Lumbosacral Foraminal Injections in Dogs: Preliminary Assessment of an Ultrasound- and Fluoroscopy-Guided Technique in a Cadaveric Model
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
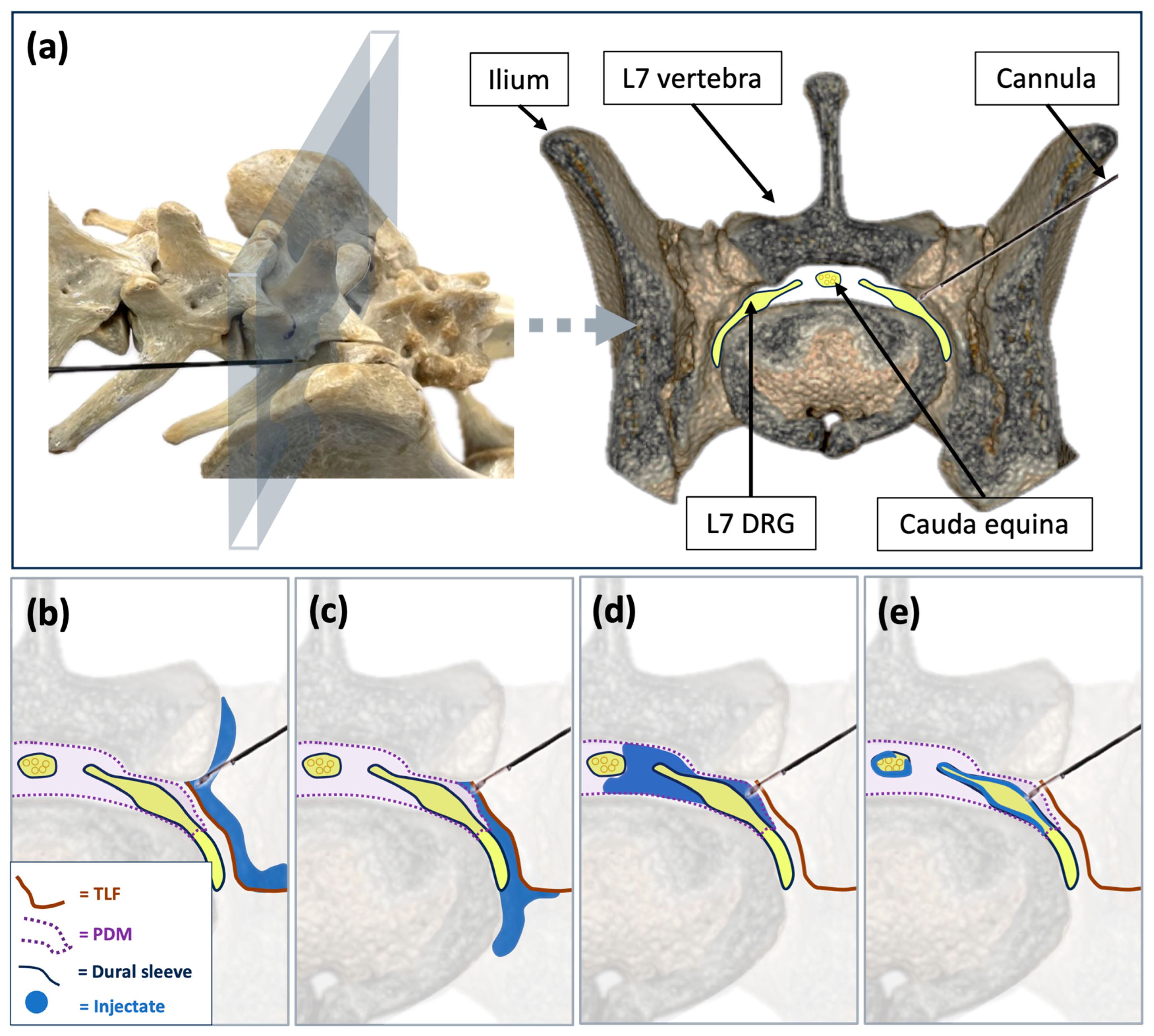
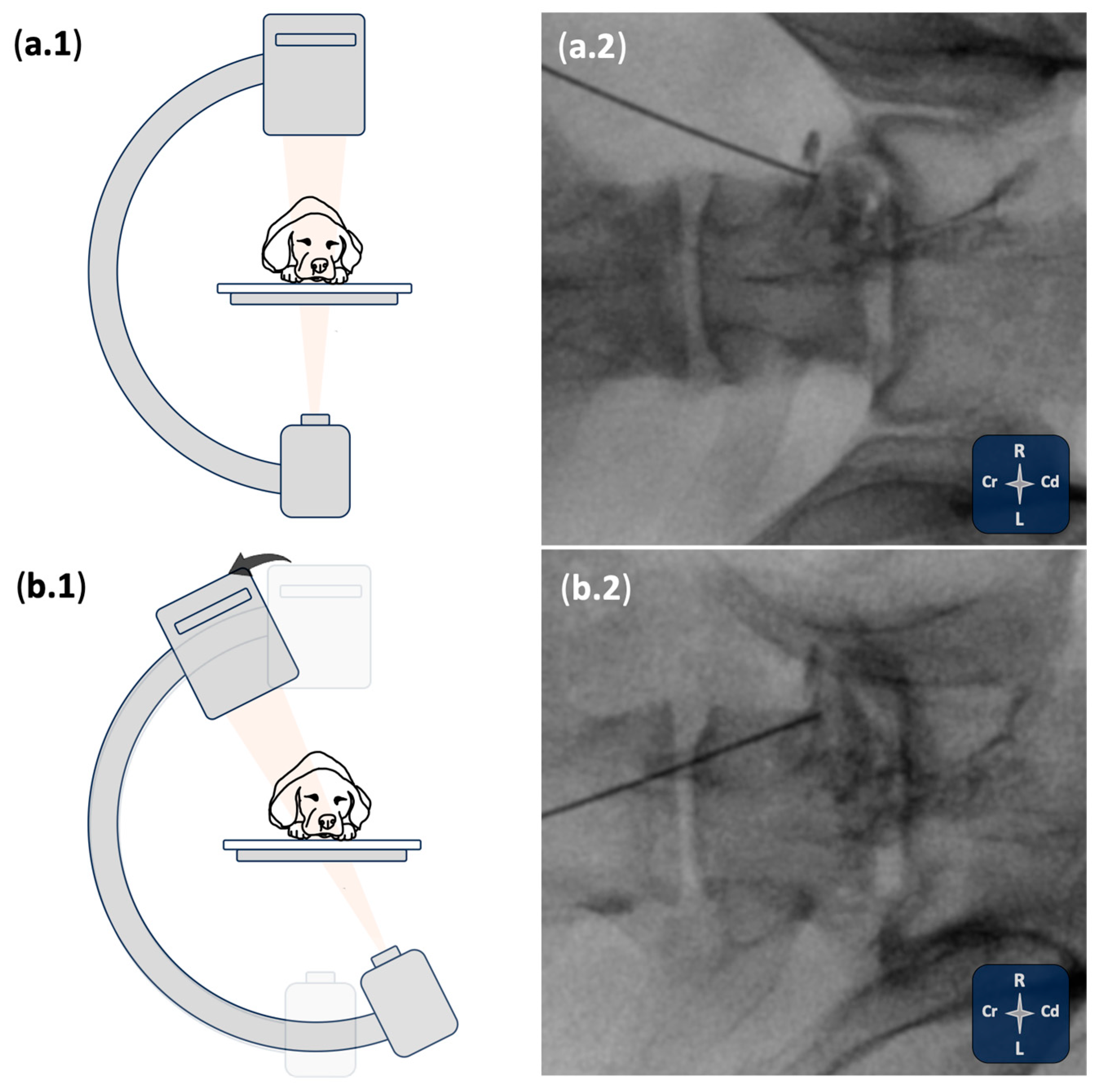
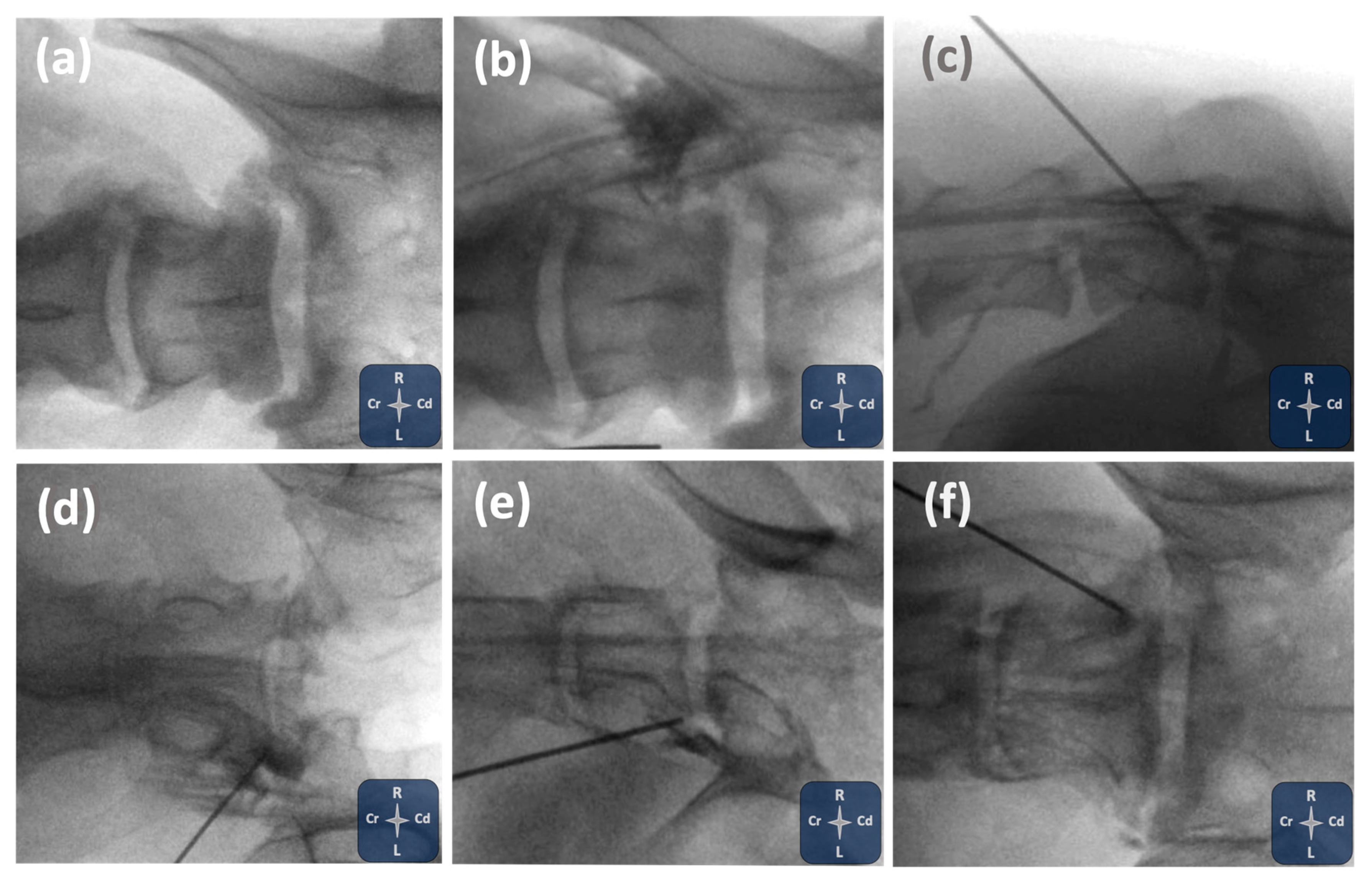
2.1. Phase I: Ultrasound- and Fluoroscopy-Guided Technique
2.2. Phase II: Imaging Study and Anatomical Dissections
2.2.1. CT Study
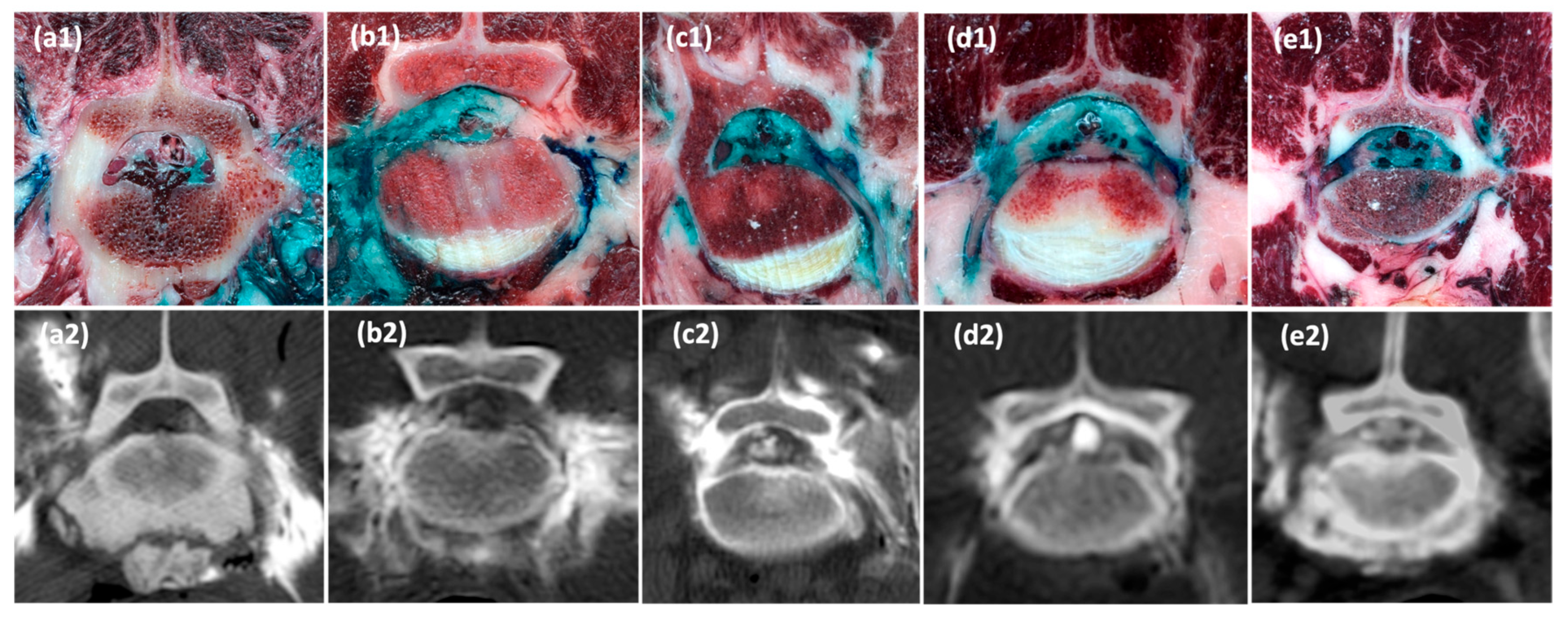
2.2.2. Anatomical Study
2.3. Statistical Analysis
3. Results
3.1. Phase I: Ultrasound- and Fluoroscopy-Guided Technique
3.2. Phase II: Imaging Study and Anatomical Dissections
3.2.1. CT Study
3.2.2. Anatomical Dissections
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low Back Pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Medina-Serra, R.; López-Abradelo, P.; Belda, E.; Riding-Medina, H.; Laredo, F.G.; Marwood, R.; Mortera, V.; Redondo, J.I. Multivariable Analysis of the Association Between Lumbar and Lumbosacral MRI-Diagnosed Spinal Pathologies and Pain in Dogs. Animals 2025, 15, 761. [Google Scholar] [CrossRef] [PubMed]
- Peene, L.; Cohen, S.P.; Kallewaard, J.W.; Wolff, A.; Huygen, F.; van de Gaag, A.; Monique, S.; Vissers, K.; Gilligan, C.; Van Zundert, J.; et al. 1. Lumbosacral Radicular Pain. Pain Pract. 2024, 24, 525–552. [Google Scholar] [CrossRef]
- Tajima, T.; Furukawa, K.; Kuramochi, E. Selective Lumbosacral Radiculography and Block. Spine 1980, 5, 68–77. [Google Scholar] [CrossRef]
- Weiner, B.K.; Fraser, R.D. Foraminal Injection for Lateral Lumbar Disc Herniation. J. Bone Jt. Surg. 1997, 79, 804–807. [Google Scholar] [CrossRef]
- Datta, S.; Manchikanti, L.; Falco, F.J.E.; Calodney, A.K.; Atluri, S.; Benyamin, R.M.; Buenaventura, R.M.; Cohen, S.P. Diagnostic Utility of Selective Nerve Root Blocks in the Diagnosis of Lumbosacral Radicular Pain: Systematic Review and Update of Current Evidence. Pain Physician 2013, 16, SE97-124. [Google Scholar] [CrossRef]
- Beynon, R.; Elwenspoek, M.M.C.; Sheppard, A.; Higgins, J.N.; Kolias, A.G.; Laing, R.J.; Whiting, P.; Hollingworth, W. The Utility of Diagnostic Selective Nerve Root Blocks in the Management of Patients with Lumbar Radiculopathy: A Systematic Review. BMJ Open 2019, 9, e025790. [Google Scholar] [CrossRef]
- Smith, C.C.; McCormick, Z.L.; Mattie, R.; MacVicar, J.; Duszynski, B.; Stojanovic, M.P. The Effectiveness of Lumbar Transforaminal Injection of Steroid for the Treatment of Radicular Pain: A Comprehensive Review of the Published Data. Pain Med. 2020, 21, 472–487. [Google Scholar] [CrossRef]
- Ackerman, W.E.; Ahmad, M. The Efficacy of Lumbar Epidural Steroid Injections in Patients with Lumbar Disc Herniations. Anesth. Analg. 2007, 104, 1217–1222. [Google Scholar] [CrossRef]
- Manchikanti, L.; Knezevic, E.; Knezevic, N.N.; Sanapati, M.R.; Thota, S.; Abd-Elsayed, A.; Hirsch, J.A. Epidural Injections for Lumbar Radiculopathy or Sciatica: A Comparative Systematic Review and Meta-Analysis of Cochrane Review. Pain Physician 2021, 24, E539–E554. [Google Scholar] [CrossRef]
- Kim, W.-J.; Shin, H.-Y.; Yoo, S.H.; Park, H.S. Comparison of Epidural Spreading Patterns and Clinical Outcomes of Transforaminal Epidural Steroid Injection with High-Volume Injectate via the Subpedicular Versus the Retrodiscal Approach. Pain Physician 2018, 21, 269–278. [Google Scholar] [CrossRef]
- Ignjatovic, S.; Omidi, R.; Kubik-Huch, R.A.; Anderson, S.; Ahlhelm, F.J. The Retroneural Approach: An Alternative Technique for Lumbar Transforaminal Epidural Steroid Injections. Acta Radiol. 2018, 59, 1508–1516. [Google Scholar] [CrossRef]
- Ghai, B.; Gupta, A.K.; Makkar, J.K.; Dhatt, S.S. Contrast Medium Volume Needed to Reach Anterior Epidural Space via the Kambin Triangle or Subpedicular Approach for Transforaminal Epidural Injection. Pain Physician 2020, 23, 383–392. [Google Scholar] [CrossRef]
- Yu, R.K.; Lagemann, G.M.; Ghodadra, A.; Agarwal, V. Extraforaminal Needle Tip Position Reduces Risk of Intravascular Injection in CT-Fluoroscopic Lumbar Transforaminal Epidural Steroid Injections. J. Spine Surg. 2016, 2, 246–255. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jin, H.S.; Bae, G.H.; Choi, E.J.; Lee, J.W.; Lee, P.B. Comparison of Effect and Contrast Spreading in Transforaminal Epidural Injection Using the Retrodiscal Versus Subpedicular Approach: A Prospective, Randomized Trial. Pain Physician 2022, 25, E1379–E1388. [Google Scholar] [PubMed][Green Version]
- Schultz, D.M. Fluoroscopy in Interventional Pain Management. In Essentials of Interventional Techniques in Managing Chronic Pain; Springer International Publishing: Cham, Switzerland, 2018; pp. 109–123. [Google Scholar][Green Version]
- Yang, S.; Chang, M.C. Efficacy of Pulsed Radiofrequency in Controlling Pain Caused by Spinal Disorders: A Narrative Review. Ann. Cardiothorac. Surg. 2020, 9, 3528–3536. [Google Scholar] [CrossRef] [PubMed]
- Vuka, I.; Marciuš, T.; Došenović, S.; Ferhatović Hamzić, L.; Vučić, K.; Sapunar, D.; Puljak, L. Efficacy and Safety of Pulsed Radiofrequency as a Method of Dorsal Root Ganglia Stimulation in Patients with Neuropathic Pain: A Systematic Review. Pain Med. 2020, 21, 3320–3343. [Google Scholar] [CrossRef]
- Park, S.; Park, J.; Jang, J.N.; Choi, S.; Song, Y.; Kim, Y.U.; Park, S. Pulsed Radiofrequency of Lumbar Dorsal Root Ganglion for Lumbar Radicular Pain: A Systematic Review and Meta-analysis. Pain Pract. 2024, 24, 772–785. [Google Scholar] [CrossRef]
- Vanneste, T.; Van Lantschoot, A.; Van Boxem, K.; Van Zundert, J. Pulsed Radiofrequency in Chronic Pain. Curr. Opin. Anaesthesiol. 2017, 30, 577–582. [Google Scholar] [CrossRef]
- Jang, J.N.; Park, S.; Park, J.-H.; Song, Y.; Kim, Y.U.; Kim, D.S.; Sohn, J.E.; Park, S. Output Current and Efficacy of Pulsed Radiofrequency of the Lumbar Dorsal Root Ganglion in Patients with Lumbar Radiculopathy: A Prospective, Double-Blind, Randomized Pilot Study. Pain Physician 2023, 26, E797–E804. [Google Scholar] [CrossRef]
- Dietrich, T.J.; Peterson, C.K.; Zeimpekis, K.G.; Bensler, S.; Sutter, R.; Pfirrmann, C.W.A. Fluoroscopy-Guided versus CT-Guided Lumbar Steroid Injections: Comparison of Radiation Exposure and Outcomes. Radiology 2019, 290, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Maino, P.; Presilla, S.; Colli Franzone, P.A.; van Kuijk, S.M.J.; Perez, R.S.G.M.; Koetsier, E. Radiation Dose Exposure for Lumbar Transforaminal Epidural Steroid Injections and Facet Joint Blocks Under CT vs. Fluoroscopic Guidance. Pain Pract. 2018, 18, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, J.; Ma, L.; Cai, Z.; Meng, C.; Qi, S.; Zhou, H. Ultrasound-Guided Versus Fluoroscopy-Controlled Lumbar Transforaminal Epidural Injections. Clin. J. Pain 2016, 32, 103–108. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, G.; Wang, Q.; Yang, L. Ultrasound-Guided Transforaminal Epidural Injection with Fluoroscopy Confirmation for the Treatment of Unilateral Lumbar Radiculopathy: A Randomized Controlled Non-Inferiority Study. Clin. Neurol. Neurosurg. 2023, 231, 107849. [Google Scholar] [CrossRef] [PubMed]
- Vydyanathan, A.; Agrawal, P.; Donia, K.; Wahezi, S.; Koushik, S.; Slinchenkova, K.; Gritsenko, K.; Shaparin, N. Feasibility of Ultrasound-Guided Lumbar Transforaminal Epidural Steroid Injections for Management of Lumbar Radicular Back Pain. J. Pain Res. 2025, 18, 759–767. [Google Scholar] [CrossRef]
- Medina-Serra, R.; Belda, E.; López-Abradelo, P.; Zoff, A.; Sanchís-Mora, S. Radiation Exposure during Ultrasound- and Fluoroscopy-Guided Spinal Interventional Pain Management Procedures in Dogs: A Retrospective Analysis in a Single Institution. Vet Anaesth Analg 2025. [Google Scholar] [CrossRef]
- Kneissl, S.; Breit, S.; Willmitzer, F.; Thalhammer, J.; Dengg, S. Dispersal pattern of injectate following CT-guided perineural infiltration in the canine thoracolumbar spine: A cadaver study. Vet. Radiol. Ultrasound 2015, 56, 212–219. [Google Scholar] [CrossRef]
- Liotta, A.; Sandersen, C.; Couvreur, T.; Bolen, G. Technique, difficulty, and accuracy of computed tomography-guided translaminar and transforaminal lumbosacral epidural and intraarticular lumbar facet joint injections in dogs. Vet. Radiol. Ultrasound 2016, 57, 191–198. [Google Scholar] [CrossRef]
- Liotta, A.P.; Girod, M.; Peeters, D.; Sandersen, C.; Couvreur, T.; Bolen, G. Clinical Effects of Computed Tomography–Guided Lumbosacral Facet Joint, Transforaminal Epidural, and Translaminar Epidural Injections of Methylprednisolone Acetate in Healthy Dogs. Am. J. Vet. Res. 2016, 77, 1132–1139. [Google Scholar] [CrossRef]
- Medina-Serra, R.; Gil-Cano, F.; Laredo, F.G.; Belda, E. Pulsed Radiofrequency for Lumbosacral Radicular Pain in Dogs: Description and Assessment of an Ultrasound- and Fluoroscopy-Guided Technique in a Cadaveric Model. Animals 2025, 15, 2586. [Google Scholar] [CrossRef]
- Breit, S.; Giebels, F.; Kneissl, S. Foraminal and Paraspinal Extraforaminal Attachments of the Sixth and Seventh Lumbar Spinal Nerves in Large Breed Dogs. Vet. J. 2013, 197, 631–638. [Google Scholar] [CrossRef]
- Hashemi, M.; Dadkhah, P.; Taheri, M.; Haji, S.M.; Abootorabi, S.; Naderi-nabi, B. Ultrasound-Guided Lumbar Transforaminal Epidural Injections; A Single Center Fluoroscopic Validation Study. Bull. Emerg. Trauma 2019, 7, 251–255. [Google Scholar] [CrossRef]
- Fishman, S.; Smith, H.; Meleger, A.; Seibert, J. Radiation Safety in Pain Medicine. Reg. Anesth. Pain Med. 2002, 27, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Rathmell, J.P.; Benzon, H.T.; Dreyfuss, P.; Huntoon, M.; Wallace, M.; Baker, R.; Riew, K.D.; Rosenquist, R.W.; Aprill, C.; Rost, N.S.; et al. Safeguards to Prevent Neurologic Complications after Epidural Steroid Injections. Anesthesiology 2015, 122, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Van Boxem, K.; Rijsdijk, M.; Hans, G.; de Jong, J.; Kallewaard, J.W.; Vissers, K.; van Kleef, M.; Rathmell, J.P.; Van Zundert, J. Safe Use of Epidural Corticosteroid Injections: Recommendations of the WIP Benelux Work Group. Pain Pract. 2019, 19, 61–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tariq, R.; Liu, G.L.; Yan, H.; Kaye, A.D. Inadvertent Intrathecal Injections and Best Practice Management. Acta Anaesthesiol. Scand. 2017, 61, 11–22. [Google Scholar] [CrossRef]
- Lima, R.M.; Navarro, L.H.; Carness, J.M.; Barros, G.A.; Marques, M.E.A.; Solanki, D.; Ganem, E.M. Clinical and Histological Effects of the Intrathecal Administration of Methylprednisolone in Dogs. Pain Physician 2010, 13, 493–501. [Google Scholar] [CrossRef]
- Rijsdijk, M.; van Wijck, A.J.M.; Kalkman, C.J.; Meulenhoff, P.C.W.; Grafe, M.R.; Steinauer, J.; Yaksh, T.L. Safety Assessment and Pharmacokinetics of Intrathecal Methylprednisolone Acetate in Dogs. Anesthesiology 2012, 116, 170–181. [Google Scholar] [CrossRef]
- Finlayson, R.J.; Etheridge, J.-P.B.; Chalermkitpanit, P.; Tiyaprasertkul, W.; Nelems, B.; Tran, D.Q.H.; Huntoon, M.A. Real-Time Detection of Periforaminal Vessels in the Cervical Spine. Reg. Anesth. Pain Med. 2016, 41, 130–134. [Google Scholar] [CrossRef]
| Assessment Method | Epidural Contrast (n = 10) | Perineural Epidural Stain of L7 at Foraminal Region (n = 10) | Paravertebral Foraminal Contrast (n = 10) | Subarachnoid Contrast (n = 10) | Intravascular Contrast (n = 10) |
|---|---|---|---|---|---|
| Fluoroscopy | 8/10 (80%) | N/A | 10/10 (100%) | 5/10 (50%) | 1/10 (10%) |
| CT | 8/10 (80%) | N/A | 10/10 (100%) | 6/10 (60%) * | 2/10 (20%) ** |
| Cryosections | 8/10 (80%) | 9/10 (90%) | 10/10 (100%) | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Serra, R.; Gil-Cano, F.; Soler, M.; Laredo, F.G.; Belda, E. Lumbosacral Foraminal Injections in Dogs: Preliminary Assessment of an Ultrasound- and Fluoroscopy-Guided Technique in a Cadaveric Model. Animals 2025, 15, 2958. https://doi.org/10.3390/ani15202958
Medina-Serra R, Gil-Cano F, Soler M, Laredo FG, Belda E. Lumbosacral Foraminal Injections in Dogs: Preliminary Assessment of an Ultrasound- and Fluoroscopy-Guided Technique in a Cadaveric Model. Animals. 2025; 15(20):2958. https://doi.org/10.3390/ani15202958
Chicago/Turabian StyleMedina-Serra, Roger, Francisco Gil-Cano, Marta Soler, Francisco G. Laredo, and Eliseo Belda. 2025. "Lumbosacral Foraminal Injections in Dogs: Preliminary Assessment of an Ultrasound- and Fluoroscopy-Guided Technique in a Cadaveric Model" Animals 15, no. 20: 2958. https://doi.org/10.3390/ani15202958
APA StyleMedina-Serra, R., Gil-Cano, F., Soler, M., Laredo, F. G., & Belda, E. (2025). Lumbosacral Foraminal Injections in Dogs: Preliminary Assessment of an Ultrasound- and Fluoroscopy-Guided Technique in a Cadaveric Model. Animals, 15(20), 2958. https://doi.org/10.3390/ani15202958








