Effects of Cool Water Supply on Laying Performance, Egg Quality, Rectal Temperature and Stress Hormones in Heat-Stressed Laying Hens in Open-Type Laying Houses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
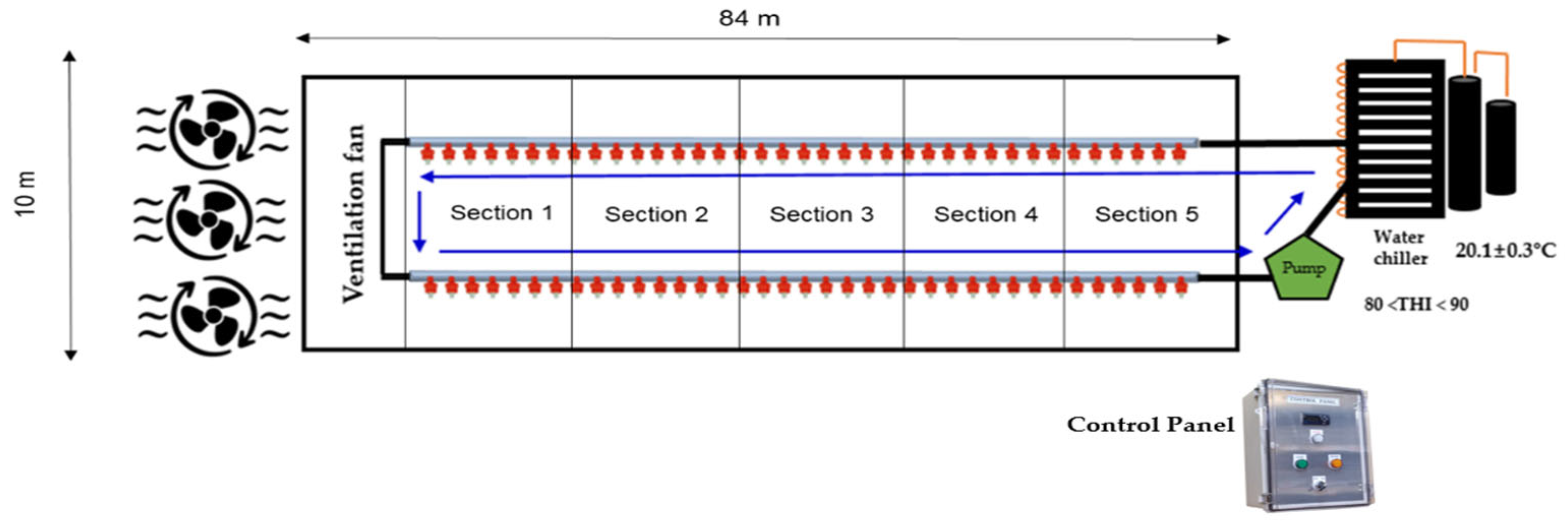
2.1. Experimental Design
2.2. Laying Performance and Economic Evaluation
2.3. Determination of Egg Quality Parameters
2.4. Measurement of Rectal Temperature
2.5. Corticosterone in Yolk Samples
2.6. Statistical Analysis
3. Results
3.1. Laying Performance
3.2. Egg Quality
3.3. Rectal Temperature
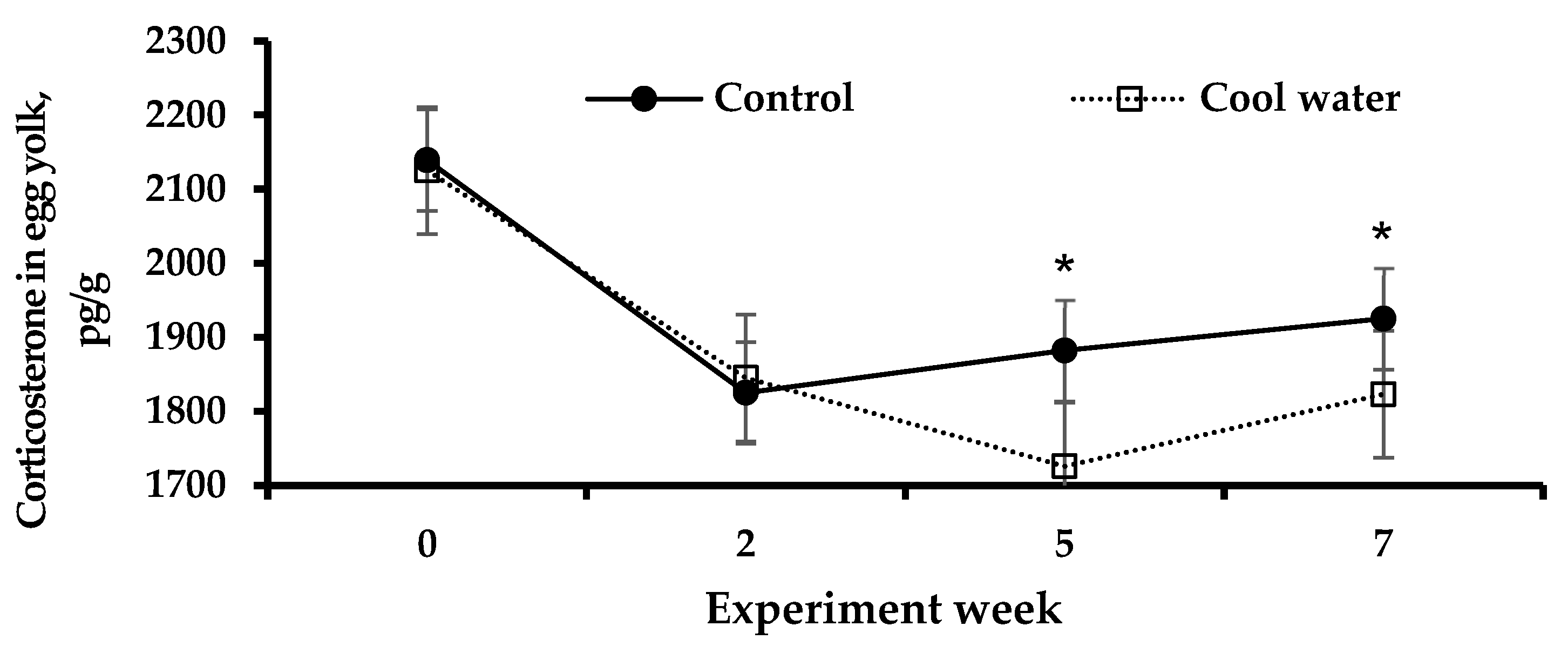
3.4. Corticosterone in Egg Yolk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oluwagbenga, E.M.; Tetel, V.; Schober, J.; Fraley, G.S. Chronic heat stress part 1: Decrease in egg quality, increase in cortisol levels in egg albumen, and reduction in fertility of breeder pekin ducks. Front. Physiol. 2022, 13, 1019741. [Google Scholar] [CrossRef] [PubMed]
- Ouzeau, G.; Soubeyroux, J.M.; Schneider, M.; Vautard, R.; Planton, S. Heat waves analysis over France in present and future climate: Application of a new method on the EURO-CORDEX ensemble. Clim. Serv. 2016, 4, 1–12. [Google Scholar] [CrossRef]
- Eltahan, H.M.; Kang, C.W.; Chowdhury, V.S.; Eltahan, H.M.; Abdel-Maksoud, M.A.; Mubarak, A.; Lim, C.I. Cold drinking water boosts the cellular and humoral immunity in heat-exposed laying hens. Animals 2023, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, H.F.; Jiao, T.; Sui, S.J.; Gu, X.H.; Zhang, Z.Y.; Buyse, J.; Decuypere, E. Thermoregulation responses of broiler chickens to humidity at different ambient temperatures. I. One week of age. Poult. Sci. 2005, 84, 1166–1172. [Google Scholar] [CrossRef]
- Ebeid, T.A.; Suzuki, T.; Sugiyama, T. High-temperature influences eggshell quality and calbindin-D28K localization of eggshell gland and all intestinal segments of laying hens. Poult. Sci. 2012, 91, 2282–2287. [Google Scholar] [CrossRef]
- Lin, H.; Mertens, K.; Kemps, B.; Govaerts, T.; De Ketelaere, B.; De Baerdemaeker, J.; Decuypere, E.; Buyse, J. New approach of testing the effect of heat stress on eggshell quality: Mechanical and material properties of eggshell and membrane. Br. Poult. Sci. 2004, 45, 476–482. [Google Scholar] [CrossRef]
- Aengwanich, W. Pathological changes and the effects of ascorbic acid on lesions scores of bursa of Fabricius in broilers under chronic heat stress. Res. J. Vet. Sci. 2009, 1, 62–66. [Google Scholar]
- Bozkurt, M.; Kucukvilmaz, K.; Catli, A.U.; Cinar, M.; Bintas, E.; Coven, F. Performance, egg quality, and immune response of laying hens fed diets supplemented with manna-oligosaccharide or an essential oil mixture under moderate and hot environmental conditions. Poult. Sci. 2012, 91, 1379–1386. [Google Scholar] [CrossRef]
- Bahry, M.A.; Yang, H.; Tran, P.V.; Do, P.H.; Han, G.; Eltahan, H.M.; Chowdhury, V.S.; Furuse, M. Reduction in voluntary food intake, but not fasting, stimulates hypothalamic gonadotropin-inhibitory hormone precursor mRNA expression in chicks under heat stress. Nruropeptides 2018, 71, 90–96. [Google Scholar] [CrossRef]
- Balnace, D.; Brake, J. Nutrition and management of heat-stressed pullets and laying hens. Worlds Poult. Sci. J. 2005, 61, 299–406. [Google Scholar] [CrossRef]
- Giannenas, I.; Sakkas, P.; Papadopoulos, G.A.; Mitsopoulos, I.; Stylianaki, I.; Dokou, S.; Tsiouris, V.; Papagrigoriou, T.; Panheleux, M.; Robert, F.; et al. The association of Curcuma and Scutellaria plant extracts improves laying hen thermal tolerance and egg oxidative stability and quality under heat stress conditions. Front. Vet. Sci. 2022, 9, 957847. [Google Scholar] [CrossRef] [PubMed]
- Cruvinel, J.M.; Urayama, P.M.G.; Dos Santos, T.S.; Denadai, J.C.; Muro, E.M.; Dornelas, L.C.; Pasquali, G.A.M.; Neto, A.C.C.; Zanetti, L.H.; Netto, R.G.F.; et al. Different dietary electrolyte balance values on performance, egg, and bone quality of Japanese quail (Coturnix Coturnix Japonica) under heat stress. Trop. Anim. Health Prod. 2021, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moller, A.P. The allometry of number of feathers in birds changes seasonally. Avian Res. 2015, 6, 2. [Google Scholar] [CrossRef]
- Igbkwe, N.A.R. Effects of environmental heat stress on reproduction and its management in chickens. Niger. Vet. J. 2018, 39, 101–104. [Google Scholar] [CrossRef]
- Tao, X.; Xin, H. Acute synergistic effects of air temperature, humidity, and velocity on homeostasis of market-size broilers. Tnans. Am. Soc. Agric. Eng. 2003, 46, 491–497. [Google Scholar]
- Gates, R.S.; Zhang, H.; Couiver, D.G.; Overhults, D.G. Regional variation in temperature humidity index for poultry housing. Trans. ASAE. 1995, 38, 197–205. [Google Scholar] [CrossRef]
- Borges, S.A.; Fischer da Silva, A.V.; Makorka, A.; Hooge, D.M.; Cummings, K.R. Physiological responses of broiler chickens to heat stress and dietary electrolyte balance (sodium plus potassium minus chloride, milliequivalents per kilogram). Poult. Sci. 2004, 83, 1551–1558. [Google Scholar] [CrossRef]
- Hy-Line, Int. Understanding Heat Stress in Layers: Management Tips to Improve Hot Weather Flock Performance; Hy-Line, Int.: West Dse Moines, IA, USA, 2016; pp. 1–8. Available online: www.hyline.com (accessed on 20 October 2019).
- Xin, H.; Gates, R.S.; Puma, M.C.; Anh, D.U. Drinking water temperature effects on laying hens subjected to warm cyclic environments. Poult. Sci. 2002, 81, 608–617. [Google Scholar] [CrossRef]
- Baker, A.; Teeter, R.G. Drinking water temperature and potassium chloride supplementation effect on broiler body temperature and performance during heat stress. J. Appl. Poult. Res. 1994, 3, 87–92. [Google Scholar] [CrossRef]
- Abioja, M.O.; Osinowo, O.A.; Smith, O.F.; Eruvbetine, D.; Abiona, J.A. Evaluation of cold water and vitamin C on broiler growth during hot-dry season in SW Nigeria. Arch. Zootec. 2011, 60, 1095–1130. [Google Scholar] [CrossRef]
- Candido, M.G.L.; Tinoco, I.F.F.; Albino, L.F.T.; Freitas, L.C.S.R.; Santos, T.C.; Cecon, P.R.; Gates, R.S. Effects of heat stress on pullet cloacal and body temperature. Poult. Sci. 2020, 99, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Im, E.S.; Thanh, N.X.; Kim, Y.H.; Ahn, J.B. 2018 summer extreme temperatures in South Korea and their intensification under 3 °C global warming. Environ. Res. Lett. 2019, 14, 094020. [Google Scholar] [CrossRef]
- Ho, C.H.; Park, C.K.; Yun, J.; Lee, E.J.; Kim, J.; Yoo, H.D. Asymmetric expansion of summer season on May and September in Korea. Asia-Pac. J. Atmos. Sci. 2021, 57, 619–627. [Google Scholar] [CrossRef]
- Kim, Y.H.; Ahn, J.B.; Suh, M.S.; Cha, D.H.; Chang, E.C.; Min, S.K.; Byun, Y.H.; Kim, J.U. Future changes in extreme heatwaves in terms of intensity and duration over the CORDEX-East Asia Phase Two domain using multi-GCM and multi-RCM chains. Environ. Res. Lett. 2023, 18, 034007. [Google Scholar] [CrossRef]
- National Institute of Animal Science (NIAS). Korean feeding standard for poultry. In Nutrient Requirements of Poultry, 4th ed.; National Institute of Animal Science: Wanju-gun, Republic of Korea, 2022. [Google Scholar]
- Kim, H.R.; Ryu, C.; Lee, S.D.; Cho, J.H.; Kang, H.K. Effects of heat stress on the laying performance, egg quality, and physiological response of laying hens. Animals 2024, 14, 1076. [Google Scholar] [CrossRef]
- Han, G.P.; Kim, J.H.; Lee, J.H.; Kim, H.W.; Kil, D.Y. Research Note: Effect of increasing fat supplementation in diets on productive performance, egg quality, and fatty liver incidence in laying hens throughout the entire laying cycle. Poult. Sci. 2023, 102, 103069. [Google Scholar] [CrossRef]
- Kang, H.K.; Park, S.B.; Jeon, J.J.; Kim, H.S.; Park, K.T.; Kim, S.H.; Hong, E.C.; Kim, C.H. Effect of increasing levels of apparent metabolizable energy on laying hens in barn system. Asian-Aust. J. Anim. Sci. 2018, 31, 1766–1772. [Google Scholar] [CrossRef]
- Anene, D.O.; Akter, Y.; Thomson, P.C.; Groves, P.; Liu, S.; O’Shea, C.J. Hens that exhibit poorer feed efficiency produce eggs with lower albumen quality and are prone to being overweight. Animals 2021, 11, 2986. [Google Scholar] [CrossRef]
- Eisen, E.J.; Bohren, B.B.; McKean, H.E. The haugh unit as a measure of egg albumen quality. Poult. Sci. 1962, 41, 1461–1468. [Google Scholar] [CrossRef]
- Son, J.; Lee, W.D.; Kim, H.; Hong, E.C.; Kim, H.J.; Yun, Y.S.; Kang, H.K. A comparative study on feeding timing and additive types of broilers in a high-temperature environment. J. Anim. Sci. 2023, 101, skad 290. [Google Scholar] [CrossRef]
- Kozlowski, C.P.; Bauman, J.E.; Caldwell Hahn, D. A simplified method for extracting androgens from avian egg yolks. Zoo Biol. 2009, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Song, J.Y.; Park, J.; Kwon, B.Y.; Lee, K.W. The effect of low temperature on laying performance and physiological stress responses in laying hens. Animals 2023, 13, 3824. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Y.; Hester, P.Y.; Makagon, M.M.; Xiong, Y.; Gates, R.S.; Cheng, H.W. Effect of cooled perches on performance, plumage condition, and foot health of cages White leghorn hens exposed to cyclic heat. Poult. Sci. 2019, 98, 2705–2718. [Google Scholar] [CrossRef]
- Barrett, N.W.; Rowland, K.; Schmidt, C.J.; Lamont, S.J.; Rothschild, M.F.; Ashwell, C.M.; Persia, M.E. Effects of acute and chronic heat stress on the performance, egg quality, body temperature, and blood gas parameters of laying hens. Poult. Sci. 2019, 98, 6684–6692. [Google Scholar] [CrossRef]
- Li, G.M.; Liu, L.P.; Yin, B.; Liu, Y.Y.; Dong, W.W.; Gong, S.; Zhang, J.; Tan, J.H. Heat stress decreases egg production of laying hens by inducing apoptosis of follicular cells via activating the FasL/Fas and TNF-α systems. Poult. Sci. 2020, 99, 6084–6093. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Hu, M.; Gu, L.; Lei, M.; Chen, Z.; Zhu, H.; Chen, R. Effect of heat stress on egg production, steroid hormone synthesis, and related gene expression in chicken preovulatory follicular granulosa cells. Animals 2022, 12, 1467. [Google Scholar] [CrossRef]
- Mashaly, M.M.; Hendricks, G.L.; Kalama, M.A.; Gehad, A.E.; Abbas, A.O.; Patterson, P.H. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004, 83, 889–894. [Google Scholar] [CrossRef]
- Gutierrez, W.M.; Min, W.; Chang, H.H. Effects of chilled drinking water on performance of laying hens during constant high ambient temperature. Asian-Aust. J. Anim. Sci. 2009, 22, 694–699. [Google Scholar] [CrossRef]
- Silva, G.F.; Pereire, D.F.; Bueno, L.C.; Santos, T.S.; Tavares, B.O. Performance of laying hens and economic viability of different climatization systems. Ital. J. Anim. Sci. 2013, 12, e47. [Google Scholar] [CrossRef]
- Glatz, P.C. Effect of cool drinking water on production and shell quality of laying hens in summer. Asian-Aust. J. Anim. Sci. 2001, 14, 850–854. [Google Scholar] [CrossRef]
- Mahmoud, K.Z.; Beck, M.M.; Scheideler, S.E.; Forman, M.F.; Anderson, K.P.; Kachman, S.D. Acute high environmental temperature and calcium-estrogen relationship in the hen. Poult. Sci. 1996, 75, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Oguntunji, A.O.; Alabi, O.M. Influence of high environmental temperature on egg production and shell quality: A review. World’s Poult. Sci. J. 2010, 66, 739–750. [Google Scholar] [CrossRef]
- Roberts, J.R. Factors affecting egg internal quality and eggshell quality in laying hens. J. Poult. Sci. 2004, 41, 161–177. [Google Scholar] [CrossRef]
- Chauhan, H.V.S.; Roy, S. Nutritional diseases. In Poultry Diseases Diagnosis and Treatment, 3rd ed.; New Age International: New Delhi, India, 2007; Volume 110002, p. 172. [Google Scholar]
- Kim, D.H.; Lee, Y.K.; Lee, S.D.; Lee, K.W. Impact of relative humidity on the laying performance, egg quality, and physiological stress responses of laying hens exposed to high ambient temperature. J. Therm. Biol. 2022, 103, 103167. [Google Scholar] [CrossRef] [PubMed]
- Cornescu, G.M.; Panaite, T.D.; Untea, A.E.; Varzaru, I.; Saracila, M.; Dumitru, M.; Vlaicu, P.A.; Gavris, T. Mitigation of heat stress effects on laying hens’ performances, egg quality, and some blood parameters by adding dietary zinc-enriched yeasts, parsley, and their combination. Front. Vet. Sci. 2023, 10, 1202058. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M. Physiological alterations of poultry to the high environmental temperature. J. Therm. Biolol. 2018, 76, 101–106. [Google Scholar] [CrossRef]
- Zhao, J.P.; Jiao, H.C.; Jiang, Y.B.; Song, Z.C.; Wang, X.J.; Lin, H. Cool perch availability improves the performance and welfare status of broiler chickens in hot weather. Poult. Sci. 2012, 91, 1775–1784. [Google Scholar] [CrossRef]
- Hu, J.Y.; Xiong, Y.; Gates, R.S.; Cheng, H.W. Perches as cooling devices for reducing heat stress in caged laying hens: A review. Animals 2021, 11, 3026. [Google Scholar] [CrossRef]
- Abioja, M.O.; Osinowo, O.A.; Smith, O.F.; Eruvbetine, D. Physiological and haematological responses of broiler chickens offered cold water and vitamin C during hot-dry season. Nigerian J. Anim. Prod. 2013, 40, 24–36. [Google Scholar] [CrossRef]
- Erensoy, K.; Noubandiguim, M.; Sarica, M.; Aslan, R. The effect of intermittent feeding and cold water on performance and carcass traits of broilers reared under daily heat stress. Asian-Aust. J. Anim. Sci. 2020, 33, 2031–2038. [Google Scholar] [CrossRef]
- Bortolotti, G.R.; Marchant, T.A.; Blas, J.; German, T. Corticosterone in feathers integrated measure of avian stress physiology. Funct. Ecol. 2008, 22, 494–500. [Google Scholar] [CrossRef]
- Freeman, N.E.; Newman, A.E. Quantifying corticosterone in feathers: Validations for an emerging technique. Conserv. Physiol. 2018, 6, coy051. [Google Scholar] [CrossRef] [PubMed]
- Carbajal, A.; Tallo-Parra, O.; Sabes-Alsina, M.; Mular, I.; Lopez-Bejar, M. Feather corticosterone evaluated by ELISA in broilers. A potential tool to evaluate broiler welfare. Poult. Sci. 2014, 93, 2884–2886. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Lee, W.D.; Kim, H.J.; Kang, B.S.; Kang, H.K. Effect of providing environmental enrichment into aviary house on the welfare of laying hens. Animals 2022, 12, 1165. [Google Scholar] [CrossRef] [PubMed]
- Downing, J.A.; Bryden, W.L. Determination of corticosterone concentrations in egg albumen: A non-invasive indicator of stress in laying hens. Physiol. Behav. 2008, 95, 381–387. [Google Scholar] [CrossRef]
- Alm, M.; Tauson, R.; Holm, L.; Wichman, A.; Kalliokoski, O.; Wall, H. Welfare indicators in laying hens in relation to nest exclusion. Poult. Sci. 2016, 95, 1238–1247. [Google Scholar] [CrossRef]
- Haffelin, K.E.; Lindenwald, R.; Kaufmann, F.; Dohring, S.; Spindler, B.; Preisinger, R.; Rautenschlein, S.; Kemper, N.; Anderson, R. Corticosterone in feathers of laying hens: An assay validation for evidence-based assessment of animal welfare. Poult. Sci. 2020, 99, 4685–4694. [Google Scholar] [CrossRef]
- Caulfield, M.P.; Padula, M.P. HPLC MS-MS analysis shows measurement of corticosterone in egg albumen is not a valid indicator of chicken welfare. Animals 2020, 10, 821. [Google Scholar] [CrossRef]
- Farghly, M.F.; Mahrose, K.M.; Galal, A.E.; Ali, R.M.; Ahmad, E.A.; Rehman, Z.U.; Ullah, Z.; Ding, C. Implementation of different feed withdrawal times and water temperatures in managing turkeys during heat stress. Poult. Sci. 2018, 97, 3076–3084. [Google Scholar] [CrossRef]
- Park, S.O.; Park, B.S.; Hwangbo, J. Effect of cold water and inverse lighting on growth performance of broiler chickens under extreme heat stress. J. Environ. Biol. 2015, 36, 865–873. [Google Scholar]


| Items | Information | |
|---|---|---|
| Farm | Animal welfare-certified farm | |
| Region | Okcheon-gun, Chungcheongbuk-do, Republic of Korea | |
| Strain | Hy-Line Brown | |
| Housing type | Free-range system | |
| Construction materials | Sandwich panel | |
| Ventilation system (type) | Mechanical ventilation system (tunnel-type) | |
| Flock size | 2800~2900 hens | |
| House size, m, m2 | 10 × 84, 840 | |
| Stocking density, hens/m2 | 7.5 | |
| Number installed | Feeders | 239 |
| Waterers | 805 | |
| Perches (length, m) | 42 (19.2), 2 (28.4) | |
| Items | Levels |
|---|---|
| Outside average temperature, °C | 33.5 ± 3.89 |
| Max temperature, °C | 39.7 |
| Min temperature, °C | 22.4 |
| Inside average temperature, °C | 32.83 ± 3.23 |
| Max temperature, °C | 41.9 |
| Min temperature, °C | 23.2 |
| Average THI | 85.21 |
| Items | Composition |
|---|---|
| Ingredients, g/kg | |
| Corn | 411.5 |
| Wheat | 150.0 |
| Soybean meal | 250.0 |
| Corn distiller’s dried grains with solubles | 50.0 |
| Canola meal | 20.0 |
| Tallow | 5.0 |
| Molasses | 5.0 |
| Limestone | 97.0 |
| Dicalcium phosphate | 7.0 |
| Sodium chloride | 2.0 |
| Vitamin premix 1 | 1.5 |
| Mineral premix 2 | 1.0 |
| Total | 1000.0 |
| Calculated nutrient composition 3 | |
| ME, MJ/kg | 11.32 |
| Crude protein, g/kg | 186.0 |
| Available phosphate, g/kg | 3.3 |
| Lysine, g/kg | 9.7 |
| Methionine, g/kg | 3.1 |
| Items | Treatments 2 | p-Value | |
|---|---|---|---|
| Control | Cool Water | ||
| Hen day egg production, % | 83.5 ± 1.8 b | 84.5 ± 0.9 a | 0.04 |
| Feed intake, g/birds | 125.9 ± 2.5 b | 129.8 ± 2.3 a | 0.03 |
| Egg weight, g | 63.1 ± 0.39 | 63.9 ± 0.47 | 0.39 |
| FCR, g/g | 2.39 ± 0.01 | 2.40 ± 0.01 | 0.28 |
| Total mortality, % | 2.39 a | 1.55 b | <0.01 |
| Economic gain per 2900 laying hens/month | |||
| Number of eggs produced, n | 72,645 | 73,515 | - |
| Egg sales revenue, KRW | 12,930,810 | 13,085,670 | - |
| Egg sales revenue, USD | 10,775.68 | 10,904.73 | - |
| Items | Treatments 2 | p-Value | |
|---|---|---|---|
| Control | Cool Water | ||
| Haugh unit | |||
| 0 weeks | 85.67 ± 8.02 | 85.17 ± 5.73 | 0.781 |
| 2 weeks | 86.75 ± 6.64 | 88.30 ± 3.71 | 0.267 |
| 5 weeks | 86.13 ± 7.12 | 88.70 ± 5.99 | 0.136 |
| 7 weeks | 86.78 ± 6.68 | 88.70 ± 5.32 | 0.226 |
| Eggshell strength, kgf | |||
| 0 weeks | 4.43 ± 0.66 | 4.15 ± 1.01 | 0.210 |
| 2 weeks | 3.87 ± 1.22 | 3.93 ± 1.28 | 0.839 |
| 5 weeks | 4.13 ± 1.02 b | 4.59 ± 0.64 a | 0.042 |
| 7 weeks | 4.07 ± 0.93 b | 4.45 ± 0.55 a | 0.047 |
| Eggshell thickness, mm | |||
| 0 weeks | 0.374 ± 0.026 | 0.378 ± 0.029 | 0.550 |
| 2 weeks | 0.394 ± 0.026 | 0.397 ± 0.030 | 0.677 |
| 5 weeks | 0.395 ± 0.029 | 0.390 ± 0.028 | 0.506 |
| 7 weeks | 0.382 ± 0.030 | 0.383 ± 0.020 | 0.801 |
| Eggshell color | |||
| 0 weeks | 27.32 ± 3.46 | 28.79 ± 3.69 | 0.115 |
| 2 weeks | 28.32 ± 3.11 | 27.96 ± 3.06 | 0.653 |
| 5 weeks | 28.50 ± 4.26 | 27.75 ± 4.33 | 0.506 |
| 7 weeks | 30.30 ± 4.57 | 27.86 ± 3.20 | 0.320 |
| Egg yolk color | |||
| 0 weeks | 6.85 ± 0.67 | 6.72 ± 0.46 | 0.360 |
| 2 weeks | 6.27 ± 0.42 | 6.62 ± 0.46 | 0.103 |
| 5 weeks | 6.61 ± 0.51 | 6.46 ± 0.47 | 0.222 |
| 7 weeks | 6.81 ± 0.42 | 6.61 ± 0.42 | 0.073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.-H.; Lee, W.-D.; Lim, S.-J.; Yang, K.-Y.; Jeon, J.-H. Effects of Cool Water Supply on Laying Performance, Egg Quality, Rectal Temperature and Stress Hormones in Heat-Stressed Laying Hens in Open-Type Laying Houses. Animals 2025, 15, 1635. https://doi.org/10.3390/ani15111635
Kim C-H, Lee W-D, Lim S-J, Yang K-Y, Jeon J-H. Effects of Cool Water Supply on Laying Performance, Egg Quality, Rectal Temperature and Stress Hormones in Heat-Stressed Laying Hens in Open-Type Laying Houses. Animals. 2025; 15(11):1635. https://doi.org/10.3390/ani15111635
Chicago/Turabian StyleKim, Chan-Ho, Woo-Do Lee, Se-Jin Lim, Ka-Young Yang, and Jung-Hwan Jeon. 2025. "Effects of Cool Water Supply on Laying Performance, Egg Quality, Rectal Temperature and Stress Hormones in Heat-Stressed Laying Hens in Open-Type Laying Houses" Animals 15, no. 11: 1635. https://doi.org/10.3390/ani15111635
APA StyleKim, C.-H., Lee, W.-D., Lim, S.-J., Yang, K.-Y., & Jeon, J.-H. (2025). Effects of Cool Water Supply on Laying Performance, Egg Quality, Rectal Temperature and Stress Hormones in Heat-Stressed Laying Hens in Open-Type Laying Houses. Animals, 15(11), 1635. https://doi.org/10.3390/ani15111635







