The Synergistic Effect of Vitamin C Supplementation and Early Feed Withdrawal on Heat Stress Mitigation in Broiler Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
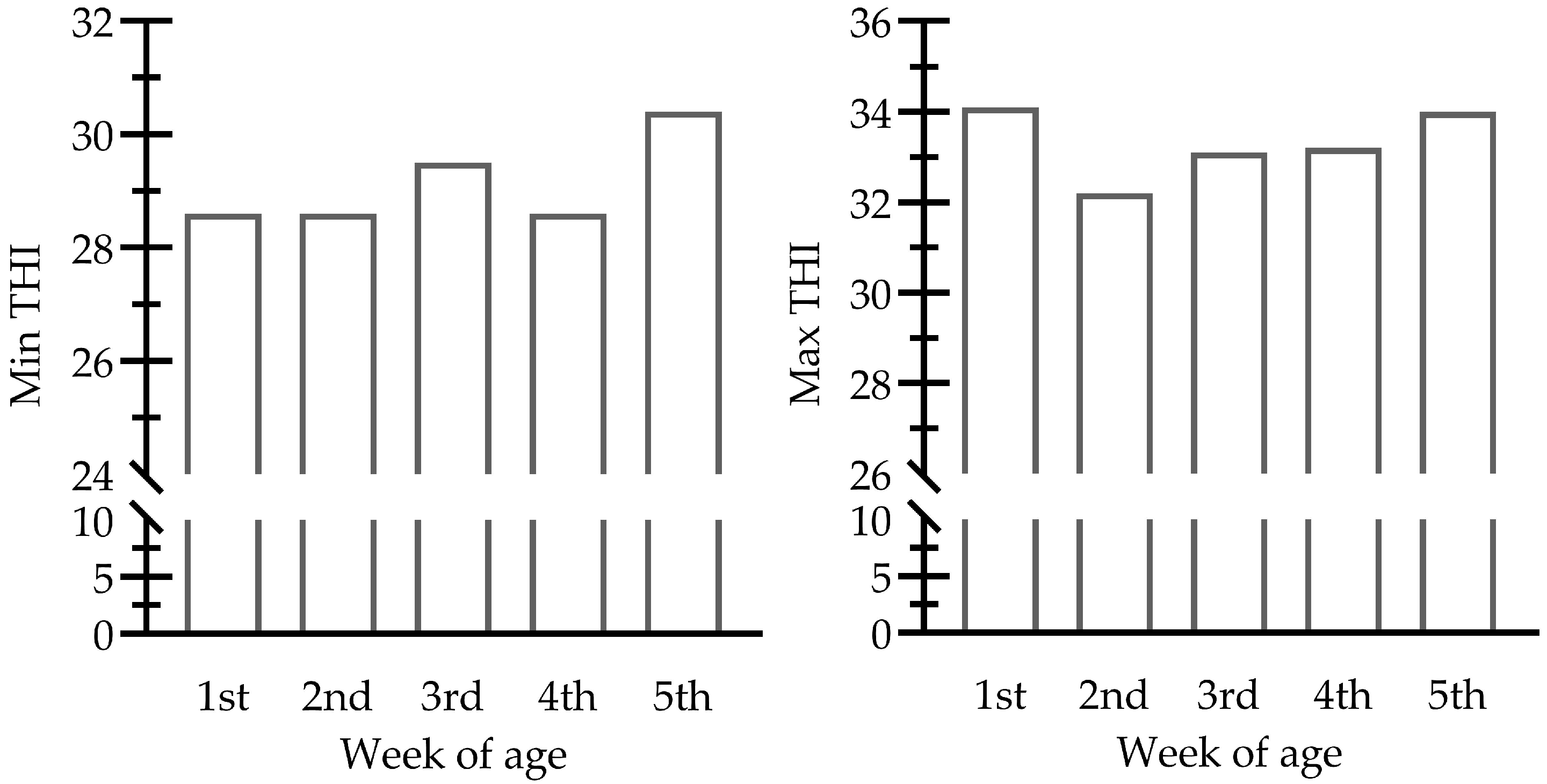
2.2. Experimental Conditions
2.3. Production Performance
2.4. Blood Sampling and Analysis
2.5. Hepatic Antioxidant Markers and Heat Shock Protein 70
2.6. Immunological Parameters
2.7. Economic Efficiency Evaluation
2.8. Statistical Analysis
3. Results
3.1. Productive Parameters
3.2. Metabolic Hormones and Blood Metabolites
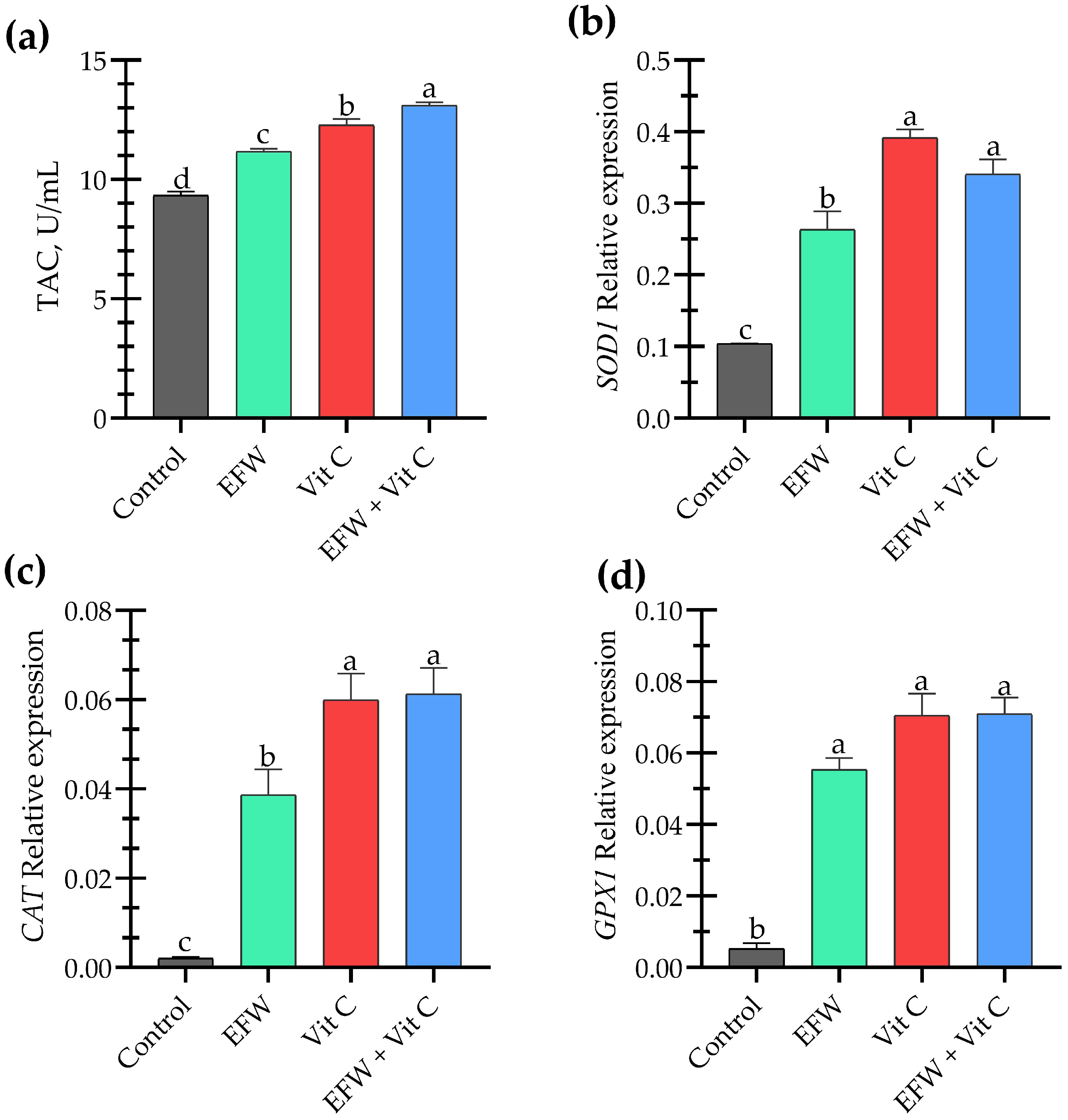
3.3. Antioxidant Status
3.4. Stress Markers, Heat Shock Protein (HSP70), Cytokines, and Immune Markers
3.5. Economic Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Alb | Albumin |
| BW | Body weight |
| BWG | Body weight gain |
| CAT | Catalase |
| Chol | Cholesterol |
| EFW | Early feed withdrawal for 24 h |
| FCR | Feed conversion ratio |
| FI | Feed intake |
| Glob | Globulin |
| GXP | Glutathione peroxidase |
| H/L ratio | Heterophils to lymphocytes ratio |
| Hb | Hemoglobin |
| HSP70 | Heat shock protein 70 |
| IFN-γ | Interferon-gamma |
| IL-10 | Interleukin 10 |
| IL-1β | Interleukin 1 Beta |
| SOD | Superoxide dismutase |
| T3 | Triiodothyronine |
| T4 | Thyroxin |
| TAC | Total antioxidant capacity |
| TP | Total protein |
| Vit C | Vitamin C |
References
- Kim, D.Y.; Kim, J.H.; Choi, W.J.; Han, G.P.; Kil, D.Y. Comparative effects of dietary functional nutrients on growth performance, meat quality, immune responses, and stress biomarkers in broiler chickens raised under heat stress conditions. Anim. Biosci. 2021, 34, 1839–1848. [Google Scholar] [CrossRef]
- Mancinelli, A.C.; Baldi, G.; Soglia, F.; Mattioli, S.; Sirri, F.; Petracci, M.; Castellini, C.; Zampiga, M. Impact of chronic heat stress on behavior, oxidative status and meat quality traits of fast-growing broiler chickens. Front. Physiol. 2023, 14, 1242094. [Google Scholar] [CrossRef] [PubMed]
- Oke, O.E.; Akosile, O.A.; Oni, A.I.; Opowoye, I.O.; Ishola, C.A.; Adebiyi, J.O.; Odeyemi, A.J.; Adjei-Mensah, B.; Uyanga, V.A.; Abioja, M.O. Oxidative stress in poultry production. Poult. Sci. 2024, 103, 104003. [Google Scholar] [CrossRef] [PubMed]
- Riber, A.B.; Wurtz, K.E. Impact of growth rate on the welfare of broilers. Animals 2024, 14, 3330. [Google Scholar] [CrossRef]
- Nassar, F.S.; Abbas, A.O.; Alaqil, A.A.; Kamel, N.N. Sustainable broilers production performance under high Stocking condition through colocynth seed supplementation. Sustainability 2023, 15, 5102. [Google Scholar] [CrossRef]
- Alzarah, M.I.; Althobiati, F.; Abbas, A.O.; Mehaisen, G.M.K.; Kamel, N.N. Citrullus colocynthis seeds: A potential natural immune modulator source for broiler reared under chronic heat stress. Animals 2021, 11, 1951. [Google Scholar] [CrossRef]
- Rezar, V.; Pal, M.P.; Leskovec, J.; Levart, A.; Salobir, J.; Lavrencic, A.; Vrecl, M.; Pirman, T. Combined effects of cyclic heat stress, dietary induced oxidative stress and different levels of antioxidant on gut fermentation activity and mucosal morphology in broiler chickens. Agriculture 2024, 14, 64. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.M.; Li, S.Y.; Wu, Q.F.; Guo, H.; Wang, H.Y.; Su, P.; Wang, J.H. Research Note: Heat stress affects immune and oxidative stress indices of the immune organs of broilers by changing the expressions of adenosine triphosphate-binding cassette subfamily G member 2, sodium-dependent vitamin C transporter-2, and mitochondrial calcium uniporter. Poult. Sci. 2023, 102, 14. [Google Scholar] [CrossRef]
- Amer, S.A.; Mohamed, W.A.M.; Gharib, H.S.A.; Al-Gabri, N.A.; Gouda, A.; Elabbasy, M.T.; Abd El-Rahman, G.I.; Omar, A.E. Changes in the growth, ileal digestibility, intestinal histology, behavior, fatty acid composition of the breast muscles, and blood biochemical parameters of broiler chickens by dietary inclusion of safflower oil and vitamin C. BMC Vet. Res. 2021, 17, 68. [Google Scholar] [CrossRef]
- Livingston, M.L.; Pokoo-Aikins, A.; Frost, T.; Laprade, L.; Hoang, V.; Nogal, B.; Phillips, C.; Cowieson, A.J. Effect of heat stress, dietary Electrolytes, and vitamins E and C on growth performance and blood biochemistry of the broiler chicken. Front. Anim. Sci. 2022, 3, 807267. [Google Scholar] [CrossRef]
- Onagbesan, O.M.; Uyanga, V.A.; Oso, O.; Tona, K.; Oke, O.E. Alleviating heat stress effects in poultry: Updates on methods and mechanisms of actions. Front. Vet. Sci. 2023, 10, 1255520. [Google Scholar] [CrossRef]
- Sahin, K.; Sahin, E.; Deeh, P.B.D.; Kayri, V.; Orhan, C.; Sahin, N. Role of the antioxidant defence system and transcription factors in preventing heat stress in poultry: A dietary approach. Worlds Poult. Sci. J. 2023, 79, 651–687. [Google Scholar] [CrossRef]
- Saeed, M.; Al-Khalaifah, H.; Al-Nasser, A.; Al-Surrayai, T. Feeding the future: A new potential nutritional impact of Lactiplantibacillus plantarum and its promising interventions in future for poultry industry. Poult. Sci. 2025, 104, 105130. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalaifa, H.; Mushtaq, M.; Shoib, M.; Ullah, I.; Shah, M.; Naz, S.; Khan, R.U.; Abudabos, A.; Alhidary, I.A. Mitigating heat stress in broilers: Effects of Bacillus subtilis probiotic and garlic (Allium sativum) supplementation on growth performance, antioxidant status, cecal microbiota and immune response. Poult. Sci. 2025, 104, 105795. [Google Scholar] [CrossRef]
- Biswas, A.; Deo, C.; Sharma, D.; Matin, A.; Tiwari, A.K. Production performance, haematological parameters, serum biochemistry, and expression of HSP-70 in broiler chickens fed dietary ascorbic acid during heat stress. Int. J. Biometeorol. 2024, 68, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Gouda, A.; Amer, S.A.; Gabr, S.; Tolba, S.A. Effect of dietary supplemental ascorbic acid and folic acid on the growth performance, redox status, and immune status of broiler chickens under heat stress. Trop. Anim. Health Prod. 2020, 52, 2987–2996. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, J.J.; Sun, S.; Ma, F.Y.; Xiong, Y.J.; He, S.J. Optimizing growth and antioxidant function in heat-stressed broilers with vitamin C and betaine supplementation. Int. J. Biometeorol. 2024, 68, 1953–1960. [Google Scholar] [CrossRef]
- Tavakolinasab, F.; Hashemi, M. Effect of using vitamin C supplementation on performance, blood parameters, carcass characteristics and meat quality of broiler chickens under heat stress condition: A meta-analysis. J. Anim. Physiol. Anim. Nutr. 2025, 109, 753–765. [Google Scholar] [CrossRef]
- Keikhosrokiani, A.; Chamani, M.; Foroudi, F.; Sadeghi, A.A.; Aminafshar, M. Effect of vitamin C and probiotics on broilers performance and Claudin-2 (CLDN-2) expression under heat stress. Philipp. Agric. Sci. 2023, 106, 223–237. [Google Scholar] [CrossRef]
- Yin, C.; Zhou, C.; Shi, Y.; Ge, Y.; Gao, X.; Wu, C.; Xu, Z.; Huang, C.; Hu, G.; Liu, P.; et al. Effects and potential mechanism of dietary vitamin C supplementation on hepatic lipid metabolism in growing laying hens under chronic heat stress. J. Anim. Sci. 2023, 101, 308. [Google Scholar] [CrossRef]
- Hieu, T.V.; Guntoro, B.; Qui, N.H.; Quyen, N.T.K.; Hafiz, F.A.A. The application of ascorbic acid as a therapeutic feed additive to boost immunity and antioxidant activity of poultry in heat stress environment. Vet. World 2022, 15, 685–693. [Google Scholar] [CrossRef]
- Albokhadaim, I.F.; Althnaian, T.A.; El-Bahr, S.M. Gene expression of heat shoc kproteins/factors (HSP60, HSP70, HSP90, HSF-1, HSF-3) and antioxidant enzyme activities in heat stressed broilers treated with vitamin C. Pol. J. Vet. Sci. 2019, 22, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, B.; Wu, M.M.; Deng, Y.F.; Li, J.; Xiong, Y.J.; He, S.J. Effect of dietary supplemental vitamin C and betaine on the growth performance, humoral immunity, immune organ index, and antioxidant status of broilers under heat stress. Trop. Anim. Health Prod. 2023, 55, 96. [Google Scholar] [CrossRef] [PubMed]
- Ncho, C.M.; Gupta, V.; Goel, A. Effect of thermal conditioning on growth performance and thermotolerance in broilers: A systematic review and meta-analysis. J. Therm. Biol. 2021, 98, 102916. [Google Scholar] [CrossRef]
- Oni, A.I.; Abiona, J.A.; Fafiolu, A.O.; Oke, O.E. Early-age thermal manipulation and supplemental antioxidants on physiological, biochemical and productive performance of broiler chickens in hot-tropical environments. Stress 2024, 27, 2319803. [Google Scholar] [CrossRef]
- Gouda, A.; Al-Khalaifah, H.; Al-Nasser, A.; Kamel, N.N.; Gabr, S.; Eid, K.M.A. Early feeding strategy mitigates major physiological dynamics altered by heat stress in broilers. Animals 2024, 14, 1485. [Google Scholar] [CrossRef]
- Kokoris, J.C.; Ruzic, Z.; Kanacki, Z.; Stojanovic, S.; Paras, S.; Milosevic, V. Effects of vitamin C and early-age thermal conditioning on pituitary adrenocorticotropic hormone cells in broilers chronically exposed to heat stress: An immunohistomorphometric and hormonal study. Vet. Res. Forum 2024, 15, 125–130. [Google Scholar] [CrossRef]
- Saiz del Barrio, A.; Mansilla, W.D.; Navarro-Villa, A.; Mica, J.H.; Smeets, J.H.; den Hartog, L.A.; García-Ruiz, A.I. Effect of mineral and vitamin C mix on growth performance and blood corticosterone concentrations in heat-stressed broilers. J. Appl. Poult. Res. 2020, 29, 23–33. [Google Scholar] [CrossRef]
- Akinyemi, F.; Adewole, D. Environmental stress in chickens and the potential effectiveness of dietary vitamin supplementation. Front. Anim. Sci. 2021, 2, 775311. [Google Scholar] [CrossRef]
- Wasti, S.; Sah, N.; Mishra, B. Impact of Heat Stress on Poultry Health and Performances, and Potential Mitigation Strategies. Animals 2020, 10, 1266. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Poultry: 9th Revised Edition; The National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Marai, I.F.M.; Ayyat, M.S.; Abd El-Monem, U.M. Growth performance and reproductive traits at first parity of New Zealand White female rabbits as affected by heat stress and its alleviation under Egyptian conditions. Trop. Anim. Health Prod. 2001, 33, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Dedousi, A.; Kritsa, M.Z.; Sossidou, E.N. Thermal comfort, growth performance and welfare of olive pulp fed broilers during hot season. Sustainability 2023, 15, 1386. [Google Scholar] [CrossRef]
- Jain, N.C. Schalm’s Veterinary Hematology, 4th ed.; Lea and Febiger: Philadelphia, PA, USA, 1986. [Google Scholar]
- Sánchez-Carbayo, M.; Mauri, M.; Alfayate, R.; Miralles, C.; Soria, F. Analytical and clinical evaluation of TSH and thyroid hormones by electrochemiluminescent immunoassays. Clin. Biochem. 1999, 32, 395–403. [Google Scholar] [CrossRef]
- Weichselbaum, T.E. An accurate and rapid method for the determination of proteins in small amounts of blood serum and plasma. Am. J. Clin. Pathol. 1946, 10, 40–49. [Google Scholar] [CrossRef]
- Doumas, B.T.; Biggs, H.G.; Arends, R.L.; Pinto, P.V.C. Determination of serum albumin. In Standard Methods of Clinical Chemistry; Cooper, G.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1972; Volume 7, pp. 175–188. [Google Scholar]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- McGowan, M.W.; Artiss, J.D.; Strandbergh, D.R.; Zak, B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin. Chem. 1983, 29, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Vassault, A.; Grafmeyer, D.; Naudin, C.; Dumont, G.; Bailly, M.; Henny, J.; Gerhardt, M.; Georges, P. Protocole de validation de techniques. Ann. Biol. Clin. 1986, 44, 45. [Google Scholar]
- Griffin, H.D.; Whitehead, C.C. Plasma lipoprotein concentration as an indicator of fatness in broilers: Development and use of a simple assay for plasma very low density lipoproteins. Br. Poult. Sci. 1982, 23, 307–313. [Google Scholar] [CrossRef]
- Henry, T.J. Determination of serum creatinine. In Clinical Chemistry, Principles and Technics, 2nd ed.; Henry, R.J., Cannon, D.C., Winkelman, J.W., Eds.; Medical Department: Hagerstown, MD, USA, 1974; 1629p. [Google Scholar]
- Sanders, G.T.; Pasman, A.J.; Hoek, F.J. Determination of uric acid with uricase and peroxidase. Clin. Chim. Acta 1980, 101, 299–303. [Google Scholar] [CrossRef]
- Janaszewska, A.; Bartosz, G. Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.E.; Hultin, H.O. Some characteristics of the enzymic lipid peroxidation system in the microsomal fraction of flounder skeletal muscle. J. Food Sci. 1987, 52, 15–21. [Google Scholar] [CrossRef]
- Anderson, R.L.; Wang, C.Y.; van Kersen, I.; Lee, K.J.; Welch, W.J.; Lavagnini, P.; Hahn, G.M. An immunoassay for heat shock protein 73/72: Use of the assay to correlate HSW3/72 levels in mammalian cells with heat response. Int. J. Hyperth. 1993, 9, 539–552. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Lie, Ø.; Syed, M.; Solbu, H. Improved agar plate assays of bovine lysozyme and haemolytic complement activity. Acta Vet. Scand. 1986, 27, 23–32. [Google Scholar] [CrossRef]
- Al-Khalaifah, H.S.; Shahin, S.E.; Omar, A.E.; Mohammed, H.A.; Mahmoud, H.I.; Ibrahim, D. Effects of graded levels of microbial fermented or enzymatically treated dried brewer’s grains on growth, digestive and nutrient transporter genes expression and cost effectiveness in broiler chickens. BMC Vet. Res. 2020, 16, 424. [Google Scholar] [CrossRef]
- Qaid, M.M.; Al-Mufarrej, S.I.; Al-Garadi, M.A.; Al-Haidary, A.A. Effects of Rumex nervosus leaf powder supplementation on carcasses compositions, small intestine dimensions, breasts color quality, economic feasibility in broiler chickens. Poult. Sci. 2023, 102, 102943. [Google Scholar] [CrossRef] [PubMed]
- Calik, A.; Emami, N.K.; White, M.B.; Walsh, M.C.; Romero, L.F.; Dalloul, R.A. Influence of dietary vitamin E and selenium supplementation on broilers subjected to heat stress, Part I: Growth performance, body composition and intestinal nutrient transporters. Poult. Sci. 2022, 101, 101857. [Google Scholar] [CrossRef] [PubMed]
- Malila, Y.; Sanpinit, P.; Thongda, W.; Jandamook, A.; Srimarut, Y.; Phasuk, Y.; Kunhareang, S. IInfluences of thermal stress during three weeks before market age on histology and expression of genes associated with adipose infiltration and inflammation in commercial broilers, native chickens, and crossbreeds. Front. Physiol. 2022, 13, 858735. [Google Scholar] [CrossRef] [PubMed]
- Zeferino, C.P.; Komiyama, C.M.; Pelícia, V.C.; Fascina, V.B.; Aoyagi, M.M.; Coutinho, L.L.; Sartori, J.R.; Moura, A.S. Carcass and meat quality traits of chickens fed diets concurrently supplemented with vitamins C and E under constant heat stress. Animal 2016, 10, 163–171. [Google Scholar] [CrossRef]
- Teyssier, J.R.; Brugaletta, G.; Sirri, F.; Dridi, S.; Rochell, S.J. A review of heat stress in chickens. Part II: Insights into protein and energy utilization and feeding. Front. Physiol. 2022, 13, 943612. [Google Scholar] [CrossRef]
- Emami, N.K.; Greene, E.S.; Kogut, M.H.; Dridi, S. Heat stress and feed restriction distinctly affect performance, carcass and meat yield, intestinal integrity, and inflammatory (chemo) cytokines in broiler chickens. Front. Physiol. 2021, 12, 707757. [Google Scholar] [CrossRef]
- Teyssier, J.R.; Preynat, A.; Cozannet, P.; Briens, M.; Mauromoustakos, A.; Greene, E.S.; Owens, C.M.; Dridi, S.; Rochell, S.J. Constant and cyclic chronic heat stress models differentially influence growth performance, carcass traits and meat quality of broilers. Poult. Sci. 2022, 101, 101963. [Google Scholar] [CrossRef]
- McKee, J.S.; Harrison, P.C. Effects of supplemental ascorbic acid on the performance of broiler chickens exposed to multiple concurrent stressors. Poult. Sci. 1995, 74, 1772–1785. [Google Scholar] [CrossRef] [PubMed]
- Oloruntola, O.D.; Ayodele, S.O.; Oloruntola, D.A.; Olarotimi, O.J.; Falowo, A.B.; Akinduro, V.O.; Adeniji, O.E.; Adu, O.A.; Gbore, F.A. Performance, HSP70 expression, antioxidant enzymes, oxidative DNA damage biomarkers, metabolic hormones, and intestinal microbiota of broiler chickens fed mistletoe leaf powder supplemented diets under tropical high ambient temperatures. J. Therm. Biol. 2024, 121, 103861. [Google Scholar] [CrossRef] [PubMed]
- Shewita, R.S.; El-Naggar, K.; Abd El Naby, W.S.H. Influence of dietary vitamin c supplementation on growth performance, blood biochemical parameters and transcript levels of heat shock proteins in high stocking density reared broiler chickens. Slov. Vet. Res. 2019, 56, 129–138. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Al-Owaimer, A.N.; Hussein, E.O.S.; Ali, M.H. Effect of natural vitamin C on performance and certain haemato-biochemical values in broiler chickens exposed to heat stress. Pak. J. Zool. 2018, 50, 951–955. [Google Scholar] [CrossRef]
- Safavinia, L.; Mazhari, M.; Esmaeilipour, O.; Ziaei, N.; Doomari, H. Study of the effect of vitamin c and Carum copticum seed powder diets on growth performance, blood metabolites, carcass characteristics, and meat quality in heat stressed broilers. J. Vet. Res. 2021, 76, 291–303. [Google Scholar] [CrossRef]
- Sahin, K.; Sahin, N.; Kucuk, O. Effects of chromium, and ascorbic acid supplementation on growth, carcass traits, serum metabolites, and antioxidant status of broiler chickens reared at a high ambient temperature (32 °C). Nutr. Res. 2003, 23, 225–238. [Google Scholar] [CrossRef]
- Seven, İ.; Aksu, T.; Tatlı Seven, P. The effects of propolis and vitamin C supplemented feed on performance, nutrient utilization and carcass characteristics in broilers exposed to lead. Livest. Sci. 2012, 148, 10–15. [Google Scholar] [CrossRef]
- Oloruntola, O.D.; Adeyeye, S.A.; Abdulkadir, M.T.; Ayodele, S.O.; Oloruntola, D.A.; Agbede, J.O.; Oladebeye, F.S.; Adeyeye, E.O. Investigating the effects of dietary supplementation with Moringa leaf powder and vitamin C in aflatoxin B1-exposed broilers. J. Agric. Rural Dev. Trop. Subtrop. 2024, 125, 127–137. [Google Scholar] [CrossRef]
- Son, J.; Lee, W.D.; Kim, H.; Hong, E.C.; Kim, H.J.; Yun, Y.S.; Kang, H.K. A comparative study on feeding timing and additive types of broilers in a high-temperature environment. J. Anim. Sci. 2023, 101, 290. [Google Scholar] [CrossRef]
- Sumanu, V.O.; Naidoo, V.; Oosthuizen, M.; Chamunorwa, J.P. A technical report on the potential effects of heat stress on antioxidant enzymes activities, performance and small intestinal morphology in broiler chickens administered probiotic and ascorbic acid during the hot summer season. Animals 2023, 13, 3407. [Google Scholar] [CrossRef]
- Ghasemi, H.A.; Nari, N. Interactive effects of methionine source and carnitine supplementation on growth performance, immunity, antioxidant status, and HSP70 gene expression in broilers reared under heat stress conditions. J. Appl. Poult. Res. 2023, 32, 100374. [Google Scholar] [CrossRef]
- Nawaz, A.H.; Amoah, K.; Leng, Q.Y.; Zheng, J.H.; Zhang, W.L.; Zhang, L. Poultry response to heat stress: Its physiological, metabolic, and genetic implications on meat production and quality including strategies to improve broiler production in a warming world. Front. Vet. Sci. 2021, 8, 699081. [Google Scholar] [CrossRef]
- Egbuniwe, I.C.; Ayo, J.O.; Kawu, M.U.; Mohammed, A. Behavioral and hematological responses of broiler chickens administered with betaine and ascorbic acid during hot-dry season. J. Appl. Anim. Welf. Sci. 2018, 21, 334–346. [Google Scholar] [CrossRef]
- Erfani, M.; Eila, N.; Zarei, A.; Noshary, A. The effects of vitamin C and methionine hydroxy analog supplementation on performance, blood parameters, liver enzymes, thyroid hormones, antioxidant activity of blood plasma, intestine morphology, and HSP70 gene expression of broilers under heat stress. Trop. Anim. Health Prod. 2021, 53, 296. [Google Scholar] [CrossRef] [PubMed]
- Hajati, H.; Ahmad, H.; Abolghasem, G.; Hassan, N.-M.; and Nassiri, M.R. The effect of grape seed extract and vitamin C feed supplementation on some blood parameters and HSP70 gene expression of broiler chickens suffering from chronic heat stress. Ital. J. Anim. Sci. 2015, 14, 3273. [Google Scholar] [CrossRef]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Izuddin, W.I.; Awad, E.A.; Idrus, Z.; Samsudin, A.A.; Mustapha, N.M. Dietary supplementation of postbiotics mitigates adverse impacts of heat stress on antioxidant enzyme activity, total antioxidant, lipid peroxidation, physiological stress indicators, lipid profile and meat quality in broilers. Animals 2020, 10, 982. [Google Scholar] [CrossRef] [PubMed]
- El-Shobokshy, S.A.; Abo-Samaha, M.I.; Khafaga, A.F.; Fakiha, K.G.; Khatab, S.A.; Abdelmaksoud, E.M.; Soltan, M.A.K.; Othman, S.I.; Rudayni, H.A.; Allam, A.A.; et al. The beneficial effect of nanomethionine supplementation on growth performance, gene expression profile, and histopathology of heat-stressed broiler chicken. Poult. Sci. 2024, 103, 103206. [Google Scholar] [CrossRef]
- Ahmad-Hanafi, S.; Zulkifli, I.; Ramiah, S.K.; Chung, E.L.T.; Kamil, R.; Sazili, A.Q.; Mashitah, J. Prenatal auditory stimulation and impacts on physiological response to feed restriction in broiler chickens at market age. Poult. Sci. 2024, 103, 103948. [Google Scholar] [CrossRef]
- Bai, H.; Zhao, N.; Li, X.; Ding, Y.; Guo, Q.; Chen, G.; Chang, G. Whole-genome resequencing identifies candidate genes associated with heat adaptation in chickens. Poult. Sci. 2024, 103, 104139. [Google Scholar] [CrossRef]
- Balakrishnan, K.N.; Ramiah, S.K.; Zulkifli, I. Heat shock protein response to stress in poultry: A review. Animals 2023, 13, 317. [Google Scholar] [CrossRef]
- Hosseindoust, A.; Kang, H.K.; Kim, J.S. Quantifying heat stress; the roles on metabolic status and intestinal integrity in poultry, a review. Domest. Anim. Endocrinol. 2022, 81, 106745. [Google Scholar] [CrossRef]
- Striz, I.; Brabcova, E.; Kolesar, L.; Sekerkova, A. Cytokine networking of innate immunity cells: A potential target of therapy. Clin. Sci. 2014, 126, 593–612. [Google Scholar] [CrossRef] [PubMed]
- Wickramasuriya, S.S.; Park, I.; Lee, Y.; Richer, L.M.; Przybyszewski, C.; Gay, C.G.; van Oosterwijk, J.G.; Lillehoj, H.S. Orally delivered Bacillus subtilis expressing chicken NK-2 peptide stabilizes gut microbiota and enhances intestinal health and local immunity in coccidiosis-infected broiler chickens. Poult. Sci. 2023, 102, 102590. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiong, M.; Hu, X.; Li, Y.; Zhang, W.; He, W.; Luo, S.; Zang, J.; Yang, W.; Chen, Y. Dietary Bacillus velezensis KNF-209 supplementation improves growth performance, enhances immunity, and promotes gut health in broilers. Poult. Sci. 2024, 103, 103946. [Google Scholar] [CrossRef]
- Jiang, L.; Bai, K.; Wang, T.; Cui, Y.; Li, Y. Uncovering the protective mechanisms of Bacillus subtilis fmbj against LPS-induced hepatic immune stress and redox imbalance in broilers through transcriptomic profiling. Poult. Sci. 2025, 104, 105774. [Google Scholar] [CrossRef] [PubMed]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Izuddin, W.I.; Zulkifli, I.; Samsudin, A.A.; Mustapha, N.M. Supplementation of postbiotic RI11 improves antioxidant enzyme activity, upregulated gut barrier genes, and reduced cytokine, acute phase protein, and heat shock protein 70 gene expression levels in heat-stressed broilers. Poult. Sci. 2021, 100, 100908. [Google Scholar] [CrossRef]
- Song, B.; Tang, D.; Yan, S.; Fan, H.; Li, G.; Shahid, M.S.; Mahmood, T.; Guo, Y. Effects of age on immune function in broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 42. [Google Scholar] [CrossRef]
- Abdel-Latif, M.A.; El-Hamid, H.S.A.; Emam, M.; Noreldin, A.E.; Helmy, Y.A.; El-Far, A.H.; Elbestawy, A.R. Dietary lysozyme and avilamycin modulate gut health, immunity, and growth rate in broilers. BMC Vet. Res. 2024, 20, 28. [Google Scholar] [CrossRef]
- Omoor, I.N.A.; Yankey, R.; Shehata, A.I.; Fang, C.H.; Hui, L.; Dongmei, L.; Ling, J.; Dosoky, W.M.; Karanja, J.K.; Dawood, M.A.O.; et al. Dietary supplement of fermented grass forage regulates growth performance, antioxidant capacity, and immune response of broiler chickens. Poult. Sci. 2024, 103, 103323. [Google Scholar] [CrossRef] [PubMed]
| Target Gene | Primers (5′–3′) | PCR Product, bp | Accession No. | |
|---|---|---|---|---|
| SOD1 | F | CACTGCATCATTGGCCGTACCA | 224 | NM_205064.1 |
| R | GCTTGCACACGGAAGAGCAAGT | |||
| CAT | F | TGGCGGTAGGAGTCTGGTCT | 112 | NM_001031215.1 |
| R | GTCCCGTCCGTCAGCCATTT | |||
| GPX1 | F | GCTGTTCGCCTTCCTGAGAG | 118 | NM_001277853.1 |
| R | GTTCCAGGAGACGTCGTTGC | |||
| β-actin | F | AGCGAACGCCCCCAAAGTTCT | 139 | NM_205518.1 |
| R | AGCTGGGCTGTTGCCTTCACA |
| Parameters | Control | EFW | Vit C | EFW + Vit C | SEM | p-Value |
|---|---|---|---|---|---|---|
| Initial BW, g | 42.67 | 42.67 | 42.67 | 42.83 | 0.16 | 0.982 |
| Final BW, g | 1917 c | 2091 b | 2164 b | 2281 a | 29.04 | <0.0001 |
| BWG, g | 1875 c | 2048 b | 2121 b | 2238 a | 29.05 | <0.0001 |
| Feed intake, g | 3172 b | 3300 ab | 3404 a | 3363 a | 24.66 | 0.001 |
| FCR | 1.69 a | 1.61 b | 1.60 b | 1.50 c | 0.02 | <0.0001 |
| Parameters | Control | EFW | Vit C | EFW + Vit C | SEM | p-Value |
|---|---|---|---|---|---|---|
| T3, ng/dL | 3.61 b | 3.32 c | 4.04 a | 4.15 a | 0.07 | <0.0001 |
| T4, ng/dL | 21.59 c | 23.59 b | 23.71 b | 24.65 a | 0.25 | <0.0001 |
| Hemoglobin, g/dL | 9.11 c | 9.74 b | 10.61 a | 11.09 a | 0.17 | <0.0001 |
| Total protein, g/dL | 5.30 c | 6.13 b | 6.38 ab | 6.73 a | 0.12 | <0.0001 |
| Ablumin, g/dL | 2.71 b | 2.80 ab | 3.36 a | 3.35 a | 0.09 | 0.0043 |
| Globulin, g/dL | 2.59 b | 3.32 a | 3.02 a | 3.37 a | 0.08 | <0.0001 |
| T-Chol, mmol/L | 3.56 a | 3.32 b | 3.23 bc | 3.20 c | 0.03 | <0.0001 |
| HDL, mmol/L | 2.03 b | 2.22 a | 2.28 a | 2.29 a | 0.02 | <0.0001 |
| LDL, mmol/L | 1.28 a | 0.88 b | 0.73 c | 0.69 c | 0.05 | <0.0001 |
| VLDL, mmol/L | 0.25 a | 0.23 b | 0.22 b | 0.22 b | 0.003 | <0.0001 |
| Triglycerides, mmol/L | 1.32 a | 1.23 b | 1.22 b | 1.18 b | 0.01 | <0.0001 |
| Uric Acid, mg/100 mL | 3.25 | 2.83 | 2.90 | 2.76 | 0.09 | 0.1943 |
| Creatinine, mg/dL | 0.280 a | 0.260 ab | 0.255 ab | 0.238 b | 0.005 | 0.045 |
| Parameters | Control | EFW | Vit C | EFW + Vit C | SEM | p-Value |
|---|---|---|---|---|---|---|
| MDA, nmol/mL | 5.61 a | 2.83 b | 2.34 c | 2.26 c | 0.29 | <0.0001 |
| H/L ratio | 0.61 a | 0.54 b | 0.52 b | 0.51 b | 0.01 | <0.0001 |
| HSP70, ng/mg | 3.81 c | 5.11 b | 6.21 a | 6.09 a | 0.21 | <0.0001 |
| IFN-γ, pg/mL | 6.70 c | 7.40 c | 9.15 b | 10.53 a | 0.34 | <0.0001 |
| IL-10, µg/mL | 1.60 c | 2.93 b | 3.38 b | 3.97 a | 0.19 | <0.0001 |
| IL-1β, µg/mL | 143.0 c | 160.2 b | 169.8 a | 170.8 a | 2.50 | <0.0001 |
| Lysozyme, µg/mL | 131.0 b | 166.3 a | 179.0 a | 184.2 a | 4.82 | <0.0001 |
| Complement C3, g/L | 1.21 b | 1.44 a | 1.51 a | 1.50 a | 0.03 | <0.0001 |
| Indicators | Control | EFW | Vit C | EFW + Vit C | SEM | p-Value |
|---|---|---|---|---|---|---|
| Feed cost/kg diet * | 0.45 | 0.45 | 0.50 | 0.500 | --- | --- |
| Feed cost/kg BWG | 0.761 b | 0.726 c | 0.802 a | 0.752 bc | 0.01 | <0.0001 |
| Total feed cost/bird | 1.427 b | 1.485 b | 1.702 a | 1.681 a | 0.03 | <0.0001 |
| Total cost/bird | 1.687 b | 1.745 b | 1.962 a | 1.941 a | 0.03 | <0.0001 |
| Total revenue/bird ** | 3.835 c | 4.182 b | 4.328 b | 4.562 a | 0.06 | <0.0001 |
| Net profit | 2.147 c | 2.437 b | 2.366 b | 2.620 a | 0.04 | <0.0001 |
| Profitability index | 0.560 bc | 0.582 a | 0.547 c | 0.574 ab | 0.004 | <0.0001 |
| Economic efficiency% | 150.6 bc | 164.3 a | 139.1 c | 155.9 ab | 2.39 | <0.0001 |
| REE% | 100 c | 114 b | 110 b | 122 a | 3.22 | <0.0001 |
| RRI% | 127.37 bc | 139.75 a | 120.63 c | 135.00 ab | 1.92 | <0.0001 |
| BCR | 2.274 bc | 2.397 a | 2.206 c | 2.350 ab | 0.019 | <0.0001 |
| Profit margin% | 56.00 bc | 58.24 a | 54.66 c | 57.43 ab | 0.35 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Khalaifah, H.; Kamel, N.N.; Gabr, S.; Gouda, A. The Synergistic Effect of Vitamin C Supplementation and Early Feed Withdrawal on Heat Stress Mitigation in Broiler Chickens. Animals 2025, 15, 2996. https://doi.org/10.3390/ani15202996
Al-Khalaifah H, Kamel NN, Gabr S, Gouda A. The Synergistic Effect of Vitamin C Supplementation and Early Feed Withdrawal on Heat Stress Mitigation in Broiler Chickens. Animals. 2025; 15(20):2996. https://doi.org/10.3390/ani15202996
Chicago/Turabian StyleAl-Khalaifah, Hanan, Nancy N. Kamel, Sherin Gabr, and Ahmed Gouda. 2025. "The Synergistic Effect of Vitamin C Supplementation and Early Feed Withdrawal on Heat Stress Mitigation in Broiler Chickens" Animals 15, no. 20: 2996. https://doi.org/10.3390/ani15202996
APA StyleAl-Khalaifah, H., Kamel, N. N., Gabr, S., & Gouda, A. (2025). The Synergistic Effect of Vitamin C Supplementation and Early Feed Withdrawal on Heat Stress Mitigation in Broiler Chickens. Animals, 15(20), 2996. https://doi.org/10.3390/ani15202996






