Effects of Olive Leaf (Olea europaea) Phenolic Extract on Zootechnical Parameters, Centesimal Composition, and Biochemical Parameters of Nile Tilapia (Oreochromis niloticus) Juveniles
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Extraction of Olive Leaf Extract (OLE)
2.3. Experimental Diets
2.4. Experimental Trial
Water Quality
2.5. Data Collection
2.6. Zootechnical Performance
2.6.1. Growth Parameters
2.6.2. Zootechnical Indices
2.7. Centesimal Composition and Nutrient Deposition
2.8. Biochemical Analyses
2.8.1. Tissue Homogenization
2.8.2. Muscle and Liver Glycogen
2.8.3. Plasma and Liver Biochemical Parameters
2.8.4. Lipid Peroxidation
2.9. Statistical Analysis
3. Results
3.1. Olive Leaf Extract (OLE)
3.2. Water Quality
3.3. Zootechnical Performance
3.3.1. Growth Parameters
3.3.2. Zootechnical Indices
3.4. Centesimal Composition and Nutrient Deposition
3.5. Biochemical Analyses
3.5.1. Muscle and Liver Glycogen
3.5.2. Plasma and Liver Biochemical Parameters
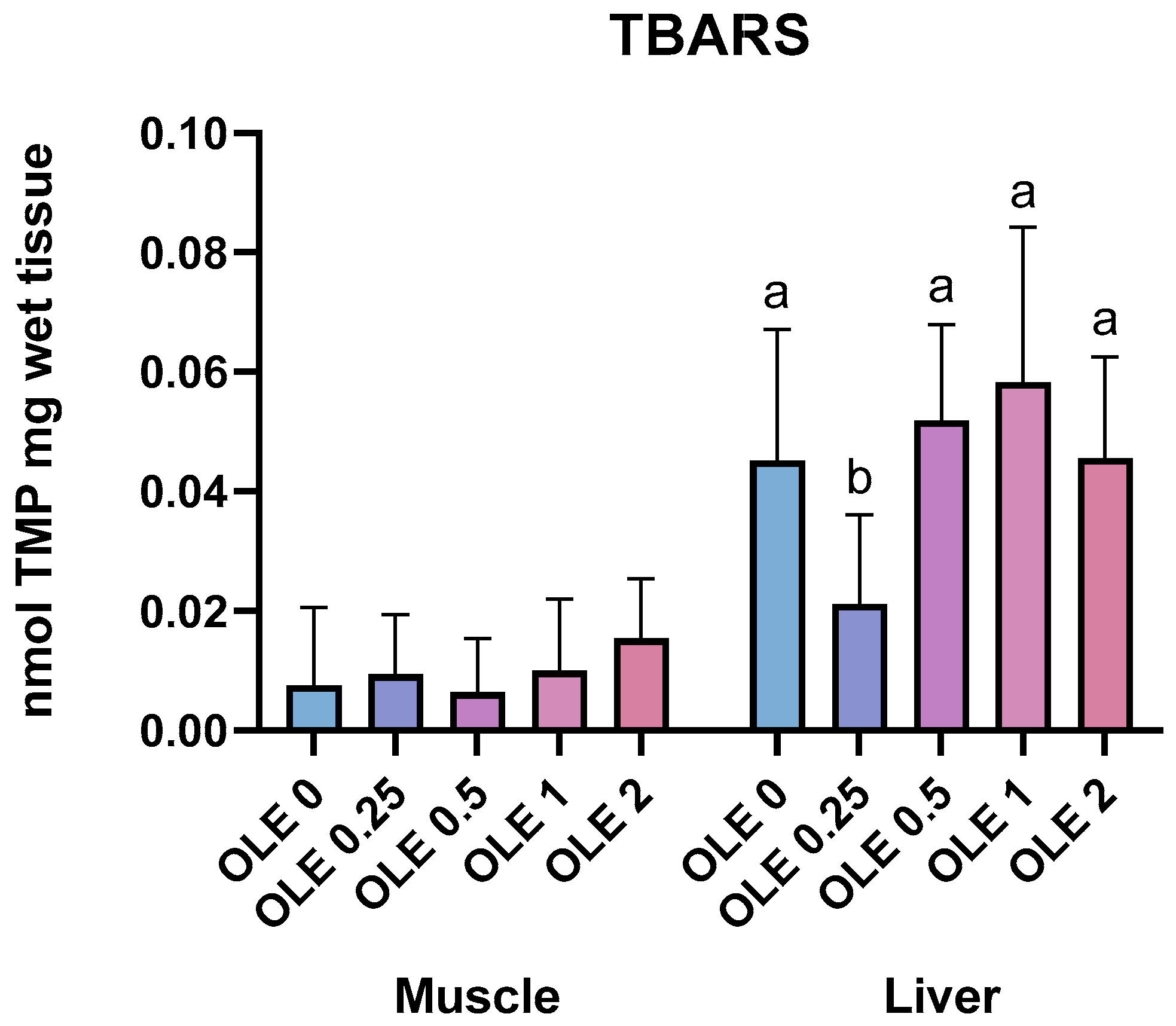
3.5.3. Lipid Peroxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verdegem, M.; Buschmann, A.H.; Latt, U.W.; Dalsgaard, A.J.T.; Lovatelli, A. The contribution of aquaculture systems to global aquaculture production. J. World Aquac. Soc. 2023, 54, 206–250. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture (SOFIA) 2024. Blue Transformation in Action; FAO: Rome, Italy, 2024; p. 264. [Google Scholar] [CrossRef]
- PeixeBR. Anuário Peixe BR da Piscicultura. 2024. Available online: https://www.peixebr.com.br/anuario-2024/ (accessed on 11 March 2025).
- El-Sayed, A.-F.; Fitzsimmons, K. From Africa to the world–The journey of Nile tilapia. Rev. Aquac. 2023, 15 (Suppl. S1), 6–21. [Google Scholar] [CrossRef]
- Beltrán, J.M.G.; Esteban, M.A. Nature-identical compounds as feed additives in aquaculture. Fish Shellfish Immunol. 2022, 23, 409–416. [Google Scholar] [CrossRef]
- Ciji, A.; Akhtar, M.S. Stress management in aquaculture: A review of dietary interventions. Rev. Aquac. 2021, 13, 2190–2247. [Google Scholar] [CrossRef]
- Gule, T.T.; Geremew, A. Dietary strategies for better utilization of aquafeeds in tilapia farming. Aquac. Nutri. 2022, 22, 9463307. [Google Scholar] [CrossRef]
- Metwaly, S.; Nasr, H.; Ahmed, K.; Fathi, M. Multifaceted stress response in Nile tilapia (Oreochromis niloticus) fingerlings: Integrative analysis of salinity, ammonia, and stocking density effects on growth, physiology, and gene expression. Fish Physiol. Biochem. 2025, 51, 48. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.V.N.; dos Santos, C.F.; dos Santos, J.M.L.; Schmitz, M.J.; Ramírez, J.R.B.; Torres, M.F.; Barbas, L.A.L.; Sampaio, L.A.; Verde, P.E.; Tesser, M.B.; et al. Effects of dietary inclusion of lyophilized açaí berries (Euterpe oleracea) on growth metrics metabolic and antioxidant biomarkers, and skin color of juvenile tambaqui (Colossoma macropomum). Aquac. Int. 2023, 31, 1031–1056. [Google Scholar] [CrossRef]
- Mohammed, E.A.H.; Kovács, B.; Kuunya, R.; Mustafa, E.O.A.; Abbo, A.S.H.; Pál, K. Antibiotic resistance in aquaculture: Challenges, trend analysis, and alternative approaches. Antibiotics 2025, 14, 598. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Bagheri, D.; Hoseinifar, S.H.; Morshedi, V.; Paolucci, M. Beneficial role of polyphenols as feed additives on growth performance, immune response and antioxidant status of Lates calcarifer (Bloch, 1790) juveniles. Aquaculture 2022, 552, 737955. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Raieni, R.F.; Dadar, M.; Yilmaz, S.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Benefits of dietary polyphenols and polyphenol-rich additives to aquatic animal health: An overview. Rev. Fish. Sci. Aquac. 2020, 29, 478–511. [Google Scholar] [CrossRef]
- Assar, D.H.; Ragab, A.E.; Abdelsatar, E.; Salah, A.S.; Salem, S.M.R.; Hendam, B.M.; Al Jaouni, S.; Al Wakeel, R.A.; AbdEl-Kader, M.F.; Elbialy, Z.I. Dietary olive leaf extract differentially modulates antioxidant defense of normal and Aeromonas hydrophila-infected common carp (Cyprinus carpio) via Keap1/Nrf2 pathway signaling: A phytochemical and biological link. Animals 2023, 13, 2229. [Google Scholar] [CrossRef]
- Mir-Cerdà, A.; Nuñez, O.; Granados, M.; Sentellas, S.; Saurina, J. An overview of the extraction and characterization of bioactive phenolic compounds from agri-food waste within the framework of circular bioeconomy. TrAC Trends Anal. Chem. 2023, 161, 116994. [Google Scholar] [CrossRef]
- Sokooti, R.; Dezfoulnejad, M.C.; Baboli, M.J. Effects of olive leaf extract (Olea europaea Leecino) on growth, haematological parameters, immune system and carcass composition in common carp (Cyprinus carpio). Aquac. Res. 2020, 52, 2415–2423. [Google Scholar] [CrossRef]
- Vijayaram, S.; Sun, Y.-Z.; Zuorro, A.; Ghafarifarsani, H.; Van Doan, H.; Hoseinifar, S.H. Bioactive immunostimulants as health-promoting feed additives in aquaculture: A review. Fish Shellfish Immunol. 2022, 130, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Fazean, Z.; El-Haroun, E.; Yousefi, M.; Yazici, M.; Van Doan, H.; Paolucci, M. The effects of grapevine (Vitis vinifera L.) leaf extract on growth performance, antioxidant status, and immunity of zebrafish (Danio rerio). Fishes 2023, 8, 326. [Google Scholar] [CrossRef]
- Razola-Díaz, M.C.; Sevenich, R.; Schluter, O.K.; Verardo, V.; Gómez-Caravaca, A.M. Improving olive leaf phenolic extraction with pulsed electric field technology pre-treatment. Foods 2025, 14, 368. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Crupi, P.; Annunziato, A.; Corbo, F. Innovative extraction technologies for development of functional ingredients based on polyphenols from olive leaves. Foods 2022, 11, 103. [Google Scholar] [CrossRef]
- Selim, S.; Albqmi, M.; Al-Sanea, M.M.; Alnusaire, T.S.; Almuhayawi, M.S.; AbdElgawad, H.; Al Jaouni, S.K.; Elkelish, A.; Hussein, S.; Warrad, M.; et al. Valorizing the usage of olive leaves, bioactive compounds, biological activities, and food applications: A comprehensive review. Front. Nutr. 2022, 9, 1008349. [Google Scholar] [CrossRef]
- Panou, A.A.; Karabagias, I.K. Olive leaf extracts as a medicinal beverage: Origin, physico-chemical properties, and bio-functionality. Beverages 2025, 11, 66. [Google Scholar] [CrossRef]
- Wan, F.; Feng, C.; Luo, K.; Cui, W.; Xia, Z.; Cheng, A. Effect of steam explosion on phenolics and antioxidant activity in plants: A review. TrAC Trends Anal. Chem. 2022, 124, 13–24. [Google Scholar] [CrossRef]
- Santos, L.G.; Silva, G.F.A.; Gomes, B.M.; Martins, V.G. A novel sodium alginate active films functionalized with purple onion peel extract (Allium cepa). Biocatal. Agric. Biotechnol. 2021, 35, 102096. [Google Scholar] [CrossRef]
- Santos, L.G.; Martins, V.G. Recovery of phenolic compounds from purple onion peel using bio-based solvents: Thermal degradation kinetics and color stability of anthocyanins. J. Food Process. Preserv. 2022, 46, e17161. [Google Scholar] [CrossRef]
- Santos, L.G.; Martins, V.G. Optimization of the green extraction of polyphenols from the edible flower Clitoria ternatea by high-power ultrasound: A comparative study with conventional extraction techniques. J. Appl. Res. Med. Aromat. Plants 2023, 34, 100458. [Google Scholar] [CrossRef]
- Naz, S.; Majeed, S.; Tasleem, S.; Jyothi, S.R.; Thakur, H.; Anuradha, J.; Ujan, J.A.; Ullah, M.; Zahid, M.; Attaullah, S.; et al. Investigating the effects of wild olive Olea europaea leaf extract on growth, body composition, digestive enzyme activity, hematological parameters, and immune function in Nile tilapia. N. Am. J. Aquac. 2024, 86, 462–474. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Rodrigues, R.B. Métodos de Análises Bromatológicas de Alimentos: Métodos Físicos, Químicos e Bromatológicos; Embrapa Clima Temperado: Pelotas, Brazil, 2010; 177p, ISSN 1516-8840. [Google Scholar]
- Dawood, M.; Madkour, K.; Mugwanya, M.; Kimera, F.; Sewilam, H. Growth performances, body composition, and blood variables of Nile tilapia (Oreochromis niloticus) juveniles grown in a recirculating aquaculture system under different water salinity levels. J. Appl. Aquac. 2023, 36, 612–626. [Google Scholar] [CrossRef]
- Intergovernmental Oceanographic Commission (IOC). Chemical Methods for Use in Marine Environment Monitoring; Manual and Guides, No. 12; UNESCO: Paris, France, 1983. [Google Scholar] [CrossRef]
- Aminot, A.; Chaussepied, M. Manuel des Analyses Chimiques en Milieu Marin; Editions Jouve; CNEXO: Paris, France, 1983; 365p. [Google Scholar]
- García-Robledo, E.; Corzo, A.; Papaspyrou, S. A fast and direct spectrophotometric method for the sequential determination of nitrate and nitrite at low concentrations in small volumes. Mar. Chem. 2014, 162, 30–36. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 22nd ed.; APHA: Washington, DC, USA, 2012. [Google Scholar]
- Santos, P.O.; Souza, E.O.; Assis, L.C.; Pierro, P.C.C.; Bolzan, R.P.; Demier, L.C.; Junior, J.G.V.; Mendonça, P.P. Uso de eugenol para anestesia de Oreochromis niloticus. Brazil J. Dev. 2022, 8, 64251–64258. [Google Scholar] [CrossRef]
- Ayres, T.S.D.M.; Christ-Ribeiro, A.; Furlong, E.B.; Monserrat, J.M.; Tesser, M.B. Use of defatted fermented rice bran in the diet of juvenile mullets Mugil liza. Aquaculture 2022, 554, 738108. [Google Scholar] [CrossRef]
- Schaubroeck, K.J.; Leitner, B.P.; Perry, R.J. An optimized method for tissue glycogen quantification. Physiol. Rep. 2022, 10, e15195. [Google Scholar] [CrossRef] [PubMed]
- Oakes, K.D.; Van Der Kraak, G.J. Utility of the TBARS assay in detecting oxidative stress in White sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat. Toxicol. 2003, 63, 447–463. [Google Scholar] [CrossRef]
- El-Hack, M.E.A.; El-Saadony, M.T.; Nader, M.M.; Salem, H.M.; El-Tahan, A.M.; Soliman, S.M.; Khafaga, A.F. Effect of environmental factors on growth performance of Nile tilapia (Oreochromis niloticus). Int. J. Biometeorol. 2022, 66, 2183–2194. [Google Scholar] [CrossRef]
- Fazio, F.; Habib, S.S.; Naz, S.; Filiciotto, F.; Cicero, N.; Rehman, H.U.; Saddozai, S.; Rind, K.H.; Rind, N.A.; Shar, A.H. Effect of fortified feed with olive leaves extract on the haematological and biochemical parameters of Oreochromis niloticus (Nile tilapia). Nat. Prod. Res. 2022, 36, 1575–1580. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Mirghaed, A.T.; Iri, Y.; Hoseinifar, S.H.; Van Doan, H.; Reverter, M. Effects of dietary Russian olive, Elaeagnus angustifolia, leaf extract on growth, hematological, immunological, and antioxidant parameters in common carp, Cyprinus carpio. Aquaculture 2021, 536, 736461. [Google Scholar] [CrossRef]
- Rajabiesterabadi, H.; Ghelichi, A.; Jorjani, S.; Hoseini, S.M.; Akrami, R. Dietary olive (Olea europaea) leaf extract suppresses oxidative stress and modulates intestinal expression of antioxidant- and tight junction-related genes in common carp (Cyprinus carpio). Aquaculture 2020, 520, 734676. [Google Scholar] [CrossRef]
- Rajabiesterabadi, H.; Yousefi, M.; Hoseini, S.M. Enhanced haematological and immune responses in common carp Cyprinus carpio fed with olive leaf extract-supplemented diets and subjected to ambient ammonia. Aquac. Nutr. 2020, 26, 763–771. [Google Scholar] [CrossRef]
- Zemheri-Navruz, F.; Acar, Ü.; Yılmaz, S. Dietary supplementation of olive leaf extract increases haematological, serum biochemical parameters and immune related genes expression level in common carp (Cyprinus carpio) juveniles. Fish Shellfish Immunol. 2019, 89, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Zemheri-Navruz, F.; Acar, Ü.; Yılmaz, S. Dietary supplementation of olive leaf extract enhances growth performance, digestive enzyme activity and growth related genes expression in common carp Cyprinus carpio. Gen. Comp. Endocrinol. 2020, 296, 113541. [Google Scholar] [CrossRef]
- Al-Sulivany, B.S.A.; Hassan, N.E.; Mhammad, H.A. Influence of dietary protein content on growth performance, feed efficiency, condition factor, and length-weight relationship in Cyprinus carpio during the summer season. Egypt. J. Aquat. Biol. Fish. 2024, 28, 505–521. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Jamil, M.; Ali, S.; Shahzad, M.M.; Hussain, M.; Ahmad, N.; Riaz, D. Gene expression, growth performance and body composition of Labeo rohita fingerlings fed on polyphenols supplement. Sains Malays. 2023, 52, 3357–3370. [Google Scholar] [CrossRef]
- Ali, M.; Hussain, S.M.; Asrar, M.; Hussain, M.; Arsalan, M.Z.H.; Yousaf, Z.; Bano, A.A. Efficacy of dietary polyphenols supplementation with soybean meal-based diet on growth, antioxidant status and carcass composition of Labeo rohita fingerlings. Pak. J. Zool. 2023, 56, 2501–3000. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Olivares-Vicente, M.; Rodríguez-Pérez, C.; Herranz-López, M.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Encinar, J.A.; Micol, V. AMPK modulatory activity of olive-tree leaves phenolic compounds: Bioassay-guided isolation on adipocyte model and in silico approach. PLoS ONE 2017, 12, e0173074. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, X.; Huang, N.; Li, H.; Tian, J.; Li, T.; Yao, K.; Nyachoti, C.M.; Kim, S.W.; Yin, Y. AMPK regulation of glucose, lipid and protein metabolism: Mechanisms and nutritional significance. Curr. Protein Pept. Sci. 2017, 18, 562–570. [Google Scholar] [CrossRef]
- Smiles, W.J.; Ovens, A.J.; Oakhill, J.S.; Kofler, B. The metabolic sensor AMPK: Twelve enzymes in one. Mol. Metab. 2024, 90, 102042. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Zhai, A.; Zhang, B.; Tian, G. AMPK-mediated regulation of lipid metabolism by phosphorylation. Biol. Pharm. Bull. 2018, 41, 985–993. [Google Scholar] [CrossRef]
- Soon, G.S.T.; Torbenson, M. The liver and glycogen: In sickness and in health. Int. J. Mol. Sci. 2023, 24, 6133. [Google Scholar] [CrossRef]
- Tan, X.; Testoni, G.; Sullivan, M.A.; López-Soldado, I.; Vilaplana, F.; Gilbert, R.G.; Guinovart, J.J.; Schulz, B.L.; Duran, J. Glycogenin is dispensable for normal liver glycogen metabolism and body glucose homeostasis. Int. J. Biol. Macromol. 2025, 291, 139084. [Google Scholar] [CrossRef] [PubMed]
- Iantomasi, M.; Terzo, M.; Tsiani, E. Anti-diabetic effects of oleuropein. Metabolites 2024, 14, 581. [Google Scholar] [CrossRef]
- Esteras, N.; Abramov, A.Y. Nrf2 as a regulator mitochondrial function: Energy metabolism and beyond. Free Radic. Biol. Med. 2022, 189, 135–153. [Google Scholar] [CrossRef]
- Da Porto, A.; Brosolo, G.; Casarsa, V.; Bulfone, L.; Scandolin, L.; Catena, C.; Sechi, L.A. The pivotal role of oleuropein in the anti-diabetic action of the Mediterranean diet: A concise review. Pharmaceutics 2022, 14, 40. [Google Scholar] [CrossRef]
- Abunab, H.; Dator, W.L.; Hawamdeh, S. Effect of olive leaf extract on glucose levels in diabetes-induced rats: A systematic review and meta-analysis. J. Diabetes 2017, 9, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Hussein, M.M.; Ahmed, O.M.; Al-Jameel, S.S.; Al-Muzafar, H.M.; Amin, K.A.; Abdou, H.M. Oleuropein ameliorates hyperlipidemia, oxidative stress, inflammatory and liver dysfunction biomarkers, in streptozotocin-induced diabetic rats. J. Appl. Pharm. Sci. 2024, 14, 227–234. [Google Scholar] [CrossRef]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant activity, metabolism, and bioavailability of polyphenols in the diet of animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Farias, D.P.; Araújo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Antidiabetic potential of dietary polyphenols: A mechanistic review. Food Res. Int. 2021, 145, 110383. [Google Scholar] [CrossRef]
- Baba, E.; Acar, Ü.; Yılmaz, S.; Zemheri, F.; Ergün, S. Dietary olive leaf (Olea europaea L.) extract alters some immune gene expression levels and disease resistance to Yersinia ruckeri infection in rainbow trout Oncorhynchus mykiss. Fish Shellfish Immunol. 2018, 79, 28–33. [Google Scholar] [CrossRef]
- Xie, N.N.; Zhang, J.M.; Jiang, M.; Meng, X.L.; Dong, L.X.; Lu, X.; Wen, H.; Tian, J. Effects of lipid levels on growth performance and glucose and lipid metabolism of adult Nile tilapia (Oreochromis niloticus) fed with high carbohydrate diets. Aquac. Rep. 2025, 40, 102639. [Google Scholar] [CrossRef]
- Yin, D.; Zhong, Y.; Liu, H.; Hu, J. Lipid metabolism regulation by dietary polysaccharides with different structural properties. Int. J. Biol. Macromol. 2024, 270, 132253. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Chen, Y.Q.; Konrad, R.J. The regulation of triacylglycerol metabolism and lipoprotein lipase activity. Adv. Biol. 2022, 6, 2200093. [Google Scholar] [CrossRef]
- Guo, J.; Chen, S.; Zhang, Y.; Liu, J.; Jiang, L.; Hu, L.; Yao, K.; Chen, X. Cholesterol metabolism: Physiological regulation and diseases. MedComm 2024, 5, e476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Chen, X.-W. The biogenesis and transport of triglyceride-rich lipoproteins. Trends Endocrinol. Metab. 2025, 36, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Fki, I.; Sayadi, S.; Mahmoudi, A.; Daoued, I.; Marrekchi, R.; Ghorbel, H. Comparative study on beneficial effects of hydroxytyrosol- and oleuropein-rich olive leaf extracts on high-fat diet-induced lipid metabolism disturbance and liver injury in rats. Biomed. Res. Int. 2020, 2020, 1315202. [Google Scholar] [CrossRef]
- Laaboudi, W.; Ashmawy, N.S.; El Hachlafi, N.; Alshabrmi, F.M.; Alnasseri, S.M.; Assaggaf, H.; Qasem, A.; AL-Fargah, A.; Al-Maaqar, S.M.; Mrabti, H.N. Impact of olive tree extract on hyperlipidemia in rats: HPLC analysis of bioactive constituents. CyTA-J. Food 2025, 23, 2463491. [Google Scholar] [CrossRef]
- Malliou, F.; Andreadou, J.; Gonzalez, F.J.; Lazou, A.; Xepapadaki, E.; Vallianou, J.; Lambrinidis, G.; Mikros, E.; Marselos, M.; Skaltsounis, A.L.; et al. The olive constituent oleuropein, as a PPARα agonist, markedly reduces serum triglycerides. J. Nutr. Biochem. 2018, 59, 17–28. [Google Scholar] [CrossRef]
- Porcu, C.; Sideri, S.; Martini, M.; Cocomazzi, A.; Galli, A.; Tarantino, G.; Balsano, C. Oleuropein induces AMPK-dependent autophagy in NAFLD mice, regardless of the gender. Int. J. Mol. Sci. 2018, 19, 3948. [Google Scholar] [CrossRef]
- Amraoui, A.; Djerrou, Z.; Haimoud, S.A.; Zerouki, K.; Elmokli, S. Antihyperlipidemic and antioxidant potential of Olea europaea L. leaves: An experimental study in vivo, in vitro and in silico. Foods Raw Mater. 2025, 13, 35–45. [Google Scholar] [CrossRef]
- Esmaeili, N. Blood performance: A new formula for fish growth and health. Biology 2021, 10, 1236. [Google Scholar] [CrossRef]
- Villasante, A.; Figueroa, E.; Godoy, K.; Dantagnan, P.; López-Polo, J.; Opazo, R.; Romero, J. Impact of plant-based diets on hepatosomatic index, circulating globulins and growth in rainbow trout (Oncorhynchus mykiss). Fishes 2025, 10, 110. [Google Scholar] [CrossRef]
- Alfonso, S.; Fiocchi, E.; Toomey, L.; Boscarato, M.; Manfrin, A.; Dimitroglu, A.; Papaharisis, L.; Passabi, E.; Stefani, A.; Lembo, G.; et al. Comparative analysis of blood protein fractions in two Mediterranean farmed fish: Dicentrarchus labrax and Sparus aurata. BMC Vet. Res. 2024, 20, 322. [Google Scholar] [CrossRef]
- Menon, S.V.; Kumar, A.; Middha, S.K.; Paital, B.; Mathur, S.; Johnson, R.; Kademan, A.; Usha, T.; Hemavathi, K.N.; Dayal, S.; et al. Water physicochemical factors and oxidative stress physiology in fish, a review. Front. Environ. Sci. 2023, 11, 1240813. [Google Scholar] [CrossRef]
- Fath El-Bab, A.; Amer, A.A.; El-Nawsany, M.M.; Ibrahim, I.H.; Gouda, A.H.; El-Bahlol, A.A.; Naiel, M.A.E. Oregano leaf extract dietary administration modulates performance, redox status, intestinal health, and expression of some related genes of Nile tilapia (Oreochromis niloticus L.). Ann. Anim. Sci. 2024, 24, 179–190. [Google Scholar] [CrossRef]
- Ying, X.; Li, X.; Deng, S.; Zhang, B.; Xiao, G.; Xu, Y.; Brennan, C.; Benjakul, S.; Ma, L. How lipids, as important endogenous nutrient component affect the quality of aquatic products: An overview of lipid peroxidation and the interaction with proteins. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70096. [Google Scholar] [CrossRef] [PubMed]
- González-Ortega, R.; Sturm, L.; Skrt, M.; Di Mattia, C.D.; Pittia, P.; Ulrih, N.P. Liposomal encapsulation of oleuropein and an olive leaf extract: Molecular interactions, antioxidant effects and applications in model food systems. Food Biophys. 2021, 16, 84–97. [Google Scholar] [CrossRef]
- Buzdar, J.A.; Shah, Q.A.; Khan, M.Z.; Zaheer, A.; Shah, T.; Ataya, F.S.; Fouad, D. Hepatoprotective effects of olive leaf extract against carbon tetrachloride-induced oxidative stress: In vivo and in-silico insights into the Nrf2-NFκB pathway. J. Mol. Histol. 2025, 56, 42. [Google Scholar] [CrossRef]


| Ingredients (g/kg) | OLE0 | OLE0.25 | OLE0.5 | OLE1.0 | OLE2.0 |
|---|---|---|---|---|---|
| Fishmeal 1 | 300 | 300 | 300 | 300 | 300 |
| Gelatin 2 | 30 | 30 | 30 | 30 | 30 |
| Soybean meal 3 | 200 | 200 | 200 | 200 | 200 |
| Corn starch 4 | 347.5 | 347.5 | 347.5 | 347.5 | 347.5 |
| Soybean oil 5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| Wheat bran 6 | 100 | 100 | 100 | 100 | 100 |
| Mineral and vitamin premix 7 | 10 | 10 | 10 | 10 | 10 |
| OLE | 0 | 0.25 | 0.5 | 1 | 2 |
| Moisture (%) | 3.37 ± 0.09 | 2.56 ± 0.15 | 2.45 ± 0.20 | 3.42 ± 0.26 | 3.31 ± 0.15 |
| Ash (%) | 9.47 ± 0.14 | 9.44 ± 0.09 | 9.46 ± 0.03 | 9.26 ± 0.16 | 9.37 ± 0.06 |
| Crude protein (%) | 33.65 ± 0.25 | 33.50 ± 0.11 | 33.59 ± 0.48 | 33.71 ± 0.35 | 33.23 ± 0.13 |
| Total lipid (%) | 7.68 ± 0.29 | 7.84 ± 0.53 | 8.49 ± 0.81 | 8.21 ± 0.42 | 8.29 ± 0.31 |
| Crude fiber (%) | 1.94 ± 0.10 | 1.45 ± 0.21 | 2.16 ± 0.77 | 1.91 ± 0.22 | 1.53 ± 0.10 |
| NNE | 43.89 | 45.21 | 43.85 | 43.49 | 44.27 |
| Parameters | Treatments | ||||
|---|---|---|---|---|---|
| OLE0 | OLE0.25 | OLE0.5 | OLE1.0 | OLE2.0 | |
| IW (g) | 0.55 ± 0.11 | 0.55 ± 0.10 | 0.55 ± 0.10 | 0.55 ± 0.10 | 0.55 ± 0.10 |
| FW (g) | 21.02 ± 0.85 | 20.60 ± 1.35 | 19.41 ± 1.78 | 21.41 ± 1.54 | 20.61 ± 0.91 |
| WG (g) | 20.47 ± 0.85 | 20.04 ± 1.35 | 18.85 ± 1.78 | 20.85 ± 1.54 | 20.05 ± 0.90 |
| DWG (g) | 0.44 ± 0.01 | 0.43 ± 0.02 | 0.40 ± 0.03 | 0.45 ± 0.03 | 0.43 ± 0.01 |
| SGR (%) | 7.88 ± 0.08 | 7.83 ± 0.13 | 7.70 ± 0.19 | 7.91 ± 0.15 | 7.84 ± 0.08 |
| SR (%) | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| FCR | 1.03 ± 0.07 | 1.06 ± 0.03 | 1.08 ± 0.02 | 1.01 ± 0.04 | 1.10 ± 0.03 |
| CF | 2.58 ± 0.46 ab | 2.64 ± 0.52 a | 2.44 ± 0.47 ab | 2.46 ± 0.41 ab | 2.33 ± 0.48 b |
| Parameters | Treatments | ||||
|---|---|---|---|---|---|
| OLE0 | OLE0.25 | OLE0.5 | OLE1.0 | OLE2.0 | |
| HSI (%) | 1.01 ± 0.18 | 1.10 ± 0.35 | 1.05 ± 0.43 | 1.12 ± 0.29 | 0.96 ± 0.32 |
| VSI (%) | 5.09 ± 0.95 | 5.81 ± 1.27 | 5.90 ± 1.50 | 6.04 ± 0.78 | 6.37 ± 1.18 |
| CY (%) | 85.42 ± 0.98 | 85.62 ± 0.78 | 85.33 ± 1.35 | 84.92 ± 1.40 | 85.61 ± 1.27 |
| IQ | 6.63 ± 1.67 | 6.33 ± 1.53 | 5.67 ± 1.23 | 5.64 ± 1.87 | 6.17 ± 1.10 |
| Parameters | Treatments | |||||
|---|---|---|---|---|---|---|
| Initial | OLE0 | OLE0.25 | OLE0.5 | OLE1.0 | OLE2.0 | |
| Moisture (%) | 81.43 ± 0.01 | 71.95 ± 0.58 a | 70.20 ± 0.55 b | 71.00 ± 0.47 ab | 71.25 ± 1.34 ab | 71.66 ± 0.81 a |
| Ash (%) | 3.82 ± 0.01 | 3.96 ± 0.31 | 3.93 ± 0.11 | 3.95 ± 0.14 | 3.90 ± 0.09 | 4.05 ± 0.22 |
| Crude protein (%) | 12.84 ± 0.04 | 14.94 ± 0.39 b | 15.73 ± 0.15 a | 15.65 ± 0.64 a | 15.64 ± 0.43 a | 15.48 ± 0.44 ab |
| Total lipid (%) | 2.16 ± 0.18 | 7.54 ± 0.68 c | 8.99 ± 0.48 a | 8.11 ± 0.31 bc | 8.28 ± 0.77 b | 7.53 ± 0.23 c |
| Protein deposition (g) | - | 3.06 ± 0.12 | 3.17 ± 0.20 | 2.95 ± 0.14 | 3.27 ± 0.14 | 3.12 ± 0.21 |
| Lipid deposition (g) | - | 1.57 ± 0.22 | 1.84 ± 0.12 | 1.56 ± 0.18 | 1.75 ± 0.06 | 1.54 ± 0.08 |
| Parameters | Treatments | ||||
|---|---|---|---|---|---|
| OLE0 | OLE0.25 | OLE0.5 | OLE1.0 | OLE2.0 | |
| Muscle glycogen (mg/g) | 0.78 ± 0.22 a | 0.54 ± 0.12 b | 0.60 ± 0.18 b | 0.59 ± 0.12 b | 0.62 ± 0.11 b |
| Hepatic glycogen (mg/g) | 0.71 ± 0.30 abc | 0.57 ± 0.43 c | 0.97 ± 0.62 a | 0.67 ± 0.31 b | 0.61 ± 0.37 b |
| Plasma glucose (mg/dL) | 78.51 ± 14.57 | 81.88 ± 10.65 | 76.69 ± 11.98 | 76.23 ± 15.48 | 76.34 ± 12.49 |
| Plasma cholesterol (mg/dL) | 161.11 ± 12.89 | 159.47 ± 16.01 | 153.84 ± 20.20 | 155.72 ± 15.00 | 152.44 ± 15.10 |
| Plasma triglycerides (mg/dL) | 141.10 ± 7.72 abc | 141.01 ± 8.02 bc | 136.30 ± 6.01 c | 147.58 ± 14.80 a | 144.93 ± 10.09 ab |
| Total plasma protein (g/dL) | 3.16 ± 0.22 ab | 3.14 ± 0.23 ab | 3.10 ± 0.18 ab | 3.21 ± 0.21 a | 2.98 ± 0.25 b |
| Hepatic glucose (mg/g) | 8.68 ± 3.80 b | 10.11 ± 2.78 b | 9.35 ± 3.55 b | 10.32 ± 5.32 b | 13.86 ± 4.43 a |
| Hepatic cholesterol (mg/g) | 4.91 ± 0.45 | 5.03 ± 0.41 | 5.16 ± 0.33 | 4.71 ± 0.86 | 4.91 ± 0.73 |
| Hepatic triglycerides (mg/g) | 28.46 ± 6.06 ab | 26.23 ± 7.05 b | 30.69 ± 6.86 a | 25.33 ± 9.21 ab | 28.59 ± 8.64 ab |
| Total hepatic protein (g/g) | 2.04 ± 0.50 | 2.00 ± 0.47 | 1.93 ± 0.45 | 2.33 ± 0.79 | 2.25 ± 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zancan, T.D.; Monserrat, J.M.; Martins, V.G.; Tesser, M.B. Effects of Olive Leaf (Olea europaea) Phenolic Extract on Zootechnical Parameters, Centesimal Composition, and Biochemical Parameters of Nile Tilapia (Oreochromis niloticus) Juveniles. Animals 2025, 15, 2935. https://doi.org/10.3390/ani15202935
Zancan TD, Monserrat JM, Martins VG, Tesser MB. Effects of Olive Leaf (Olea europaea) Phenolic Extract on Zootechnical Parameters, Centesimal Composition, and Biochemical Parameters of Nile Tilapia (Oreochromis niloticus) Juveniles. Animals. 2025; 15(20):2935. https://doi.org/10.3390/ani15202935
Chicago/Turabian StyleZancan, Thaise Dalferth, José María Monserrat, Vilásia Guimarães Martins, and Marcelo Borges Tesser. 2025. "Effects of Olive Leaf (Olea europaea) Phenolic Extract on Zootechnical Parameters, Centesimal Composition, and Biochemical Parameters of Nile Tilapia (Oreochromis niloticus) Juveniles" Animals 15, no. 20: 2935. https://doi.org/10.3390/ani15202935
APA StyleZancan, T. D., Monserrat, J. M., Martins, V. G., & Tesser, M. B. (2025). Effects of Olive Leaf (Olea europaea) Phenolic Extract on Zootechnical Parameters, Centesimal Composition, and Biochemical Parameters of Nile Tilapia (Oreochromis niloticus) Juveniles. Animals, 15(20), 2935. https://doi.org/10.3390/ani15202935









