamate Supplementation Enhances Fetal Growth in Underfed Ewes. Reprod. Fertil. Dev. 2018, 30, 1116–1127. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, H.; Fan, Y.; Guo, Y.; Zhang, G.; Nie, H.; Wang, F. Metabolomic Profiling in Umbilical Venous Plasma Reveals Effects of Dietary Rumen-Protected Arginine or N-Carbamylglutamate Supplementation in Nutrient-Restricted Hu Sheep during Pregnancy. Reprod. Domest. Anim. 2017, 52, 376–388. [Google Scholar] [CrossRef]
- Parraguez, V.H.; McCoard, S.; Sandoval, C.; Candia, F.; Maclean, P.; Mace, W.; Liu, X.; Sales, F. The Effect of N-Carbamylglutamate Supplementation during the Last Third of Gestation on the Growth and Development of Fetuses Born to Nutrient-Restricted Twin-Bearing Ewes. Animals 2024, 14, 946. [Google Scholar] [CrossRef]
- Jefferies, B.C. Body Condition Scoring and Its Use in Management. Tasmanian J. Agric. 1961, 32, 19–21. [Google Scholar]
- Zhang, H.; Sun, L.W.; Wang, Z.Y.; Deng, M.T.; Zhang, G.M.; Guo, R.H.; Ma, T.W.; Wang, F. Dietary N-Carbamylglutamate and Rumen-Protected L-Arginine Supplementation Ameliorate Fetal Growth Restriction in Undernourished Ewes. J. Anim. Sci. 2016, 94, 2072–2085. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.B.; Griffiths, P.H. Guidelines on the Recognition of Pain, Distress and Discomfort in Experimental Animals and an Hypothesis for Assessment. Vet. Rec. 1985, 116, 431–436. [Google Scholar] [CrossRef]
- van der Linden, D.S.; Sciascia, Q.; Sales, F.; Wards, N.J.; Oliver, M.H.; McCoard, S.A. Intravenous Maternal L-Arginine Administration to Twin-Bearing Ewes during Late Pregnancy Enhances Placental Growth and Development. J. Anim. Sci. 2015, 93, 4917–4925. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, P.; Hidalgo, A.I.; Manosalva, C.; Cristi, R.; Teuber, S.; Hidalgo, M.A.; Burgos, R.A. Metabolic Disturbances in Synovial Fluid Are Involved in the Onset of Synovitis in Heifers with Acute Ruminal Acidosis. Sci. Rep. 2019, 9, 5452. [Google Scholar] [CrossRef] [PubMed]
- Turín, J.; Sales, F.; Peralta, O.A.; De los Reyes, M.; Borie, C.; Carrasco, A.; González-Bulnes, A.; Parraguez, V.H. Colostrum Traits and Newborn Body Weight and Growth: Comparison between Single and Twin Underfed Sheep Pregnancies. Front. Vet. Sci. 2023, 10, 1256989. [Google Scholar] [CrossRef]
- Wittwer, F. Manual de Patología Clínica Veterinaria, 3rd ed.; Ediciones Universidad Austral de Chile: Valdivia, Chile, 2021. [Google Scholar]
- Satterfield, M.C.; Dunlap, K.A.; Keisler, D.H.; Bazer, F.W.; Wu, G. Arginine Nutrition and Fetal Brown Adipose Tissue Development in Nutrient-Restricted Sheep. Amino Acids 2011, 45, 489–499. [Google Scholar] [CrossRef]
- Peine, J.L.; Jia, G.Q.; Van Emon, M.L.; Neville, T.L.; Kirsch, J.D.; Hammer, C.J.; O’Rourke, S.T.; Reynolds, L.P.; Caton, J.S. Effects of Maternal Nutrition and Rumen-Protected Arginine Supplementation on Ewe and Postnatal Lamb Performance; Western Section; American Society of Animal Science: Bozeman, MT, USA, 2013; Volume 64. [Google Scholar]
- Ehrhardt, R.A.; Bella, A.W. Growth and Metabolism of the Ovine Placenta during Mid-Gestation. Placenta 1995, 16, 727–741. [Google Scholar] [CrossRef]
- Morrison, J.L. Sheep Models of Intrauterine Growth Restriction: Fetal Adaptations and Consequences. Clin. Exp. Pharmacol. Physiol. 2008, 35, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, L.; Wang, Z.; Deng, M.; Nie, H.; Zhang, G.; Tiewei, M.; Wang, F. N-Carbamylglutamate and L-Arginine Improved Maternal and Placental Development in Underfed Ewes. Reproduction 2016, 151, 730–743. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, F.; Nie, H.; Ma, T.; Wang, Z.; Wang, F.; Loor, J.J. Dietary N -Carbamylglutamate and Rumen-Protected l—Arginine Supplementation during Intrauterine Growth Restriction in Undernourished Ewes Improve Fetal Thymus Development and Immune Function. Reprod. Fertil. Dev. 2018, 30, 1522–1531. [Google Scholar] [CrossRef]
- Gu, F.; Jiang, L.; Xie, L.; Wang, D.; Zhao, F.; Liu, J. Supplementing N-Carbamoylglutamate in Late Gestation Increases Newborn Calf Weight by Enhanced Placental Expression of MTOR and Angiogenesis Factor Genes in Dairy Cows. Anim. Nutr. 2021, 7, 981–988. [Google Scholar] [CrossRef]
- Stelwagen, K.; Carpenter, E.; Haigh, B.; Hodgkinson, A.; Wheeler, T.T. Immune Components of Bovine Colostrum and Milk. J. Anim. Sci. 2009, 87, 3–9. [Google Scholar] [CrossRef]
- Hernandez-Castellano, L.; Almeida, A.; Castro, N.; Arguello, A. The Colostrum Proteome, Ruminant Nutrition and Immunity: A Review. Curr. Protein Pept. Sci. 2014, 15, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Miao, C.; Jiang, L.; Wang, D.; Liu, H.; Liu, J. Dietary Supplementation with N-Carbamoylglutamate Initiated from the Prepartum Stage Improves Lactation Performance of Postpartum Dairy Cows. Anim. Nutr. 2021, 7, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Chacher, B.; Zhu, W.; Ye, J.A.; Wang, D.M.; Liu, J.X. Effect of Dietary N-Carbamoylglutamate on Milk Production and Nitrogen Utilization in High-Yielding Dairy Cows. J. Dairy Sci. 2014, 97, 2338–2345. [Google Scholar] [CrossRef]
- Ma, N.; Li, Y.; Ren, L.; Hu, L.; Xu, R.; Shen, Y.; Cao, Y.; Gao, Y.; Li, J. Effects of Dietary N-Carbamylglutamate Supplementation on Milk Production Performance, Nutrient Digestibility and Blood Metabolomics of Lactating Holstein Cows under Heat Stress. Anim. Feed Sci. Technol. 2021, 273, 114797. [Google Scholar] [CrossRef]
- De Jonge, W.J.; Kwikkers, K.L.; Te Velde, A.A.; Van Deventer, S.J.H.; Nolte, M.A.; Mebius, R.E.; Ruijter, J.M.; Lamers, M.C.; Lamers, W.H. Arginine Deficiency Affects Early B Cell Maturation and Lymphoid Organ Development in Transgenic Mice. J. Clin. Inve. 2002, 110, 1539–1548. [Google Scholar] [CrossRef][Green Version]
- Groth, D.; Hartmann, S.; Klie, S.; Selbig, J. Principal Components Analysis. Methods Mol. Biol. 2013, 930, 303–342. [Google Scholar]
- Martínez-Sena, T.; Luongo, G.; Sanjuan-Herráez, D.; Castell, J.V.; Vento, M.; Quintás, G.; Kuligowski, J. Monitoring of System Conditioning after Blank Injections in Untargeted UPLC-MS Metabolomic Analysis. Sci. Rep. 2019, 9, 9822. [Google Scholar] [CrossRef]
- Wallace, E.D.; Oberlies, N.H.; Cech, N.B.; Kellogg, J.J. Detection of Adulteration in Hydrastis Canadensis (Goldenseal) Dietary Supplements via Untargeted Mass Spectrometry-Based Metabolomics. Food Chem. Toxicol. 2018, 120, 439–447. [Google Scholar] [CrossRef]
- Yao, S.; Colangelo, L.A.; Perry, A.S.; Marron, M.M.; Yaffe, K.; Sedaghat, S.; Lima, J.A.C.; Tian, Q.; Clish, C.B.; Newman, A.B.; et al. Implications of Metabolism on Multi-Systems Healthy Aging across the Lifespan. Aging Cell 2024, 23, e14090. [Google Scholar] [CrossRef]
- Fthenakis, G.; Van Saun, R.J. 12 Pregnancy Toxemia in Sheep and Goats. MSD Veterinary Manual. Available online: https://www.msdvetmanual.com/metabolic-disorders/hepatic-lipidosis/pregnancy-toxemia-in-sheep-and-goats (accessed on 12 July 2025).
- Neu, J. Glutamine in the Fetus and Critically Ill Low Birth Weight Neonate: Metabolism and Mechanism of Action. J. Nutr. 2001, 131, 2585S–2589S. [Google Scholar] [CrossRef]
- Regnault, T.R.H.; Friedman, J.E.; Wilkening, R.B.; Anthony, R.V.; Hay, J.W.W. Fetoplacental Transport and Utilization of Amino Acids in IUGR—A Review. Placenta 2005, 26, S52–S62. [Google Scholar] [CrossRef]
- Chung, M.; Teng, C.; Timmerman, M.; Meschia, G.; Battaglia, F.C. Production and Utilization of Amino Acids by Ovine Placenta In Vivo. Am. J. Physiol. Metab. 1998, 274, E13–E22. [Google Scholar] [CrossRef]
- Pan, S.; Zou, J.; Mao, H.; Hu, Z.; Sun, S.; Wu, W.; Yang, J.; An, Z.; Wang, C. Available Phosphorus Levels Modulate Growth Performance, Serum Indices, Metabolome, Rumen Fermentation, and Microorganism in Hu Lambs. Anim. Feed Sci. Technol. 2025, 322, 116259. [Google Scholar] [CrossRef]
- Holeček, M. Serine Metabolism in Health and Disease and as a Conditionally Essential Amino Acid. Nutrients 2022, 14, 1987. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.N.; Lopreiato, V.; Alharthi, A.; Loor, J.J. Amino Acids and the Regulation of Oxidative Stress and Immune Function in Dairy Cattle. J. Anim. Sci. 2020, 98, S175–S193. [Google Scholar] [CrossRef] [PubMed]
- Cardellini, M.; Ballanti, M.; Davato, F.; Cardolini, I.; Guglielmi, V.; Rizza, S.; Pecchioli, C.; Casagrande, V.; Mavilio, M.; Porzio, O.; et al. 2-Hydroxycaproate Predicts Cardiovascular Mortality in Patients with Atherosclerotic Disease. Atherosclerosis 2018, 277, 179–185. [Google Scholar] [CrossRef]
- Ehling, S.; Reddy, T.M. Investigation of the Presence of β-Hydroxy-β-Methylbutyric Acid and α-Hydroxyisocaproic Acid in Bovine Whole Milk and Fermented Dairy Products by a Validated Liquid Chromatography-Mass Spectrometry Method. J. Agric. Food Chem. 2014, 62, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- HMDB Metabocard for 2-Hydroxycaproic Acid (HMDB0001624). Available online: https://www.hmdb.ca/metabolites/HMDB0001624?utm_source=chatgpt.com#references (accessed on 9 July 2025).
- Appuhamy, J.A.D.R.N.; Knapp, J.R.; Becvar, O.; Escobar, J.; Hanigan, M.D. Effects of Jugular-Infused Lysine, Methionine, and Branched-Chain Amino Acids on Milk Protein Synthesis in High-Producing Dairy Cows. J. Dairy Sci. 2011, 94, 1952–1960. [Google Scholar] [CrossRef]
- Mackle, T.R.; Dwyer, D.A.; Bauman, D.E. Effects of Branched-Chain Amino Acids and Sodium Caseinate on Milk Protein Concentration and Yield from Dairy Cows. J. Dairy Sci. 1999, 82, 161–171. [Google Scholar] [CrossRef] [PubMed]
- An, J.; He, H.; Lan, X.; Liu, L.; Wang, Z.; Ge, Y.; Shen, W.; Cheng, A.; Wan, F. Branched-Chain Amino Acids in Ruminant Nutrition: Function Effects and Summary of Recent Advances. Anim. Feed Sci. Technol. 2024, 312, 115972. [Google Scholar] [CrossRef]
- El-Loly, M.M. Colostrum Ingredients, Its Nutritional and Health Benefits—An Overview. Clin. Nutr. Open Sci. 2022, 44, 126–143. [Google Scholar] [CrossRef]
| CON | NCG | p-Value | |||
|---|---|---|---|---|---|
| Treatment | Sex | T × S | |||
| Body Weight (kg) and Dimensions (cm) | |||||
| Body weight | 3.05 ± 0.10 | 2.95 ± 0.09 | 0.45 | 0.13 | 0.94 |
| Body length | 35.22 ± 0.63 | 36.21 ± 0.62 | 0.26 | 0.40 | 0.72 |
| Biparietal diameter | 6.04 ± 0.06 | 6.08 ± 0.06 | 0.65 | 0.12 | 0.11 |
| Thorax diameter | 34.01 ± 0.49 | 36.62 ± 0.48 | 0.58 | 0.91 | 0.78 |
| Front leg length | 30.23 ± 0.35 | 29.53 ± 0.34 | 0.16 | 0.02 | 0.07 |
| Hind leg length | 35.50 ± 0.46 | 35.57 ± 0.46 | 0.91 | 0.04 | 0.49 |
| Other newborn traits | |||||
| Gestation length (days) | 143.90 ± 0.84 | 143.50 ± 0.84 | 0.74 | 0.97 | 0.98 |
| Lamb rectal temperature (°C) | 36.67 ± 0.78 | 38.21 ± 0.75 | 0.12 | 0.17 | 0.22 |
| Fetal membranes and cotyledons | |||||
| Total p weight (g) | 554.50 ± 18.95 | 536.00 ± 18.47 | 0.49 | ||
| Total cotyledon weight (g) | 208.10 ± 8.35 | 181.8 ± 7.92 | 0.16 | ||
| Total number of cotyledons | 90.10 ± 2.09 | 85.40 ± 2.04 | 0.12 | ||
| Metabolite | Days | p-Value | Normal Range | ||||
|---|---|---|---|---|---|---|---|
| 130 dg | 145 dg | Post Lambing | Group | Time | Group × Time | ||
| β-hydroxybutyrate (mmol/L) | |||||||
| CON | 1.06 ± 0.05 | 1.09 ± 0.06 | 0.87 ± 0.05 | 0.99 | 0.01 | 0.07 | 0.2–0.6 |
| NCG | 1.04 ± 0.05 | 0.99 ± 0.06 | 0.97 ± 0.05 | ||||
| Total protein (g/L) | |||||||
| CON | 61.00 ± 1.57 | 49.62 ± 1.66 | 57.42 ± 1.6 | 0.57 | <0.001 | 0.90 | 68–88 |
| NCG | 62.05 ± 1.57 | 51.20 ± 1.66 | 58.03 ± 1.6 | ||||
| Albumin (g/L) | |||||||
| CON | 25.45 ± 0.60 | 23.64 ± 0.64 | 24.20 ± 0.61 | 0.87 | <0.001 | 0.16 | 26–42 |
| NCG | 26.50 ± 0.60 | 23.01 ± 0.64 | 23.95 ± 0.61 | ||||
| Urea (mmol/L) | |||||||
| CON | 4.21 ± 0.21 | 4.59 ± 0.24 | 4.19 ± 0.22 | 0.03 | 0.01 | 0.50 | 4.0–10.0 |
| NCG | 4.73 ± 0.21 | 5.30 ± 0.24 | 4.44 ± 0.22 | ||||
| CON | NCG | p-Value | |
|---|---|---|---|
| Fat (%) | 13.67 ± 0.89 | 14.99 ± 0.89 | 0.30 |
| Non-fat solids (%) | 26.11 ± 1.66 | 29.84 ± 1.66 | 0.12 |
| Density (g/cm3) | 1.08 ± 0.06 | 1.09 ± 0.06 | 0.23 |
| Protein (%) | 13.59 ± 0.94 | 16.45 ± 0.94 | 0.04 |
| IgG (mg/mL) | 57.48 ± 2.82 | 66.49 ± 2.89 | 0.03 |
| Metabolite | CON | NCG | Percent Difference | p-Value | FDR | Fold Change | log2(FC) |
|---|---|---|---|---|---|---|---|
| Phosphate | 52.27 ± 33.12 | 0.01 ± 0.001 | −99.98 | <0.001 | 0.0085 | −5227 | −12.5 |
| Glycine | 31.17 ± 16.27 | 0.03 ± 0.01 | −99.90 | <0.001 | 0.0010 | −706 | −10.1 |
| Proline | 0.63 ± 0.26 | 0.02 ± 0.01 | −96.83 | <0.001 | 0.0010 | −32 | −5.0 |
| Isoleucine | 2.03 ± 1.30 | 0.14 ± 0.07 | −93.10 | 0.002 | 0.0284 | −15 | −3.9 |
| Valine | 1.04 ± 0.57 | 0.08 ± 0.03 | −92.31 | <0.001 | 0.0010 | −13 | −3.7 |
| Leucine | 2.53 ± 1.24 | 0.43 ± 0.09 | −83.00 | <0.001 | 0.0046 | −28 | −2.5 |
| Methanolphosphate | 0.07 ± 0.19 | 0.51 ± 0.12 | 628.57 | <0.001 | 0.0010 | 2 | 2.8 |
| 2-hydroxypyridine | 0.09 ± 0.16 | 0.41 ± 0.10 | 355.56 | <0.001 | 0.0072 | 1 | 2.1 |
| Serine | 6.63 ± 6.06 | 30.56 ± 13.78 | 360.94 | <0.001 | 0.0072 | 2 | 2.2 |
| Ethanolamine | 10.43 ± 29.28 | 68.62 ± 14.89 | 557.91 | <0.001 | 0.0010 | 1 | 2.7 |
| 2-hydroxyhexanoic acid | 0.96 ± 0.43 | 69.44 ± 10.66 | 7133.33 | <0.001 | 1.52 × 10−11 | 11 | 6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales, F.; McCoard, S.; Alarcón, P.; Sandoval, C.; Silva, C.; Rojas, C.; Parraguez, V.H. Effect of N-Carbamylglutamate Supplementation in Late Pregnancy on Nutrient-Restricted Twin-Bearing Ewes on the Pre-Lambing Maternal Metabolome, Colostrum Quality and Lamb Birth Weight. Animals 2025, 15, 2998. https://doi.org/10.3390/ani15202998
Sales F, McCoard S, Alarcón P, Sandoval C, Silva C, Rojas C, Parraguez VH. Effect of N-Carbamylglutamate Supplementation in Late Pregnancy on Nutrient-Restricted Twin-Bearing Ewes on the Pre-Lambing Maternal Metabolome, Colostrum Quality and Lamb Birth Weight. Animals. 2025; 15(20):2998. https://doi.org/10.3390/ani15202998
Chicago/Turabian StyleSales, Francisco, Susan McCoard, Pablo Alarcón, Camila Sandoval, Claudia Silva, Carolina Rojas, and Víctor H. Parraguez. 2025. "Effect of N-Carbamylglutamate Supplementation in Late Pregnancy on Nutrient-Restricted Twin-Bearing Ewes on the Pre-Lambing Maternal Metabolome, Colostrum Quality and Lamb Birth Weight" Animals 15, no. 20: 2998. https://doi.org/10.3390/ani15202998
APA StyleSales, F., McCoard, S., Alarcón, P., Sandoval, C., Silva, C., Rojas, C., & Parraguez, V. H. (2025). Effect of N-Carbamylglutamate Supplementation in Late Pregnancy on Nutrient-Restricted Twin-Bearing Ewes on the Pre-Lambing Maternal Metabolome, Colostrum Quality and Lamb Birth Weight. Animals, 15(20), 2998. https://doi.org/10.3390/ani15202998






