Simple Summary
Growth performance represents a key metric in piscine genetic breeding. The growth-related gene spexin (spx) critically regulates appetite and metabolic homeostasis. This study characterized the open reading frame (ORF) of spx in mandarin fish (Siniperca chuatsi), an economically significant teleost, revealing its peak expression in the liver. We further elucidated the regulatory profile of spx during starvation and feeding adaptation. During a 7-day fasting regimen, spx expression in muscle, liver, and intestine exhibited an initial increase followed by a decline, whereas an inverse expression trajectory was noted in the brain and stomach. Larger individuals displayed significantly diminished spx expression in the liver and brain relative to their smaller counterparts, yet elevated expression was detected in the stomach. Using glutathione S-transferase (GST) pull-down assays, fatty acid-binding protein 2 was identified as a novel interaction partner of SPX, suggesting a potential mechanism whereby SPX modulates lipid metabolism via the peroxisome proliferator-activated receptor (PPAR) signaling pathway to mediate feeding adaptation. This study provides novel mechanistic insights into the role of SPX in nutritional adaptation in S. chuatsi, and establishes a foundational framework for subsequent genetic breeding initiatives.
Abstract
Neuropeptide Q (spexin, spx) is a pleiotropic signalling molecule that regulates appetite and metabolism primarily via activation of galanin and melanocortin receptors. Here, we cloned the open reading frame (ORF) of spx from Siniperca chuatsi (Scspx), characterised its spatiotemporal expression, elucidated spx regulatory features during starvation and feed adaptation, and identified SPX-interacting proteins using glutathione S-transferase pull-down and mass spectrometry. The Scspx ORF was 312 bp, encoding 103 amino acids. The predominant expression of spx was found in the liver of feed-trained S. chuatsi, where it was 17.36-fold greater than in muscle. During fasting (0, 3, 5, and 7 d), spx expression in the muscle, liver, and intestine initially increased and then declined, whereas brain and stomach tissues exhibited the opposite tendency. Compared to the smallest individuals, hepatic and brain spx expression was substantially lower in the largest individuals, whereas stomach expression was higher (p < 0.05). Fatty acid binding protein 2 was identified as a novel SPX-interacting partner, implicating SPX in feed adaptation through lipid metabolic regulation via the peroxisome proliferator-activated receptor signalling pathway. Our results provide the first evidence of a direct SPX-FABP2 interaction in fish, pointing to a coordinated role in downstream gene regulation. This work hereby uncovers a novel regulatory axis within the piscine energy metabolism network. These findings provide new insight into the regulatory role of SPX in feed adaptation in S. chuatsi, offering a foundation for genetic analysis.
1. Introduction
Siniperca chuatsi is an economically important freshwater species in East Asia, priced for its delicate flavour and boneless flesh, which drives high market demand. However, large-scale aquaculture of S. chuatsi is hindered by its obligate reliance on live prey, a consequence of its benthic, piscivorous feeding habits [1]. Although the development of formulated feeds has alleviated this dependency, considerable challenges persist, including low voluntary feed intake, substantial variation in growth performance, and hepatic metabolic dysfunction, which are key bottlenecks to production at a commercial scale. Starvation challenge trials are essential during the habituation of S. chuatsi to formulated feed. Studies have established the involvement of spx in fasting responses across fish species, but the specific regulatory mechanisms during fasting in mandarin fish need to be elucidated [2,3,4]. SPX is proposed to exert beneficial effects via multiple mechanisms: (1) inhibition of feeding behaviour through downregulation of orexigenic factors [5]; (2) improvement of hepatic lipid metabolism by modulating lipid-related enzyme and gene expression [6]; and (3) stimulation of lipolysis through enhanced phosphorylation of hormone-sensitive lipase (HSL) [7]. These mechanisms likely interact synergistically to maintain systemic metabolic homeostasis.
The spx gene was first identified in the human genome using bioinformatic analyses in 2007. Its cDNA was subsequently cloned in Carassius auratus and Danio rerio in 2013 [8] and later characterised in mammals, birds, and additional teleost species [9,10,11]. The genomic architecture of spx varies among teleosts. In both C. auratus and D. rerio, the gene comprises five exons and four introns. In C. auratus, alternative splicing generates three distinct transcripts (847 bp, 805 bp, and 574 bp), although only the 574 bp variant contains a complete open reading frame (ORF) encoding the mature SPX peptide. The remaining transcripts contain premature stop codons in the second intron, resulting in truncated, nonfunctional products [8,12]. In contrast, the spx genes of S. chuatsi and Epinephelus coioides exhibit a mammal-like structure, comprising six exons and five introns. This structural divergence is hypothesized to result from the insertion of a novel intron within the fifth exon [13,14].
Neuropeptide Q (spexin, SPX) has attracted attention for its distinct signalling features. The mature SPX peptide comprises a conserved 14-amino-acid core flanked by dibasic proteolytic cleavage sites [15]. Structural analyses in goldfish indicate two functional domains: Asn1–Pro4 and Gln5–Gln14 extending to the COOH terminus. The molecular surface exhibits high hydrophobicity. Lys11, the only charged residue, is essential for high-affinity receptor interaction [12]. SPX shares evolutionary ancestry with galanin (GAL) and kisspeptin (KISS), originating from a common ancestral peptide module in early vertebrates [16]. SPX activates GALR2 and GALR3 receptors in the central nervous system. Selective GALR2 activation engages the PI3K/AKT and L-type voltage-dependent calcium channel (VDCC) pathways, whereas binding to both GALR2 and GALR3 suppresses the cAMP/protein kinase A (PKA) signalling cascade [17,18]. Disruption of GALR2/3 signalling in hypothalamic nuclei, such as the arcuate nucleus (ARC), ventromedial hypothalamus (VMH), and dorsomedial hypothalamus (DMH), induces a positive energy balance, leading to glucose intolerance, lipid accumulation, and weight gain [19]. However, the structure and function of the spx gene in S. chuatsi remain uncharacterized. Furthermore, although the role of spexin in feeding is well-established, its direct influence on growth variation and lipid metabolism in teleosts is poorly understood, particularly on a mechanistic basis. To investigate the potential role of the spx gene (Scspx) in nutrient metabolism regulation in mandarin fish (Siniperca chuatsi), we cloned Scspx from feed-adapted fish, profiled its tissue expression patterns, and examined its expression in response to starvation and in populations with divergent feed-adaptation phenotypes. Furthermore, we screened for potential interacting proteins to elucidate the regulatory function of Scspx in feeding and metabolic pathways during the feed adaptation process.
2. Materials and Methods
2.1. Animals
Mandarin fish (S. chuatsi) fingerlings (53.12 ± 10.35 g; SL, ~12 cm; 5-month-old), designated for cloning, tissue expression profiling, and starvation experiments, were cultured for 120 d in aquaculture ponds (78 m × 36 m × 2.3 m) at Heyang Aquatic Co., Ltd. (Foshan, China). During this period, they were fed a formulated floating feed purchased from CP Group (diameter: 0.55 cm, crude protein ≥ 60%; total phosphorus ≥ 1.8%; crude fibre ≤ 7.0%; calcium ≤ 0.6–2.0%; crude fat ≥ 5.0%; lysine ≥ 1.8%; coarse grey powder ≤ 13.0%; moisture content ≤ 12.0%). The daily feed ration for each experimental group was adjusted based on the individual size of the mandarin fish: a feeding rate of 4.2% of body weight per day was applied during the early grow-out phase (50–200 g), and a rate of 2.8% of body weight was used during the late grow-out phase (>200 g).
A net cage (1.8 m × 1.8 m × 1.4 m; 0.3 cm mesh size) was installed within the pond, and 25 individuals were randomly selected from the main stock and transferred into the cage. Healthy mandarin fish were selected from net-pen cultured populations. Following dissection and sex identification, the subjects were randomly assigned to groups with a 1:1 male-to-female ratio, thereby minimizing any confounding effects attributable to sex. To minimize the potential influence of circadian rhythms on spexin gene expression, the sampling time for all experimental groups (including the various fasting groups and the control group) was strictly restricted to between 9:00 and 10:00 a.m. each day. Experimental groups underwent starvation conditioning with sampling at 3, 5, and 7 days post-starvation, with five fish randomly collected from the net cage at each time point. A total of five mandarin fish (S. chuatsi) maintained under routine feeding conditions were selected from the pond. After a 30 min feed withdrawal, surplus feed was removed upon cessation of feeding activity to establish a fasting condition for the net-caged cohort. Prior to sampling, all mandarin fish in net cages were confirmed to have ventral concavity, and the absence of intestinal contents was verified to strictly ensure that they were in a fasting state. The caged individuals underwent a 3 d acclimatization period prior to the initiation of complete feed deprivation to prevent stress-induced mortality, whereas the pond population continued daily feeding. No mortality was observed during the culture period.
To examine phenotypic variation in feed adaptation, ten feed-trained overwintering S. chuatsi individuals were collected from a commercial aquaculture facility in Longjiang, Shunde (Guangzhou, China). Sexually mature fish were classified into two groups based on body size: a large-body phenotype group (798.0 ± 23.10 g; n = 5; 14-month-old) and a small-body phenotype group (336.67 ± 14.70 g; n = 5; 14-month-old). Each phenotype cohort (large-body and small-body) included representative males and females demonstrating differential adaptation to feeding regimes.
All experiments were carried out in accordance with the “Guidelines for the Protection and Use of Laboratory Animals in China”. All experimental procedures and sample collection were approved by the Animal Experimentation Ethics Committee of the Pearl River Fisheries Research, Chinese Academy of Fishery Sciences.
2.2. Cloning of the Scspx ORF Sequence
The ORF of the spx gene was identified from the S. chuatsi genome. Two specific primer pairs (spx-F1/R1 and spx-F2/R2; Table 1) were designed using the PrimerQuest Tool (accessed on 25 September 2024) and synthesized by Sangon Biotech (Shanghai, China). Total RNA was extracted from liver tissue using TRIzol® Reagent (Ambion, Austin, TX, USA), and first-strand cDNA was synthesized using the ToloScript RT EasyMix for qPCR Kit (Novoprotein, Shanghai, China). Gene amplification was performed using a nested PCR approach. PCR products were separated on 1.5% agarose gels, and bands of the expected size were excised and purified using the Wizard® SV Gel and PCR Clean-Up System (Promega, Madison, WI, USA).

Table 1.
Primer sequence for Siniperca chuatsi spx gene cloning.
Purified fragments were ligated into the pBM23 vector via one-step TOPO-TA cloning at 25 °C for 4 h. Positive transformants were identified using colony PCR. Plasmids containing inserts of the expected size were confirmed using Sanger sequencing (IGE Biotechnology Ltd., Guangzhou, China).
2.3. SPX Protein Sequence Analysis in S. Chuatsi
The Scspx ORF sequence was translated into its corresponding amino acid sequence using DNAMAN software 8.0 version (Lynnon Biosoft). Homology searches were conducted via BLAST (-2.14.0) against the National Center for Biotechnology Information (NCBI) non-redundant protein database. Prior to analysis, homogeneity of variance analysis was performed, and outliers were removed to minimize experimental error. Multiple sequence alignments of SPX peptides from 12 teleost species were performed using ClustalW, with reference sequences obtained from GenBank.
Physicochemical properties, conserved domains, and predicted tertiary structures of SPX were analysed using ExPASy ProtParam (accessed on 5 October 2024), SMART(accessed on 5 October 2024), and SPOMA, respectively. A phylogenetic tree was constructed using the neighbour-joining method in MEGA 11. All quantitative data are presented as mean ± standard error. Statistical analyses were performed using SPSS 21.0 (SPSS, Armonk, NY, USA), and differences were considered significant at p < 0.05.
2.4. qRT-PCR Detection of spx Tissue Expression Profiles
The transcript abundance of the spx gene in various tissues of S. chuatsi was assessed using quantitative real-time PCR (qRT-PCR). β-Actin was used as the internal reference gene. Primers targeting spx (qspx-F/R) were designed and synthesized by Sangon Biotech (Shanghai, China; Table 1). Five healthy S. chuatsi individuals (238.2 ± 51.39 g; 8-month-old) were euthanised following approved ethical protocols. Approximately 20 mg of tissue from the liver, muscle, brain, stomach, intestine, and gill was aseptically collected using autoclaved instruments. Total RNA extraction and cDNA synthesis were performed as previously described. The qRT-PCR was conducted using the LightCycler® 96 System (Roche, Shanghai, China). Relative gene expression levels were quantified using the 2−ΔΔCt method.
2.5. spx Gene Expression During Fasting and Feed Adaptation
To investigate temporal spx expression during fasting and dietary adaptation, S. chuatsi individuals were euthanised from both net-cage (fasting 3, 5, and 7 d, n = 5 per time point) and pond-fed (control) groups. Tissue samples (~20 mg each) from the telencephalon, liver, intestine, skeletal muscle, and gastric epithelium were collected under aseptic conditions, immediately snap-frozen in liquid nitrogen, and stored at −80 °C. The qRT-PCR was used to analyse spx expression at 3, 5, and 7 d post-treatment. Total RNA was isolated, reverse transcribed, and subjected to qRT-PCR as described above. Data were statistically analysed using one-way analysis of variance in GraphPad Prism 10 version (GraphPad Software, San Diego, CA, USA), followed by Fisher’s least significant difference test for multiple comparisons. An independent-sample t-test was used to compare the differences in spx expression between the large- and small-body size groups of S. chuatsi, differences were considered significant at p < 0.05. The relative expression of spx in liver and brain tissues was compared between large-body and small-body phenotype groups to assess differential expression associated with feed adaptation.
2.6. GST Pull-Down Assay
The ORF of spx was amplified from liver-derived cDNA of feed-trained S. chuatsi and cloned into the pGEX-6P-1 vector (digested with BamHI and XhoI). The recombinant plasmid (pGEX-6P-SPX) was transformed into Escherichia coli BL21 (DE3) cells. Positive clones were induced using isopropyl β-D-1-thiogalactopyranoside (IPTG)and cultured for 8 h. Bacterial pellets were resuspended in STET buffer containing lysozyme, lysed by sonication, and centrifuged. The resulting supernatant was purified using a glutathione S-transferase (GST) affinity column. Protein purity was verified using sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE). Total protein was extracted from the liver of feed-ingesting S. chuatsi and examined using Western blot assays with a 12% SDS-PAGE gel. Proteins were transferred onto polyvinylidene difluoride membranes, blocked overnight at 4 °C, and sequentially incubated with sheep anti-GST (1:10,000), followed by anti-rabbit IgG secondary antibody. Signals were visualised using the Clarity Max™ Western ECL substrate (Bio-Rad, Hercules, CA, USA).
A total of 500 μg of GST–SPX fusion protein (experimental group) or GST alone (control group) was incubated with magnetic beads for 2 h at room temperature. Liver total protein extract was added to each group and adjusted to 1 mL with immunoprecipitation (IP) dilution buffer, followed by incubation at 4 °C for 1 min. Subsequently, 100 μL of elution buffer was added to each group and incubated in a 95 °C water bath for 5 min. After centrifugation at 12,000× g for 5 min, 20 μL of 6× loading buffer was added to the supernatants. For the input group, 100 μL of liver total protein was mixed with 20 μL of 6× loading buffer and denatured in the same way. Western blotting was used to assess protein profiles in the marker, control, experimental, and input groups. Following SDS-PAGE and silver staining, protein bands were excised and subjected to in-gel tryptic digestion. Proteins were reduced, alkylated, digested overnight with trypsin, and desalted using an SDB column. Peptides were analysed using an UltiMate 3000 RSLCnano system coupled with a Q Exactive HF mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Raw MS data were searched against the UniProt database using MaxQuant (v2.2.0.0). Differentially interacting proteins were identified and annotated using Gene Ontology (GO) analysis.
3. Results
3.1. Sequence Analysis of spx in S. chuatsi
Specific primers were designed based on the S. chuatsi genome to amplify a 312 bp ORF encoding a 103-amino acid protein with an ATG start codon and a TGA stop codon. SignalP 4.1 analysis predicted the absence of a signal peptide in the SPX protein; however, a 14-amino acid mature peptide (NWTPQAMLYLKGTQ) was identified. Physicochemical characterisation using the ExPASy ProtParam tool indicated that the SPX protein had a molecular formula of C536H817N151O162S2, a molecular weight of 12.03 kDa, and a theoretical isoelectric point (pI) of 5.81.
Phylogenetic analysis was conducted using MEGA 11.0, incorporating SPX amino acid sequences from S. chuatsi and 11 other teleost species obtained from the NCBI database. The resulting neighbour-joining phylogenetic tree resolved two major clades. S. chuatsi clustered most closely with Oncorhynchus mykiss and Cynoglossus semilaevis, and more distantly from D. rerio and Acipenser baerii (Figure 1).
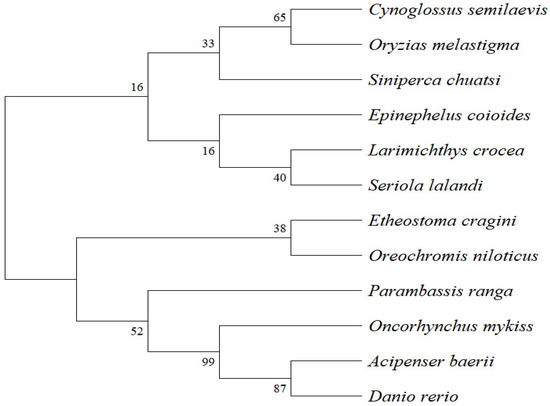
Figure 1.
Phylogenetic tree of SPX protein sequences constructed using MEGA 11.0 (neighbour-joining method).
3.2. Tissue Distribution of Scspx
To investigate the tissue-specific expression of Scspx, qRT-PCR was conducted on six tissues from S. chuatsi reared on formulated feeds. Normalised expression data indicated ubiquitous spx expression, with the highest levels detected in liver tissue and the lowest in muscle. Hepatic spx expression was 17.36-fold higher than that in muscle. However, spx mRNA levels in the liver and other tissues, excluding muscle, were similar (p > 0.05) (Figure 2).
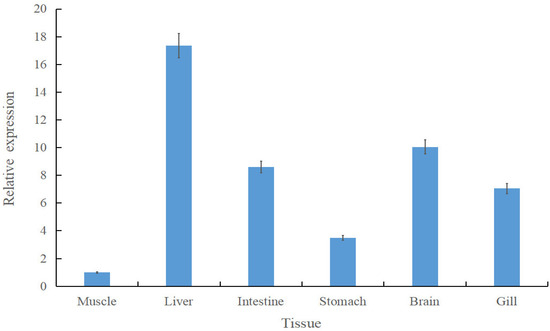
Figure 2.
Tissue-specific expression of Scspx in Siniperca chuatsi. Data are presented as mean ± standard deviation.
3.3. Regulation of spx Expression During Fasting and Feed Adaptation
To investigate spx transcriptional dynamics during fasting and in individuals with divergent growth phenotypes, qRT-PCR was used to quantify spx expression in liver, brain, and stomach tissues. During fasting (0, 3, 5, and 7 d), the liver exhibited the highest spx expression, whereas the stomach had the lowest. A conserved temporal expression pattern was observed across tissues, characterised by an initial increase followed by a decline. In the liver, spx expression peaked at day 3 and was lowest on day 5. Intestinal and gastric spx levels on day 7 exceeded those on day 3 (Figure 3). In the feed adaptation studies, individuals with the largest body size had substantially lower spx expression in the liver and brain compared to the smallest group, whereas expression in the stomach was elevated (p < 0.05) (Figure 4).
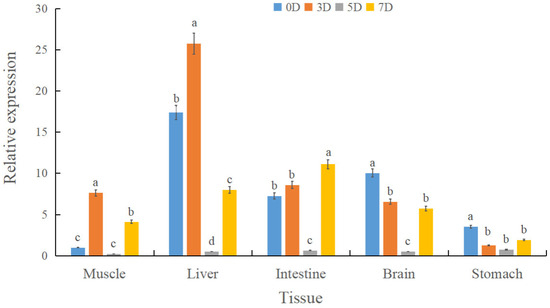
Figure 3.
Relative spx expression in liver, brain, stomach, and intestine of Siniperca chuatsi under graded starvation (0, 3, 5, and 7 d). Different letters (a, b, c, d) above the bars indicate statistically significant differences among groups as determined by a one-way analysis of variance (ANOVA). The assignment of letters was performed such that groups sharing a common letter are not significantly different from one another at the p > 0.05 level. Conversely, groups labeled with different letters exhibit a statistically significant difference (p < 0.05). The comparisons were conducted across all groups simultaneously.
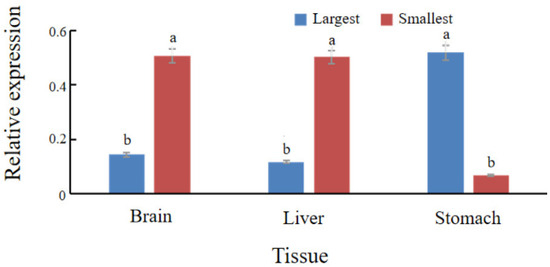
Figure 4.
Comparative spx expression in liver, brain, and stomach tissues between the largest and smallest size groups of S. chuatsi reared on formulated feeds. Mean values assigned different letters (a, b) differ significantly (p < 0.05). The absence of a common letter indicates a statistically significant difference in the pairwise comparison.
3.4. Identification of Expression Vectors and Purification of Fusion Proteins
To identify SPX-interacting proteins in S. chuatsi, GST pull-down assays were conducted using Mag-Beads conjugated with GST-tagged fusion proteins. Liver tissue lysates from individuals fed a standard formulated diet served as the protein source. The ORF of Scspx (324 bp) was inserted into the pGEX-6P-1 vector (4984 bp), yielding the recombinant plasmid, pGEX-6P-SPX (5308 bp). DNA sequencing confirmed the correct insertion of the Scspx sequence, verifying the successful construction of the expression vector.
Following transformation into Escherichia coli BL21, expression of the GST–SPX fusion protein was induced by isopropyl β-D-1-thiogalactopyranoside (IPTG). SDS-PAGE analysis indicated a distinct band at ~38 kDa, corresponding to the expected size of the GST–SPX fusion protein (Figure 5). The fusion protein was purified using GST affinity chromatography. Elution fractions exhibited markedly improved purity on SDS-PAGE, confirming successful enrichment of the recombinant GST-SPX protein.
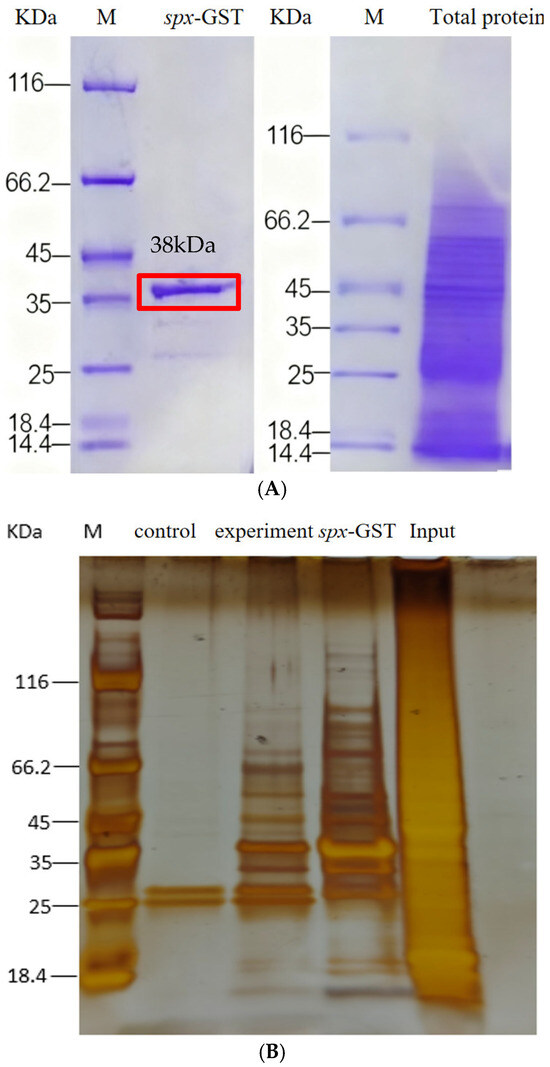
Figure 5.
Isolation and identification of ScSPX-interacting proteins. (A) SDS-PAGE analysis of the expression of recombinant ScSPX in E. coli. (B) The fusion proteins GST or GST-ScSPX were used as bait, with the total protein extract from S. chuatsi serving as the input. The results are shown from a silver-stained SDS-PAGE gel.
3.5. GST Pull-Down Identification and Characterisation of SPX-Interacting Proteins
Total liver proteins were extracted from S. chuatsi specimens adapted to artificial feed. Western blot analysis confirmed uniform protein distribution and suitability for subsequent pull-down assays. The GST–SPX fusion protein and a control GST protein were incubated with Mag-Beads, alongside liver protein extracts from the same dietary group. The experimental group consisted of GST–SPX + Mag-Beads + total protein; the control group consisted of GST + Mag-Beads + total protein. Following incubation and washing, bound proteins were eluted and analysed using SDS-PAGE, silver staining, and liquid chromatography–tandem mass spectrometry (LC–MS/MS). Differential bands, those not attributable to nonspecific GST-tag interactions, were further verified using Western blot with anti-GST antibodies. Under stringent identification criteria (≥2 unique peptides per protein), a total of 63 high-confidence SPX-interacting proteins were detected in the experimental group, comprising 17 annotated proteins and 46 uncharacterised proteins. By comparison, 74 proteins were identified in the control group, with 58 proteins common to both. Detailed information on SPX-specific interactors is summarised in Table 2.

Table 2.
Information on candidate proteins that interact with SPX proteins.
To functionally annotate the putative SPX-binding proteins, GO enrichment analysis was performed across three primary categories: molecular function (MF), cellular component (CC), and biological process (BP). A total of 21 significantly enriched Level 2 GO terms were identified. Interacting proteins were predominantly associated with ribosomal structure and binding activities, including RNA and adenyl nucleotide binding. These proteins were localised in the cytoplasm, cytosol, and ribonucleoprotein complexes, and were implicated in critical processes, such as translation, protein folding, and cellular homeostasis (Figure 6).
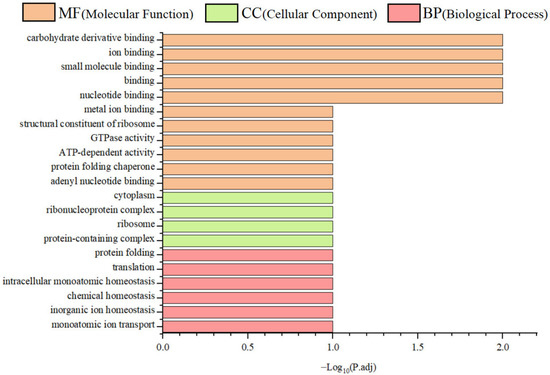
Figure 6.
Gene ontology (GO) enrichment analysis of SPX-interacting proteins.
4. Discussion
The SPX prepropeptide conforms to the structural characteristics of a canonical secretory protein, comprising a conserved 14-amino-acid mature peptide with C-terminal amidation and several non-conserved signal peptides. A paralogous gene, designated spx2, has been identified in non-mammalian vertebrates [16], whereas the original spx was subsequently renamed spx1. To date, spx2 has not been identified in mammalian genomes. Zhao et al. (2022) cloned the full-length cDNA of spx2 in D. rerio, indicating a 288 bp ORF encoding a 95-amino-acid precursor protein [20]. The mature SPX2 peptide, characterised in Oryzias latipes, Latimeria chalumnae, and D. rerio, shares a conserved sequence: NWGPQSMLYLKGRYGR [16]. Investigation of the spx gene in S. chuatsi led to the cloning of its ORF. Comparative analysis determined that the deduced protein sequence is highly conserved across teleost fish, with maximum sequence identity observed in O. niloticus. Furthermore, based on conserved domain analysis, the gene was definitively assigned to the spx1 subtype.
The spx1 is broadly expressed in the central nervous system, peripheral tissues, and reproductive organs across species, such as humans, rodents, and goldfish, and is found in the cerebral cortex, liver, and ovary, inter alia [9]. This widespread and evolutionarily conserved expression pattern suggests multifunctional regulatory roles for spx1. Expression levels of the spx gene vary markedly across species and tissues. In A. baerii, spx mRNA is predominantly detected in the hypothalamus, intestine, and liver [10], whereas in E. coioides, high expression is observed in the brain, liver, and ovary, with minimal expression in the intestine [14]. In D. rerio, in situ hybridisation indicated that spx was expressed in the mesencephalon and metencephalon, whereas spx2 was localised to the preoptic hypothalamus [21], indicating that the two paralogs may serve distinct physiological functions. In S. chuatsi, qRT-PCR analysis showed abundant spx expression in the liver, intestine, and telencephalon under both basal and fasting conditions. This tissue distribution suggests a role in regulating digestion, metabolism, and neural activity, aligning with observations in A. baerii, E. coioides, and rodents [10,11,14]. Interspecific variability in tissue-specific expression is evident [10]. For instance, in Scatophagus argus, spx is most highly expressed in the ovary, with minimal expression in the liver and brain [4]. Similarly, Kim et al. (2019) localised spx expression in D. rerio to the mesencephalon and metencephalon [21]. The observed variations in spx expression across species and tissues may be attributed to ecological adaptations associated with energy metabolism. As typical carnivorous fish, S. chuatsi and A. baerii exhibit metabolic traits adapted to high-protein and high-lipid diets, resulting in elevated energy metabolic demands on organs such as the liver and intestine. The high expression of spx in these tissues aligns with its putative roles in appetite suppression and lipid metabolism regulation, suggesting an evolutionary adaptation to a carnivorous ecological niche. In contrast, the omnivorous S. argus has a broader dietary spectrum, which may have driven the evolution of distinct energy utilization strategies. Such ecological divergence likely underlies the differential spx expression profiles: in S. argus, spx expression is diminished in conventional metabolic tissues but enhanced in regions associated with other physiological processes. These divergent expression profiles suggest species-specific adaptation of spx function to distinct physiological roles.
In mammals, spx has been implicated in regulating intestinal motility, glucose and lipid metabolism, and hepatic function [22]. Functional studies have shown that SPX modulates glycemia by promoting insulin release, influences gastric contractions, and regulates adrenal cortex cell proliferation [2,9,18,23]. However, several SPX functions characterised in fish, such as suppression of luteinizing hormone (LH), appetite inhibition, and energy balance regulation, are not conserved in mammals. Fasting experiments in E. coioides, S. argus, and Cynoglossus semilaevis have consistently shown substantial upregulation of hypothalamic spx expression in response to nutrient deprivation [4,12,24].
The present study demonstrated that spx expression in S. chuatsi liver was substantially elevated during the early phase of fasting. We propose that this upregulation contributes to appetite suppression and reduced energy intake, thereby promoting metabolic homeostasis under starvation conditions. Supporting this, spx1 knockout models in zebrafish have shown that SPX mediates appetite inhibition via suppression of AgRP1 expression [24]. Moreover, SPX–insulin interactions within islet cells establish a paracrine feedback loop modulating insulin secretion [9]. The findings of the current study suggest that SPX plays a conserved role in maintaining energy balance during fasting. In S. chuatsi, the observed downregulation of neural spx expression during prolonged starvation (5 d) may reflect an adaptive shift in response to sustained energy deficiency.
Observations during the domestication of S. chuatsi to formulated feed indicated marked phenotypic variation among individuals, despite overall successful domestication. Although some individuals exhibited robust growth performance and good feed adaptation, others showed signs of growth retardation and poor adaptation to the feed. Although multiple studies have established that the spx gene regulates feeding behaviour and satiety in teleosts [2,10,12], its role in the dietary adaptation of S. chuatsi remains incompletely understood. The present study provides the first experimental evidence supporting spx involvement in the dietary adaptation of S. chuatsi. Comparative expression analysis indicated substantially lower spx levels in the liver and brain of large-size individuals relative to small-size counterparts, whereas spx expression in the stomach was markedly upregulated in the larger group.
Using C. auratus as a model, Wong et al. (2013) demonstrated that spx suppressed food intake via insulin-mediated signalling in hepatic and cerebral tissues [12]. In vitro experiments indicated a dual regulatory mechanism of insulin on spx expression: insulin acts locally as an autocrine/paracrine signal to stimulate spx expression, and systemically as an endocrine factor to induce cerebral spx transcription [12]. Similarly, in E. coioides, peripheral administration of SPX-14 substantially upregulated pro-opiomelanocortin (pomc) expression while downregulating growth hormone (gh) and orexin mRNA levels in pituitary cells [14]. In D. rerio, spx1 knockout models exhibited markedly increased food intake compared with wild-type controls. Intracranial injection of SPX1 suppressed agouti-related peptide 1 (AgRP1) expression, implicating SPX1 as a satiety signal that exerts appetite-suppressive effects via downregulation of AgRP1 [5]. In mammals, Mirabeau et al. (2007) localised spx expression to the oesophageal and gastric submucosa, where the propeptide was shown to induce gastric smooth muscle contraction [25]. In contrast, injection of SPX in A. baerii paradoxically led to a reduction in endogenous spx expression, while simultaneously increasing mRNA levels of nucleobindin 2 (nucb2) and peptide YY (PYY), consistent with an anorexigenic regulatory role of SPX in sturgeon [9]. Collectively, these findings suggest that downregulation of spx in the liver and brain of large-sized S. chuatsi may facilitate increased feed intake by modulating hormonal profiles and repressing anorexigenic gene expression. Conversely, elevated spx expression in the stomach may enhance digestive efficiency by promoting smooth muscle contractility, thereby indirectly contributing to growth performance.
Fatty acid-binding proteins (FABPs) represent a conserved multigene family of intracellular lipid-binding proteins (iLBPs), derived via duplication and diversification of an ancestral ilbp gene [26]. To date, 12 distinct FABP isoforms have been identified, which reversibly bind hydrophobic ligands and facilitate their transport between cellular compartments to support diverse physiological processes [27,28]. Kaitetzidou et al. established a fasting-refeeding experimental model in gilthead sea bream and European sea bass, systematically revealing a dynamic regulatory relationship between FABP2 gene expression and nutritional status in fish [29]. The study demonstrated that fasting significantly down-regulated FABP2 mRNA expression in the intestinal tissues of both species, while refeeding promptly restored expression to baseline levels. This dynamic expression pattern indicates that FABP2 is directly regulated by the nutritional state of the organism. Notably, this regulatory mechanism appears highly conserved across teleost species. Similarly, Xia et al. observed in Asian sea bass that short-term fasting (3d, 6d, 12d) markedly suppressed the expression of both fabp2a and fabp2b genes in the intestine [30]. Furthermore, studies in zebrafish confirmed that increasing dietary lipid content significantly up-regulated intestinal fabp2 transcript levels. The results suggest that the regulation of fabp2 gene transcription by fatty acids is mediated by the interaction of the peroxisome proliferator-activated receptor (PPAR) with a peroxisome proliferator response element (PPRE) in its promoter region [31]. These cross-species findings collectively suggest that FABP2 plays a critical role in nutrient metabolism in teleosts, likely participating in the regulation of lipid metabolic pathways through similar molecular mechanisms across different fish species, thereby supporting the view that this protein serves a core function in lipid absorption and transport. Based on the observed upregulation of spx expression in the intestine of S. chuatsi subjected to short-term starvation after feeding, this study proposes a regulatory hypothesis wherein spx may indirectly facilitate fabp2 gene transcription via the upregulation of PPAR-α expression. This signalling cascade potentially represents a key mechanistic axis through which spx exerts its pivotal role in modulating lipid metabolism in fish.
In the current study, GST pull-down assays confirmed a specific interaction between SPX and FABP2. Further analysis indicated that FABP2 was enriched within the peroxisome proliferator-activated receptor (PPAR) signalling pathway, which governs lipid oxidation, adipocyte differentiation, hepatic lipid metabolism, cellular proliferation, and glucose uptake [32]. Zheng et al. demonstrated that SPX positively upregulated hepatic AdipoQ expression via activation of the PLC/PKC-Ca2+/CaMKII-MEK/ERK signalling pathway, thereby enhancing postprandial satiety. Conversely, adiponectin (AdipoQ) negatively suppressed SPX expression through the AMPK/PPAR-PI3K/Akt-p38 MAPK pathway, establishing a self-terminating, localized feedback loop within the liver [33]. As the most abundant circulating adipokine, adiponectin (AdipoQ) critically governs lipid/glucose metabolism and may directly influence feeding regulation [34]. In Siberian sturgeon (Acipenser baerii), intraperitoneal administration of recombinant globular adiponectin protein (SsgAd) acutely inhibited feeding. This effect was mediated by upregulation of the valvular intestinal anorexigenic peptide, PYY; modulation of hypothalamic appetite-regulating genes (POMC, NPY, AGRP, and CART); and activation of the hypothalamic AMPK/mTOR signalling pathway. Specifically, SsgAd administration promoted the expression of AdipoR1, AMPKα2, Akt, and mTOR, while suppressing the expression of AMPKβ1, AMPKβ2, and AMPKγ2 [35]. The PPAR signalling cascade is regulated downstream of the AMP-activated protein kinase (AMPK) pathway. Phosphorylated AMPK stimulates PPARα, which upregulates genes involved in fatty acid transport and mitochondrial β-oxidation, whereas PPAR-γ activation enhances insulin sensitivity and facilitates glucose uptake [36]. Acting as a cellular energy sensor, AMPK responds to increased AMP/ATP ratios by activating catabolic pathways, such as fatty acid oxidation and autophagy, and suppressing anabolic processes, such as fatty acid and cholesterol biosynthesis [37].
Thus, we hypothesized that the bidirectional regulation between SPX and AdipoQ activated AMPK, triggering a cascade of AMPK-signalling-regulated reactions that further modulated FABP2 in the PPAR signalling pathway. This SPX–AMPK–PPAR–FABP2 axis may represent a key regulatory route through which SPX influences lipid metabolism and energy homeostasis in S. chuatsi. While the current study provides compelling evidence for the association between Scspx expression and growth characteristics in S. chuatsi, it is important to acknowledge its limitations. The functional inferences regarding Scspx are primarily derived from correlative observations in expression profiling. To advance from correlation to causation, direct functional validation is essential. Future investigations utilizing gene knockout/knockdown or overexpression approaches, both in vitro and in vivo, will be crucial to unequivocally establish the physiological role of Scspx in regulating lipid metabolism and its interaction with FABP2. Such studies will ultimately determine whether the observed expression changes represent a cause or a consequence of the metabolic alterations.
5. Conclusions
Previous genome-wide association analyses identified the spx gene as being closely associated with feed adaptation in S. chuatsi. In the current study, the ORF of the spx gene was successfully cloned and characterised in feed-trained S. chuatsi. Transcriptional profiling indicated that spx expression was modulated by fasting and was associated with the degree of feed adaptation. Furthermore, in vitro GST pull-down assays demonstrated a direct interaction between SPX and FABP2 proteins. These findings provide novel insights into the functional role of ScSPX in the PPAR signalling pathway and its potential involvement in the feed domestication process of S. chuatsi.
Author Contributions
Conceptualization, X.C. and Y.Y.; Methodology, X.C.; Software, X.C.; Validation, X.C. and Y.Y.; Formal analysis, X.C. and Y.Y.; Investigation, J.D. and H.Z.; Resources, Y.Z.; Data curation, X.C.; Writing—original draft, X.C.; Writing—review & editing, C.S.; Visualization, X.C.; Supervision, C.S.; Project administration, F.G.; Funding acquisition, C.S. and X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by Guangdong Provincial Natural Science Foundation General Project (2023A1515011543), Special project of Guangdong Province’s scientific and technological achievements (2025b02010004), the National Key R&D Program of China (2024YFD2401500, 2023YFD2402900), the Central Public-interest Scientific Institution Basal Research Fund, CAFS (No. 2023TD95), the China Agriculture Research System of MOF and MARA (CARS-46), Guangdong Province Modern Agricultural Industry Technology System (Freshwater Fish System) Innovation Team Construction Project (2024CXTD26), the National Natural Science Foundation of China (No. 32002385, 32303030), and the Guangdong Rural Science and Technology Special Envoy Project (KTP20240752).
Institutional Review Board Statement
All experimental procedures were conducted in accordance with the Chinese Guidelines for the Protection and Use of Experimental Animals and were approved by the Animal Experiment Ethics Committee of the Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences (approval number: LAEC-PRFRI-2023-03-02).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Conflicts of Interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AgRP1 | agouti-related peptide 1 |
| AMPK | AMP-activated protein kinase |
| ARC | arcuate nucleus |
| BP | biological process |
| CC | cellular component |
| DMH | dorsomedial hypothalamus |
| FABP2 | fatty acid-binding protein 2 |
| GAL | Galanin |
| gh | growth hormone |
| GO | Gene Ontology |
| HSL | hormone-sensitive lipase |
| KISS | Kisspeptin |
| LH | luteinizing hormone |
| MF | molecular function |
| nucb2 | nucleobindin 2 gene |
| PKA | protein kinase A |
| PI3K | phosphoinositide 3-kinase |
| PPAR | peroxisome proliferator-activated receptor |
| PYY | peptide YY |
| Scspx | Siniperca chuatsi spx gene |
| SPX | Spexin |
| VDCC | voltage-dependent calcium channel |
| VMH | ventromedial hypothalamus |
References
- Sun, C.F.; Sun, H.L.; Dong, J.J.; Tian, Y.Y.; Hu, J.; Ye, X. Correlation analysis of mandarin fish (Siniperca chuatsi) growth hormone gene polymorphisms and growth traits. J. Genet. 2019, 98, 58. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lin, F.; Chen, H.; Liu, J.; Gao, Y.; Zhang, X.; Hao, J.; Chen, D.; Yuan, D.; Wang, T. Ya-fish (Schizothorax prenanti) spexin: Identification, tissue distribution and mRNA expression responses to periprandial and fasting. Fish Physiol. Biochem. 2016, 42, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, B.; Chen, S. Spexin in the half-smooth tongue sole (Cynoglossus semilaevis): Molecular cloning, expression profiles, and physiological effects. Fish Physiol. Biochem. 2018, 44, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.P.; Chen, H.P.; Zhai, Y.; Jia, L.Y.; Liu, J.Y.; Wang, M.; Jiang, D.N.; Wu, T.L.; Zhu, C.H.; Li, G.L. Molecular cloning, characterization and expression analysis of spexin in spotted scat (Scatophagus argus). Gen. Comp. Endocrinol. 2018, 266, 60–66. [Google Scholar] [CrossRef]
- Zheng, B.; Li, S.; Liu, Y.; Li, Y.; Chen, H.; Tang, H.; Liu, X.; Lin, H.; Zhang, Y.; Cheng, C.H.K. Spexin suppress food Intake in zebrafish: Evidence from gene knockout study. Sci. Rep. 2017, 7, 14643. [Google Scholar] [CrossRef]
- Kumar, S.; Mankowski, R.T.; Anton, S.D.; Babu Balagopal, P. Novel insights on the role of spexin as a biomarker of obesity and related cardiometabolic disease. Int. J. Obes. 2021, 45, 2169–2178. [Google Scholar] [CrossRef]
- Kolodziejski, P.A.; Pruszynska-Oszmalek, E.; Micker, M.; Skrzypski, M.; Wojciechowicz, T.; Szwarckopf, P.; Skieresz-Szewczyk, K.; Nowak, K.W.; Strowski, M.Z. Spexin: A novel regulator of adipogenesis and fat tissue metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1228–1236. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Qi, X.; Zhou, W.; Liu, X.; Lin, H.; Zhang, Y.; Cheng, C.H. A novel neuropeptide in suppressing luteinizing hormone release in goldfish, Carassius auratus. Mol. Cell. Endocrinol. 2013, 374, 65–72. [Google Scholar] [CrossRef]
- Mills, E.G.; Izzi-Engbeaya, C.; Abbara, A.; Comninos, A.N.; Dhillo, W.S. Functions of galanin, spexin and kisspeptin in metabolism, mood and behaviour. Nat. Rev. Endocrinol. 2021, 17, 97–113. [Google Scholar] [CrossRef]
- Tian, Z.; Xu, S.; Wang, M.; Li, Y.; Chen, H.; Tang, N.; Wang, B.; Zhang, X.; Li, Z. Identification, tissue distribution, periprandial expression, and anorexigenic effect of spexin in Siberian sturgeon, Acipenser baeri. Fish Physiol. Biochem. 2020, 46, 2073–2084. [Google Scholar] [CrossRef]
- Porzionato, A.; Rucinski, M.; Macchi, V.; Stecco, C.; Malendowicz, L.K.; De Caro, R. Spexin expression in normal rat tissues. J. Histochem. Cytochem. 2010, 58, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.K.; Sze, K.H.; Chen, T.; Cho, C.K.; Law, H.C.; Chu, I.K.; Wong, A.O. Goldfish spexin: Solution structure and novel function as a satiety factor in feeding control. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E348–E366. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhang, X.; Zhao, X.; Jing, W.; Cao, Z.; Li, J.; Huang, Y.; You, X.; Wang, M.; Shi, Q. A Chromosome-level genome assembly of the mandarin fish (Siniperca chuatsi). Front. Genet. 2021, 12, 671650. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Q.; Xiao, L.; Chen, H.; Li, G.; Zhang, Y.; Lin, H. Molecular cloning and functional characterization of spexin in orange-spotted grouper (Epinephelus coioides). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 196–197, 85–91. [Google Scholar] [CrossRef]
- Lim, C.H.; Lee, M.Y.M.; Soga, T.; Parhar, I. Evolution of structural and functional diversity of spexin in mammalian and non-mammalian vertebrate species. Front. Endocrinol. 2019, 10, 379. [Google Scholar] [CrossRef]
- Kim, D.K.; Yun, S.; Son, G.H.; Hwang, J.I.; Park, C.R.; Kim, J.I.; Kim, K.; Vaudry, H.; Seong, J.Y. Coevolution of the spexin/galanin/kisspeptin family: Spexin activates galanin receptor type II and III. Endocrinology. 2014, 155, 1864–1873. [Google Scholar] [CrossRef]
- Fang, P.; Yu, M.; Shi, M.; Bo, P.; Zhang, Z. Galanin peptide family regulation of glucose metabolism. Front. Neuroendocrinol. 2020, 56, 100801. [Google Scholar] [CrossRef]
- Lin, C.Y.; Zhang, M.; Huang, T.; Yang, L.L.; Fu, H.B.; Zhao, L.; Zhong, L.L.; Mu, H.X.; Shi, X.K.; Leung, C.F. Spexin enhances bowel movement through activating L-type voltage-dependent calcium channel via Galanin receptor 2 in Mice. Sci. Rep. 2015, 5, 12095. [Google Scholar] [CrossRef]
- Fang, P.; She, Y.; Yu, M.; Yan, J.; Yu, X.; Zhao, J.; Jin, Y.; Min, W.; Shang, W.; Zhang, Z. Novel hypothalamic pathways for metabolic effects of spexin. Pharmacol. Res. 2024, 208, 107399. [Google Scholar] [CrossRef]
- Zhao, T.; Ye, Z.; Liu, Y.; Lin, H.; Li, S.; Zhang, Y. Mutation of spexin2 promotes feeding, somatic growth, adiposity, and insulin resistance in zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 322, R454–R465. [Google Scholar] [CrossRef]
- Kim, E.; Jeong, I.; Chung, A.Y.; Kim, S.; Kwon, S.H.; Seong, J.Y.; Park, H.C. Distribution and neuronal circuit of spexin 1/2 neurons in the zebrafish CNS. Sci. Rep. 2019, 9, 5025. [Google Scholar] [CrossRef]
- Wong, M.K.H.; Chen, Y.; He, M.; Lin, C.; Bian, Z.; Wong, A.O.L. Mouse Spexin: (II) Functional role as a satiety factor inhibiting food intake by regulatory actions within the hypothalamus. Front. Endocrinol. 2021, 12, 681647. [Google Scholar] [CrossRef]
- Rucinski, M.; Porzionato, A.; Ziolkowska, A.; Szyszka, M.; Macchi, V.; De Caro, R.; Malendowicz, L.K. Expression of the spexin gene in the rat adrenal gland and evidences suggesting that spexin inhibits adrenocortical cell proliferation. Peptides 2010, 31, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zahir, I.; Ogawa, S.; Dominic, N.A.; Soga, T.; Parhar, I.S. Spexin and galanin in metabolic functions and social behaviors with a focus on non-mammalian vertebrates. Front. Endocrinol. 2022, 13, 882772. [Google Scholar] [CrossRef]
- Mirabeau, O.; Perlas, E.; Severini, C.; Audero, E.; Gascuel, O.; Possenti, R.; Birney, E.; Rosenthal, N.; Gross, C. Identification of novel peptide hormones in the human proteome by hidden Markov model screening. Genome Res. 2007, 17, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Schaap, F.G.; van der Vusse, G.J.; Glatz, J.F. Evolution of the family of intracellular lipid binding proteins in vertebrates. Mol. Cell. Biochem. 2002, 239, 69–77. [Google Scholar] [CrossRef] [PubMed]
- McKillop, I.H.; Girardi, C.A.; Thompson, K.J. Role of fatty acid binding proteins (FABPs) in cancer development and progression. Cell. Signal. 2019, 62, 109336. [Google Scholar] [CrossRef]
- Smathers, R.L.; Petersen, D.R. The human fatty acid-binding protein family: Evolutionary divergences and functions. Hum. Genom. 2011, 5, 170–191. [Google Scholar] [CrossRef]
- Kaitetzidou, E.; Chatzifotis, S.; Antonopoulou, E.; Sarropoulou, E. Identification, phylogeny, and function of fabp2 paralogs in two non-model teleost fish species. Mar. Biotechnol. 2015, 17, 663–677. [Google Scholar] [CrossRef]
- Xia, J.H.; Lin, G.; He, X.; Liu, P.; Liu, F.; Sun, F.; Tu, R.; Yue, G.H. Whole genome scanning and association mapping identified a significant association between growth and a SNP in the IFABP-a gene of the Asian seabass. BMC Genom. 2013, 14, 295. [Google Scholar] [CrossRef]
- Venkatachalam, A.B.; Sawler, D.L.; Wright, J.M. Tissue-specific transcriptional modulation of fatty acid-binding protein genes, fabp2, fabp3 and fabp6, by fatty acids and the peroxisome proliferator, clofibrate, in zebrafish (Danio rerio). Gene 2013, 520, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2017, 13, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Bai, J.; He, M. Functional interaction of Spexin and Adiponectin forming a local feedbackin goldfish hepatocytes for feeding regulation. J. Endocr. Soc. 2021, 5 (Suppl. S1), A47. [Google Scholar] [CrossRef]
- Zheng, Y.; Ye, C.; He, M.; Ko, W.K.W.; Chan, Y.W.; Wong, A.O.L. Goldfish adiponectin: (I) molecular cloning, tissue distribution, recombinant protein expression, and novel function as a satiety factor in fish model. Front. Endocrinol. 2023, 14, 1283298. [Google Scholar] [CrossRef]
- Tang, N.; Li, Y.Z.; Wu, Y.R.; Wu, H.W.; Kang, Q.; Yao, Q.; Chen, S.H.; Liu, Y.L.; Jiang, K.Z.; Xiong, Y.X. Globular adiponectin, acting via AdipoR1, regulates food intake of Siberian sturgeon (Acipenser baerii) in a mTOR dependent manner. Aquaculture 2025, 595, 741594. [Google Scholar] [CrossRef]
- Ruderman, N.; Prentki, M. AMP kinase and malonyl-CoA: Targets for therapy of the metabolic syndrome. Nat. Rev. Drug Discov. 2004, 3, 340–351. [Google Scholar] [CrossRef]
- Ke, R.; Xu, Q.; Li, C.; Luo, L.; Huang, D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol. Int. 2018, 42, 384–392. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).