1. Introduction
Livestock production is an essential component of agricultural production, as livestock consume natural agricultural resources in animal protein production [
1,
2,
3,
4]. Livestock production represents about 30% of the total agricultural income and is important for achieving food security, especially from animal protein [
5,
6,
7]. Native breeds are well adapted to agricultural climatic conditions, resistant to many tropical diseases, and can survive and produce milk on poor forage and fodder resources [
7,
8]. Some of these breeds are known for their high milk and fat production. Agriculture faces a great challenge due to the increasing global population, which requires increasing food production while implementing effective and sustainable production techniques. The ability of ruminants, especially small ruminants, to use low-value inputs and produce high-value outputs is well-known [
9].
Growth traits are economically significant in sheep breeding, influenced by multiple genes governing growth, bone formation, birth weight, weaning weight, and muscle development. Growth hormone, a 191-amino acid polypeptide secreted by the somatotropic cells of the anterior pituitary gland, is encoded by the
GH (growth hormone) gene [
10,
11]. The ovine growth hormone gene is found on chromosome 11 (11q25), consisting of five exons, 1795 bp in size.
GH is pivotal for postnatal growth, tissue development, lactation, reproduction, and the metabolism of proteins, lipids, and carbohydrates [
12]. There have been reported associations between polymorphic variants of the
GH gene and production traits such as live weight [
13,
14,
15], body measurements [
16,
17], birth weight [
18,
19], and weaning weight [
20,
21,
22]. Given that one of the main functions of growth hormone is to promote body and muscle development, the
GH gene was selected as a candidate marker in this study [
16].
The calpastatin gene (CAST) is important for regulation of meat tenderization, birth weight, and weaning weight. The CAST gene is found on chromosome 5 (5q15) and consists of 29 exons, 89,553 bp in size.
Many researchers have determined differences in growth rate, fat deposition, and productivity and their dependence on the diet, species, breed, and gender of lambs [
23,
24,
25,
26,
27]. However, due to a poor selection method, these animals’ productive capacity has deteriorated over time [
28]. Moreover, genetic studies on sheep breeds have revealed gene candidates associated with productivity [
29]. The presence of
GH and Calpastatin (
CAST) influence growth rate [
30,
31]. The height of degree of heterozygosity of
GH/HaeIII polymorphisms can be a genetic marker in a sheep selection programme for improvement of growth rate and weaning weight.
Growth hormone (GH) and Calpastatin (CAST) gene polymorphisms can affect not only productivity but also meat fat composition, which affects not only meat’s sensory properties but also physiological benefits for human health.
Lamb meat is rich in fatty acids (FAs). Also, the proportions of saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), and polyunsaturated fatty acid (PUFA) in lamb meat are of great significance for maintaining normal physiological function in the human body [
32,
33]. The flavour of lamb perceived by consumers is generally closely related to the content of certain beneficial FAs [
34,
35].
Ruminant meat is a source of proteins and minerals for human nutrition. However, high levels of saturated fatty acids (SFAs) in livestock meat are associated with chronic and heart diseases [
36]. Many efforts have been made to improve animal fat content in terms of management and genetic improvement [
37,
38]. Intramuscular fat contributes to tenderness and can be affected by selection. Many polymorphisms of the
CAST gene have been related to meat quality indicators and characteristics, such as tenderness, colour, and intramuscular fat (IMF) content in cattle and pig carcasses [
39,
40,
41,
42,
43,
44]. It has been reported that several genes related to lipid metabolism regulate the composition of FAs in ruminant meats [
45]. The FA composition of lamb meat is an important trait for the determination of flavour and nutrition, and this meat can provide essential FAs for human health [
46]. The types and levels of FAs are influenced by polygenic and environmental factors [
47,
48]. A better knowledge of the molecular architecture of meat FA composition is important as it may generate new opportunities for more effective marker-assisted breeding, leading to economic benefits for the sheep industry [
49]. Nevertheless, compared to beef and pork, the studies of genetic effects, including the mutation and expression of candidate genes, on the FA composition of lamb are few. Calpastatin (
CAST) is involved in the calpain–calpastatin system, which influences many important processes, including muscle development and growth [
30], and is also known to regulate the degradation of myofibrillar proteins both in living and in post-mortem muscle tissue [
50,
51].
Recently, a special interest in developing and improving native breeds has increased. The important role of these genes in animal growth is well-known, so their polymorphisms and interactions with growth and meat characteristics are likely to be markers in a sheep breeding programme to improve productivity in particular. However, research focusing on the relationship between the CAST or GH genes and meat quality, especially FA composition, in sheep meat is limited. Since the GH gene affects lipid synthesis and mobilization, its genetic variations can determine which fatty acids are stored in the muscles. The CAST gene regulates muscle protein degradation and structural changes and therefore affects meat texture. By studying the polymorphisms of these genes, it is possible to identify the genetic factor that determines a healthier and higher-quality fatty acid profile of meat, and the results obtained can be applied in breeding to improve the nutritional value of meat and provide economic production value. Considering the lack of studies on the influence of these gene polymorphisms on the fat quality of ovine, the association of GH and CAST gene polymorphisms with the fatty acid profile of meat will be determined. The aim of this study was to investigate the relationship between breed, GH and CAST genotypes, and FA composition in the ovine intramuscular fat of musculus Semimembranosus.
2. Materials and Methods
This study was conducted at the sheep farm, located in Lithuania (55°46′06.2″ N 23°44′08.4″ E). A total of 175 blood samples were drawn by jugular vein puncture from Lithuanian Black-Headed (N43), Lithuanian Black-Headed *Ile de France (N43), Lithuanian Black-Headed *Suffolk (N44), and Lithuanian Black-Headed *Texel (N45) lambs from the 2024 season. The meat of the studied sheep breeds is characterized by good muscle mass and flavour: Lithuanian Black-Headed meat is medium-fat and tender, Ile de France—lean, with a high percentage of muscle yield, Suffolk—juicy and tender, and Texel—extremely lean, very muscular, and firm.
Two feeding systems were applied depending on the season: indoor feeding (late November to early May) and pasture-based feeding (mid-May to early November). During the indoor period, lambs received hay, haylage, 0.3–0.4 kg of concentrated feed per day, and mineral licks; before weaning, additional protein-rich feed (0.1–0.2 kg per lamb) was provided. Pasture-based feeding consisted of ad libitum fresh grass, water, and mineral licks. After weaning, female lambs grazed on cultivated pastures, while ram lambs remained indoors and were fed hay, haylage, and concentrated feed containing a pea–oat–vetch mixture, barley, and protein supplements.
The lambs were born in January, and weaning took place in May. Approximately 30% of the lambs were from twin births.
The lambs were weighed weekly, and the growth rate was calculated using birth weight and month weight values.
The investigation was carried out in the Lithuanian University of Health Sciences, K. Janušauskas Laboratory of Genetic (Kaunas, Lithuania). In the present study, 175 animals were tested. Genomic DNA was extracted from blood samples taken into EDTA containing tubes, using a “GeneJET Genomic DNA Purification Kit” (Thermo Scientific, Waltham, MA, USA).
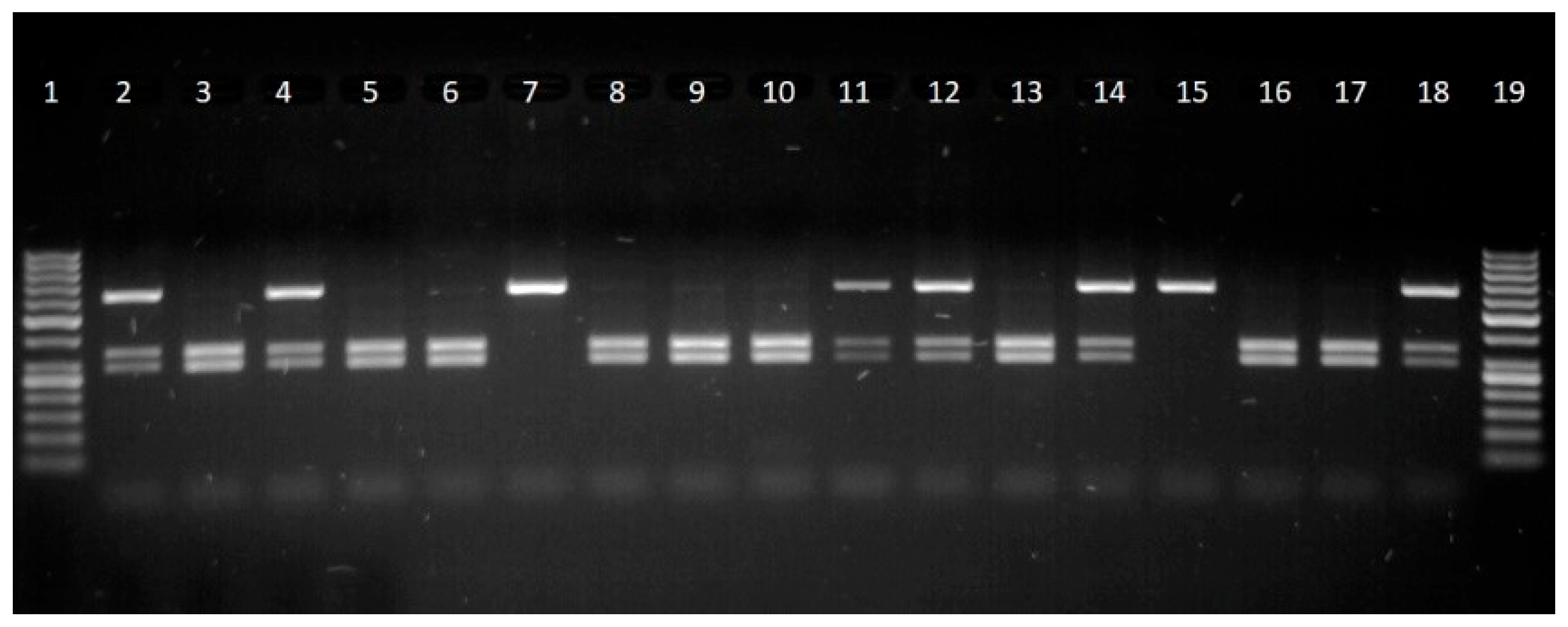
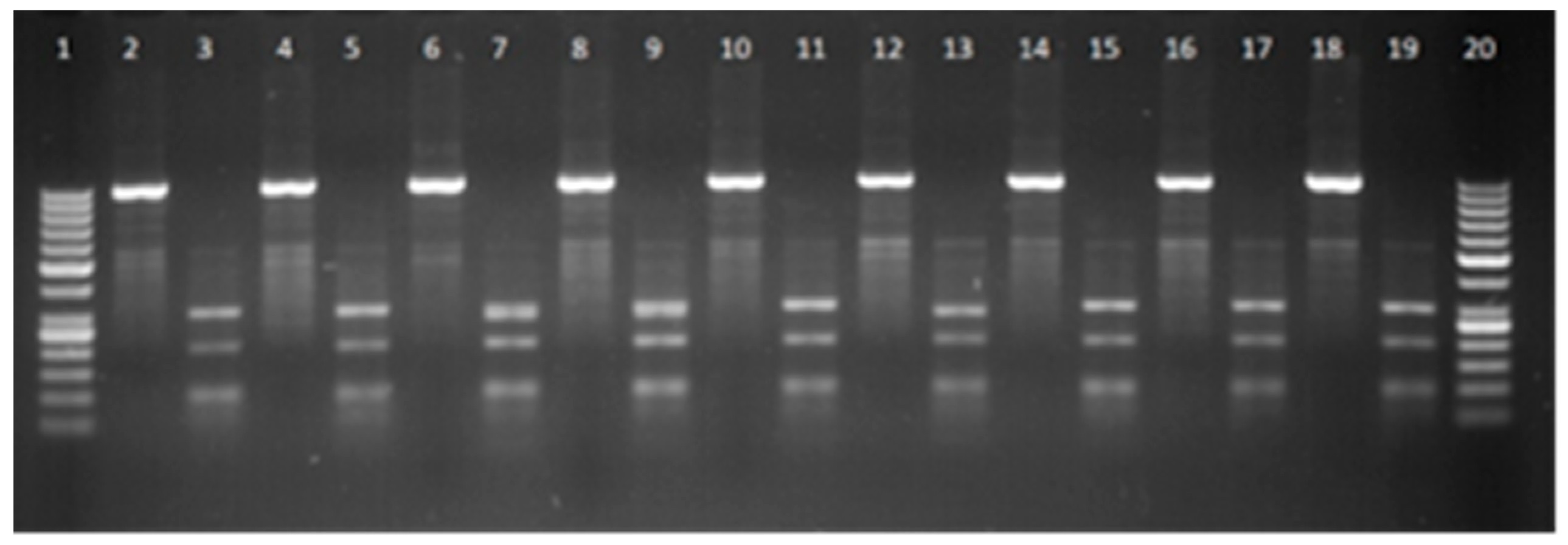
The methods of polymerase chain reaction and restriction length polymorphism were used to genotype growth hormone (
GH) (C → G substitution) and (
CAST) (A → G substitution at position 286) gene polymorphisms [
52,
53]. The characteristics of the primers used for amplification and restriction analysis are presented in
Table 1.
The fragment sizes were determined by agarose gel electrophoresis in 2% agarose gel (Thermo Scientific, Waltham, MA, USA), stained with ethidium bromide (Thermo Scientific, Waltham, MA, USA). Fragment identification was performed in ultraviolet light, using a MiniBIS Pro Video Documentation System (DNR Bio Imaging System, Neve Yamin, Israel).
Meat samples of four lamb breeds were taken for analysis: Lithuanian Black-Headed (N43), Lithuanian Black-Headed *Ile de France (N43), Lithuanian Black-Headed *Suffolk (N44), and Lithuanian Black-Headed *Texel (N45), from the slaughterhouse (X) located in Radviliškis district, Lithuania. The samples of 200–250 g from musculus. Semimembranosus were taken 48 h after carcass meat cooling. The samples were kept in a refrigerator at +4 °C temperature 24 h until the homogenization and extraction of intramuscular fat. Fatty acids extracted with n-hexane were methylated with a 2 mol/L KOH solution in anhydrous methanol.
The fatty acid methyl esters (FAMEs) were analyzed using a gas chromatography mass spectrometry (GC/MS) instrumental technique, in accordance with LST EN ISO 12966—2:2017 [
54]. Chromatographic analysis of fatty acid methyl esters was performed using a PerkinElmer Clarus 680 gas chromatograph and a PerkinElmer Clarus SQ8T mass spectrometer with a capillary column SP-2560, 100 m × 0.25 mm × 0.20 µm. Conditions for chromatographic analysis were as follows: the injector and detector temperatures were maintained at 250 °C; the oven temperature was initially 60 °C for 1 min and was increased to 180 °C at a rate of 12 °C/min and held for 30 min. The spectrometer temperature mode was 5 °C/min. up to 300 °C, holding for 2 min. The samples of FAME in hexane (1.0 μL) were injected through the split injection port (10:1). Carrier gas (He) flow rate was 1 mL/min. A Supelco 37 Component FAME Mix fatty acid kit (Merck, Darmstadt, Germany) was used for fatty acid identification. Individual FAMEs were identified by comparing their retention times with those of the standard, and the results were calculated as a percentage of the total FAMEs (% of TFA). Individual FAs were used to calculate the sums of saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs).
Statistical data analysis was performed using SPSS 25.0 analytical software (IBM Corp., Armonk, New York, NY, USA). The data were presented using descriptive statistics. Normal distributions of variables were assessed using the Kolmogorov–Smirnov test. One-way analysis of variance (ANOVA) was used to determine statistically significant differences between the means. Multiple comparisons of groups mean were calculated using the post hoc Tukey test. The differences were considered significant at p < 0.05.
4. Conclusions
Based on the research, it can be concluded that the purebred Lithuanian Black-Headed lambs had higher birth weight, better intramuscular fat, and an FA profile associated with lower SFA and higher PUFA contents; therefore, it is not appropriate to cross them with Ile de France, Suffolk or Texel breeds. However, better average daily gain results were obtained by crossing Lithuanian Black-Headed sheep with Ile de France or Suffolk breeds. The genetic influence of the meat-variety breeds was responsible for this, most likely due to positive heterosis, which is advantageous for meat production.
Our research suggests that the most useful to grow lambs who have the BB genotype of the GH gene and the MN genotype of the CAST gene, because these lambs have the highest birth weight and weight gain. An AA genotype of the GH gene and an MN genotype of the CAST gene could be applied for genetic selection, improving the quality and nutritional value of ovine intramuscular fat with optimal an omega 6 and omega 3 ratio, lower SFA, and higher PUFA. Our results will allow us to increase the productivity and nutritional value of ovine meat and to meet the demands of consumers in the future.