Effect of FABP4 Gene Polymorphisms on Fatty Acid Composition, Chemical Composition, and Carcass Traits in Sonid Sheep
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Statement of Ethics
2.2. Lamb Sample Preparation
2.3. Real-Time Quantitative PCR (RT-qPCR)
2.4. Resequencing and Variant Detection in FABP4
2.5. Polymorphism Genotyping Using iPLEX MassARRAY
2.6. Determination of Fatty Acid Composition
2.7. Measurements of Carcass Traits
2.8. Measurements of Chemical Composition
2.9. Bioinformatics Analysis
2.10. Statistical Analysis
3. Results
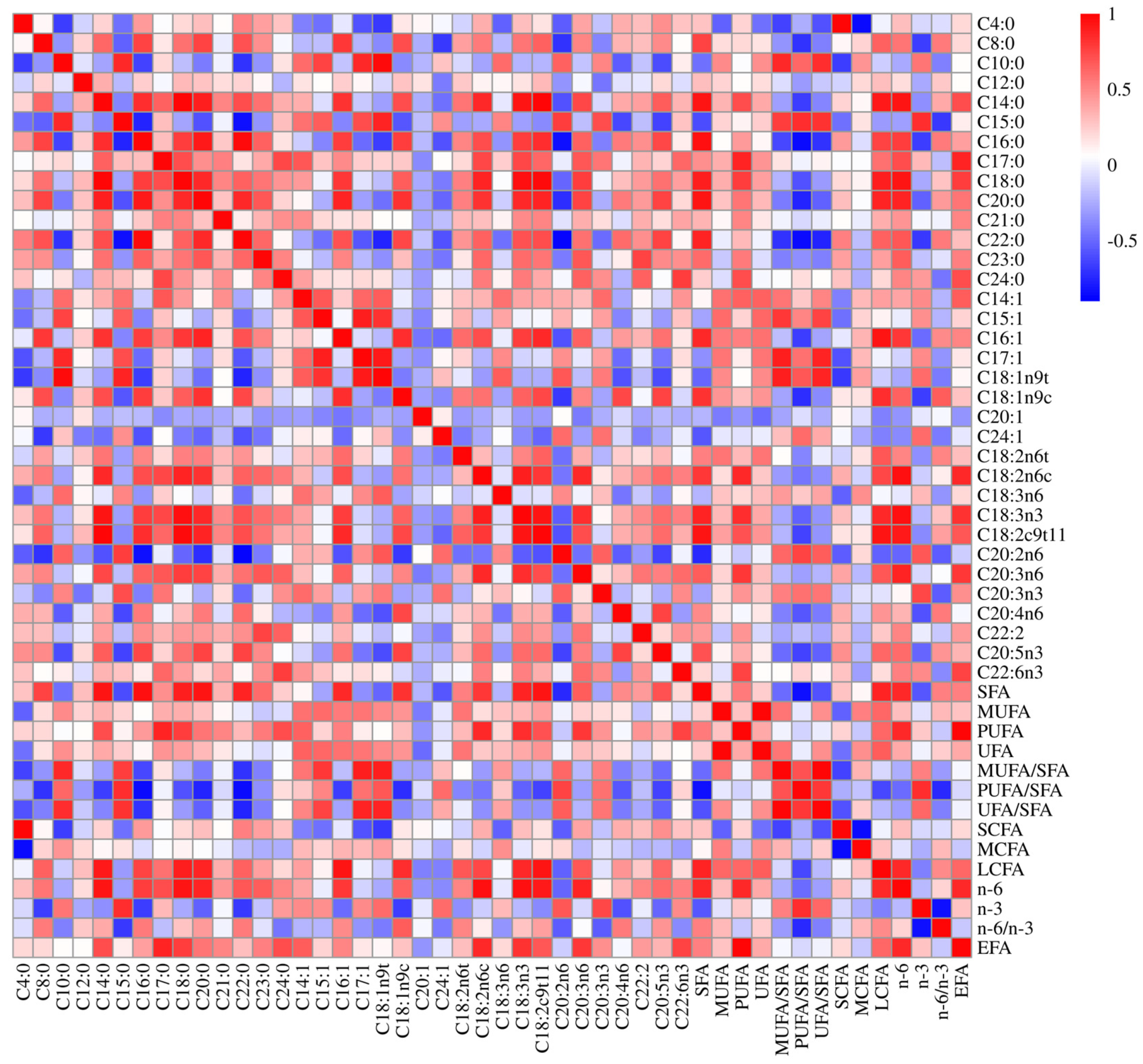
3.1. Fatty Acid Profiles of Longissimus Thoracis Muscle Tissue
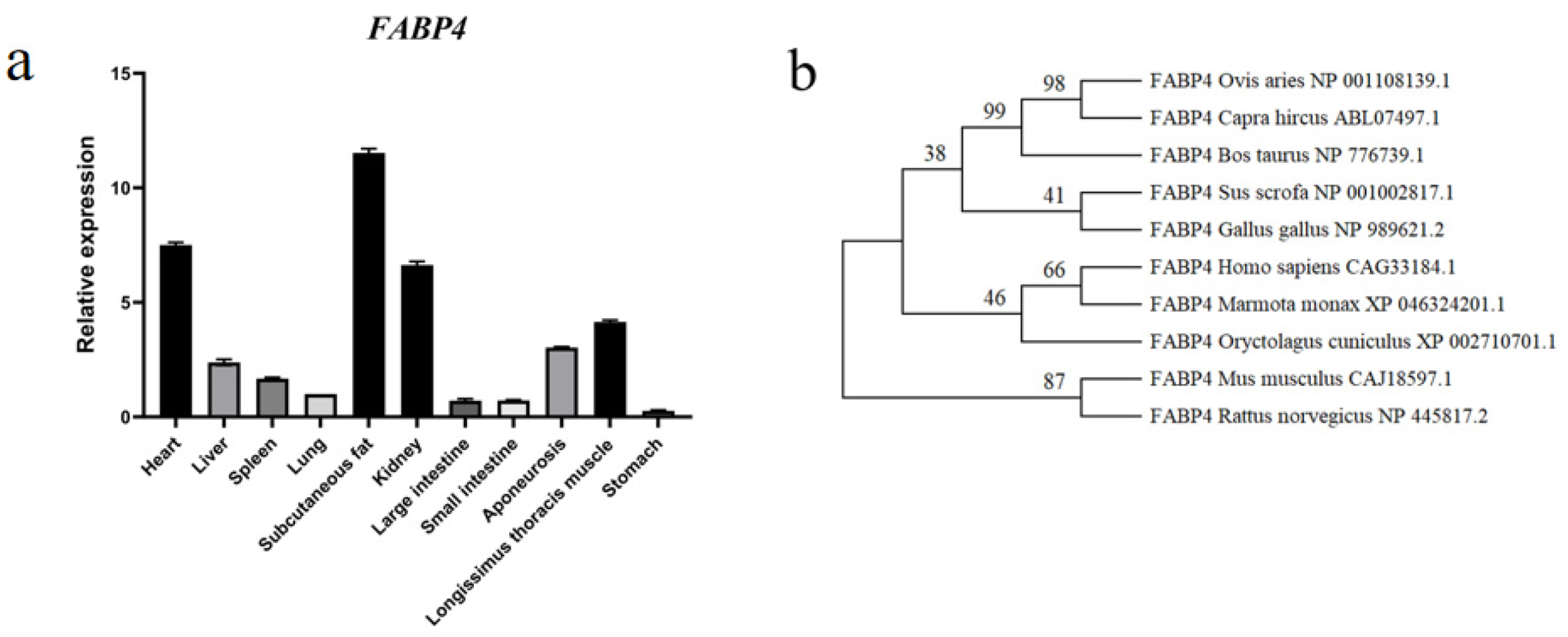
3.2. FABP4 Gene Expression Profile and Phylogenetic Tree
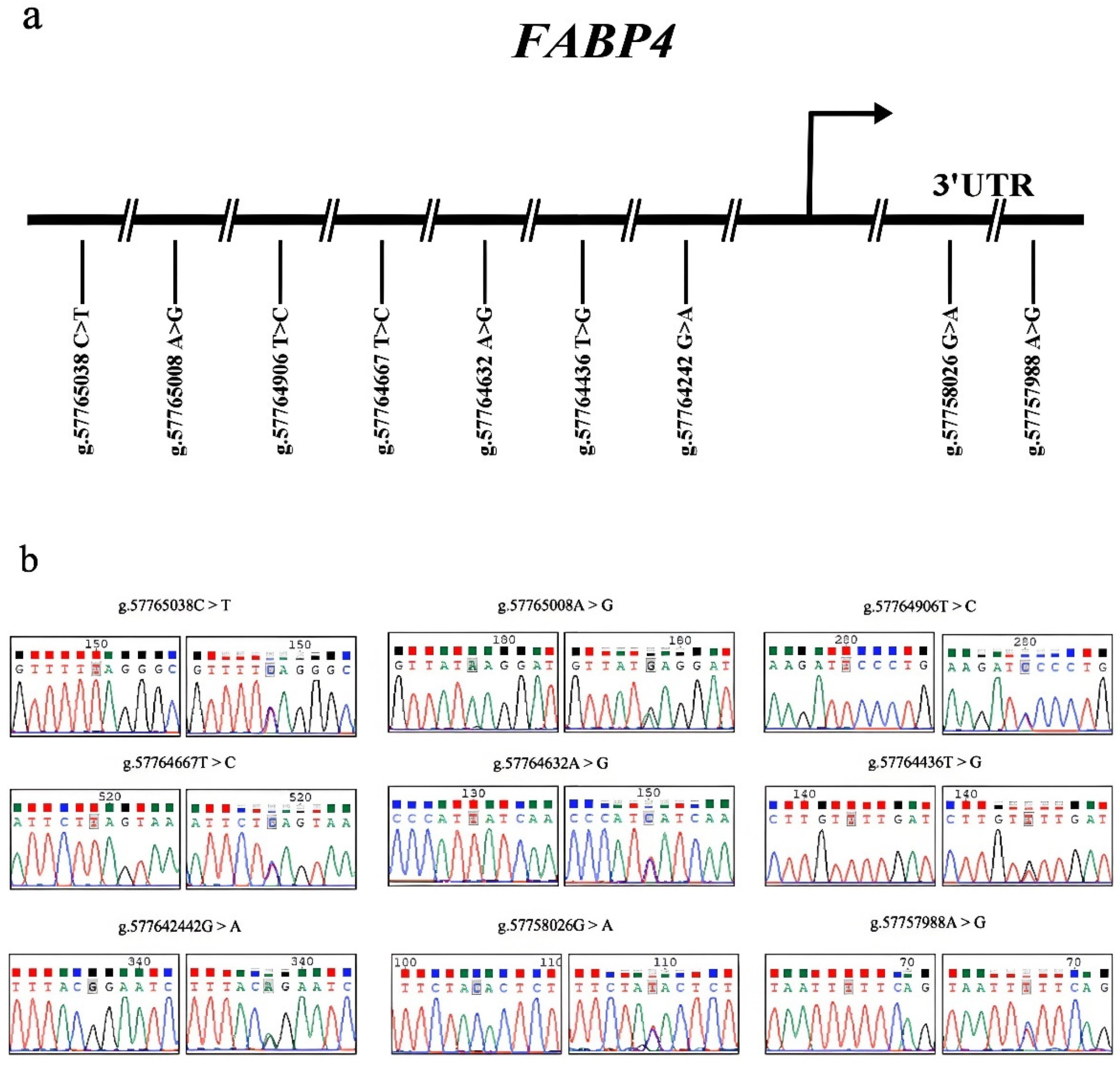
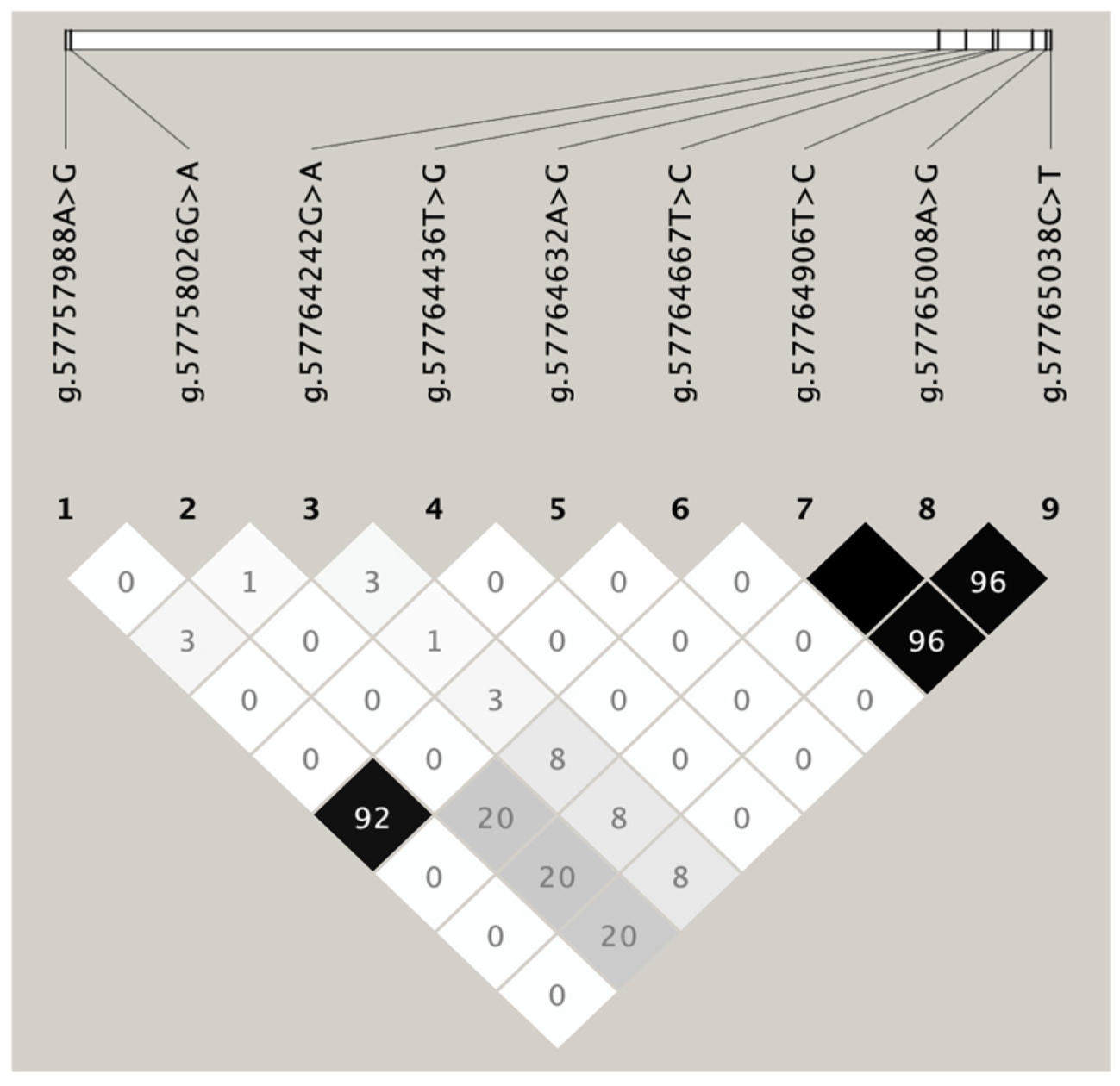
3.3. Variant Identification in Sonid Sheep FABP4 Gene
3.4. Association Analysis of Novel Variants in FABP4 with Fatty Acid
3.5. Association Analysis of Variants in FABP4 with Chemical Composition
3.6. Association Analysis of Variants in FABP4 with Carcass Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Si, Z.; Qu, C.; Zhu, C. (Eds.) Prospects for Supply and Demand of Mutton in China in the Next 10 Years; Beef and mutton Industry Chain team of Ministry of Agriculture and Rural Areas, Agricultural Information Institute, Chinese Academy of Agricultural Sciences: Qingyang, China, 2019; pp. 2–4. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. Available online: https://www.stats.gov.cn/sj/ (accessed on 10 October 2024).
- Panea, B.; Ripoll, G. Quality and Safety of Meat Products. Foods 2020, 9, 803. [Google Scholar] [CrossRef] [PubMed]
- China national commission of animal genetic resources (CNCAGR). Animal Genetic Resources in China: Sheep and Goats; China Agriculture Press: Beijing, China, 2011; pp. 40–42. [Google Scholar]
- Wang, B.; Luo, Y.; Wang, Y.; Wang, D.; Hou, Y.; Yao, D.; Tian, J.; Jin, Y. Rumen bacteria and meat fatty acid composition of Sunit sheep reared under different feeding regimens in China. J. Sci. Food Agric. 2021, 101, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, C.; Dou, L.; Chen, X.; Zhao, L.; Su, L.; Jin, Y. Effects of Feeding Regimes and Postmortem Aging on Meat Quality, Fatty Acid Composition, and Volatile Flavor of Longissimus Thoracis Muscle in Sunit Sheep. Animals 2022, 12, 3081. [Google Scholar] [CrossRef] [PubMed]
- Tvrzicka, E.; Kremmyda, L.S.; Stankova, B.; Zak, A. Fatty acids as biocompounds: Their role in human metabolism, health and disease—A review. Part 1: Classification, dietary sources and biological functions. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2011, 155, 117–130. [Google Scholar] [CrossRef]
- Bobiński, R.; Bobińska, J. Fatty acids of human milk—A review. J. Int. Vitaminol. Nutr. 2022, 92, 280–291. [Google Scholar] [CrossRef]
- Gravador, R.S.; Harrison, S.M.; Monahan, F.J.; Gkarane, V.; Farmer, L.J.; Brunton, N.P. Validation of a Rapid Microwave-Assisted Extraction Method and GC-FID Quantification of Total Branched Chain Fatty Acids in Lamb Subcutaneous Adipose Tissue. J. Food Sci. 2019, 84, 80–85. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Xu, J.; Yang, Q.; Sha, Y.; Jiao, T.; Zhao, S. Effects of yeast cultures on meat quality, flavor composition and rumen microbiota in lambs. Curr. Res. Food Sci. 2024, 9, 100845. [Google Scholar] [CrossRef]
- Coyne, J.M.; Evans, R.D.; Berry, D.P. Dressing percentage and the differential between live weight and carcass weight in cattle are influenced by both genetic and non-genetic factors1. J. Anim. Sci. 2019, 97, 1501–1512. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Prache, S.; Schreurs, N.; Guillier, L. Review: Factors affecting sheep carcass and meat quality attributes. Animal 2022, 16 (Suppl. S1), 100330. [Google Scholar] [CrossRef]
- Leiferman, A.; Shu, J.; Grove, R.; Cui, J.; Adamec, J.; Zempleni, J. A diet defined by its content of bovine milk exosomes and their RNA cargos has moderate effects on gene expression, amino acid profiles and grip strength in skeletal muscle in C57BL/6 mice. J. Nutr. Biochem. 2018, 59, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.H.; Zhu, Y.J.; Tang, S.Q.; Wei, X.J.; Sheng, Z.H.; Jiao, G.A.; Hu, P.S.; Zhuang, J.Y. Pleiotropic Effects of Rice Florigen Gene RFT1 on the Amino Acid Content of Unmilled Rice. Front. Genet. 2020, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Choi, T.J.; Park, M.N.; Oh, S.H. Estimation of environmental effects and genetic parameters of carcass traits on Chikso (Korean brindle cattle). Asian-Australas. J. Anim. Sci. 2020, 33, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, S.I.; van der Werf, J.H.; Jacob, R.H.; Hopkins, D.L.; Pannier, L.; Pearce, K.L.; Gardner, G.E.; Warner, R.D.; Geesink, G.H.; Edwards, J.E.; et al. Genetic parameters for meat quality traits of Australian lamb meat. Meat Sci. 2014, 96, 1016–1024. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Cao, Y.; Hou, M.; Zhao, R.; Chen, Y.; Li, J. Genome-wide association analysis identifies genetic variants associated with muscle fatty acids and amino acids in grass carp (Ctenopharyngodon idella). Agric. Commun. 2024, 2, 100043. [Google Scholar] [CrossRef]
- González-Becerra, K.; Ramos-Lopez, O.; Barrón-Cabrera, E.; Riezu-Boj, J.I.; Milagro, F.I.; Martínez-López, E.; Martínez, J.A. Fatty acids, epigenetic mechanisms and chronic diseases: A systematic review. Lipids Health Dis. 2019, 18, 178. [Google Scholar] [CrossRef]
- Ji, X.; Cao, Z.; Hao, Q.; He, M.; Cang, M.; Yu, H.; Ma, Q.; Li, X.; Bao, S.; Wang, J.; et al. Effects of New Mutations in BMPRIB, GDF9, BMP15, LEPR, and B4GALNT2 Genes on Litter Size in Sheep. Vet. Sci. 2023, 10, 258. [Google Scholar] [CrossRef]
- Pothakam, N.; Supakankul, P.; Norseeda, W.; Liu, G.; Teltathum, T.; Naraballobh, W.; Khamlor, T.; Sringarm, K.; Mekchay, S. Association of adipocytokine IL-1A and IL-6 genes with intramuscular fat content and fatty acid composition in pigs. Meat Sci. 2021, 179, 108554. [Google Scholar] [CrossRef]
- Zappaterra, M.; Luise, D.; Zambonelli, P.; Mele, M.; Serra, A.; Costa, L.N.; Davoli, R. Association study between backfat fatty acid composition and SNPs in candidate genes highlights the effect of FASN polymorphism in large white pigs. Meat Sci. 2019, 156, 75–84. [Google Scholar] [CrossRef]
- Kim, J.S.; Ingale, S.L.; Lee, S.H.; Choi, Y.H.; Kim, E.H.; Lee, D.C.; Chae, B.J. Impact of dietary fat sources and feeding level on adipose tissue fatty acids composition and lipid metabolism related gene expression in finisher pigs. Anim. Feed Sci. Technol. 2014, 196, 60–67. [Google Scholar] [CrossRef]
- Zhao, Z.; Bai, Y.; Tian, H.; Shi, B.; Li, X.; Luo, Y.; Wang, J.; Hu, J.; Abbas Raza, S.H. Interference with ACSL1 gene in bovine adipocytes: Transcriptome profiling of circRNA related to unsaturated fatty acid production. Genomics 2021, 113, 3967–3977. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Abbas Raza, S.H.; Tian, H.; Shi, B.; Luo, Y.; Wang, J.; Liu, X.; Li, S.; Bai, Y.; Hu, J. Effects of overexpression of ACSL1 gene on the synthesis of unsaturated fatty acids in adipocytes of bovine. Arch. Biochem. Biophys. 2020, 695, 108648. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Bernlohr, D.A. Metabolic functions of FABPs—Mechanisms and therapeutic implications. Nat. Reviews. Endocrinol. 2015, 11, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Nowowiejska, J.; Baran, A.; Flisiak, I. Fatty Acid-Binding Proteins in Psoriasis—A Review. Metabolites 2022, 12, 833. [Google Scholar] [CrossRef]
- Veerkamp, J.H.; Van-Kuppevelt, T.H.; Maatman, R.G.; Prinsen, C.F. Structural and functional aspects of cytosolic fatty acid-binding proteins. Prostaglandins Leukot. Essent. Fat. Acids 1993, 49, 887–906. [Google Scholar] [CrossRef]
- Lee, S.H.; Werf, J.H.; Lee, S.H.; Park, E.W.; Oh, S.J.; Gibson, J.P.; Thompson, J.M. Genetic polymorphisms of the bovine fatty acid binding protein 4 gene are significantly associated with marbling and carcass weight in Hanwoo (Korean Cattle). Anim. Genet. 2010, 41, 442–444. [Google Scholar] [CrossRef]
- Floresta, G.; Patamia, V.; Zagni, C.; Rescifina, A. Adipocyte fatty acid binding protein 4 (FABP4) inhibitors. An update from 2017 to early 2022. Eur. J. Med. Chem. 2022, 240, 114604. [Google Scholar] [CrossRef]
- Storch, J.; Thumser, A.E. The fatty acid transport function of fatty acid-binding proteins. Biochim. Biophys. Acta 2000, 1486, 28–44. [Google Scholar] [CrossRef]
- Yonekura, S.; Hirota, S.; Miyazaki, H.; Tokutake, Y. Subcellular Localization and Polymorphism of Bovine FABP4 in Bovine Intramuscular Adipocytes. Anim. Biotechnol. 2016, 27, 96–103. [Google Scholar] [CrossRef]
- Yin, B.Z.; Fang, J.C.; Zhang, J.S.; Zhang, L.M.; Xu, C.; Xu, H.Y.; Shao, J.; Xia, G.J. Correlations between single nucleotide polymorphisms in FABP4 and meat quality and lipid metabolism gene expression in Yanbian yellow cattle. PLoS ONE 2020, 15, e0234328. [Google Scholar] [CrossRef]
- Ockner, R.K.; Manning, J.A.; Poppenhausen, R.B.; Ho, W.K. A binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science 1972, 177, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Y.; Cheng, G.; Cheng, Z.X.; Bao, C.; Yamada, T.; Cao, G.F.; Bao, S.Q.; Schreurs, N.M.; Zan, L.S.; Tong, B. Association of variants in FABP4, FASN, SCD, SREBP1 and TCAP genes with intramuscular fat, carcass traits and body size in Chinese Qinchuan cattle. Meat Sci. 2022, 192, 108882. [Google Scholar] [CrossRef] [PubMed]
- Gerbens, F.; Jansen, A.; Van Erp, A.J.; Harders, F.; Meuwissen, T.H.; Rettenberger, G.; Veerkamp, J.H.; Te Pas, M.F. The adipocyte fatty acid-binding protein locus: Characterization and association with intramuscular fat content in pigs. Mamm. Genome Off. J. Int. Mamm. Genome Soc. 1998, 9, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhou, H.; Hu, J.; Luo, Y.; Hickford, J.G.H. Variation in the FABP4 gene affects carcass and growth traits in sheep. Meat Sci. 2018, 145, 334–339. [Google Scholar] [CrossRef]
- Pei, J.H.; Li, J.W.; Han, G.D.; He, B.Y.; Ye, Q.R. Responses of plant functional traits to stocking rate in Stipa breviflora desert steppe. Chin. J. Grassl. 2024, 1–10. [Google Scholar] [CrossRef]
- NY/T 1564-2021; Code of Practice for Livestock and Poultry Meat Fabrication Sheep and Goat Meat. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2021.
- Wang, J.; Yu, X.; Cao, X.; Tan, L.; Jia, B.; Chen, R.; Li, J. GAPDH: A common housekeeping gene with an oncogenic role in pan-cancer. Comput. Struct. Biotechnol. J. 2023, 21, 4056–4069. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gabriel, S.; Ziaugra, L.; Tabbaa, D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr. Protoc. Hum. Genet. 2009, 60, 2.12.1–2.12.18. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- He, P.; Lei, Y.; Zhang, K.; Zhang, R.; Bai, Y.; Li, Z.; Jia, L.; Shi, J.; Cheng, Q.; Ma, Y.; et al. Dietary oregano essential oil supplementation alters meat quality, oxidative stability, and fatty acid profiles of beef cattle. Meat Sci. 2023, 205, 109317. [Google Scholar] [CrossRef]
- Dugan, M.E.R.; Wood, J.D. Letter to the editor. Meat Sci. 2018, 143, 268. [Google Scholar] [CrossRef] [PubMed]
- NY/T 3469-2019; Operating Procedures of Livestock and Poultry Slaughtering Sheep and Goat. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2019.
- Giller, K.; Sinz, S.; Messadene-Chelali, J.; Marquardt, S. Maternal and direct dietary polyphenol supplementation affect growth, carcass and meat quality of sheep and goats. Animal 2021, 15, 100333. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.5-2016; National Standard for Food Safety-Determination of Protein in Food. National Health and Family Planning Commission and the State Food and Drug Administration of the People’s Republic of China: Beijing, China, 2016.
- Lorenzo, J.M.; Carballo, J. Changes in physico-chemical properties and volatile compounds throughout the manufacturing process of dry-cured foal loin. Meat Sci. 2015, 99, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.H.; Zhou, Y.; Wang, G.Y.; Zhu, R.J.; Ge, C.R.; Liao, G.Z. Changes in the physicochemical properties and volatile flavor compounds of dry-cured Chinese Laowo ham during processing. J. Food Process. Preserv. 2020, 44, e14593. [Google Scholar] [CrossRef]
- Khan, A.; Fornes, O.; Stigliani, A.; Gheorghe, M.; Castro-Mondragon, J.A.; Van-Der Lee, R.; Bessy, A.; Chèneby, J.; Kulkarni, S.R.; Tan, G.; et al. JASPAR 2018: Update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 2018, 46, D260–D266. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
- Guo, X.; Li, T.; Lu, D.; Yamada, T.; Li, X.; Bao, S.; Liu, J.; Borjigin, G.; Cang, M.; Tong, B. Effects of the Expressions and Variants of the CAST Gene on the Fatty Acid Composition of the Longissimus Thoracis Muscle of Grazing Sonid Sheep. Animals 2023, 13, 195. [Google Scholar] [CrossRef]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef]
- Stejskal, D.; Karpisek, M. Adipocyte fatty acid binding protein in a Caucasian population: A new marker of metabolic syndrome? Eur. J. Clin. Investig. 2006, 36, 621–625. [Google Scholar] [CrossRef]
- Shin, S.C.; Heo, J.P.; Chung, E.R. Genetic variants of the FABP4 gene are associated with marbling scores and meat quality grades in Hanwoo (Korean cattle). Mol. Biol. Rep. 2012, 39, 5323–5330. [Google Scholar] [CrossRef]
- Pu, M.R.; Zhang, D.Y.; Zhao, L.M.; Xu, D.; Ma, Z.W.; Han, K.C.; Tian, H.B. Polymorphisms of PRKAA1 and FABP4 genes and their association with feed efficiency in Hu sheep. Small Rumin. Res. 2024, 240, 107377. [Google Scholar] [CrossRef]
- He, S.; Zhang, Z.; Lu, W. Natural promoters and promoter engineering strategies for metabolic regulation in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2023, 50, kuac029. [Google Scholar] [CrossRef] [PubMed]
- Fitz, E.; Wanka, F.; Seiboth, B. The Promoter Toolbox for Recombinant Gene Expression in Trichoderma reesei. Front. Bioeng. Biotechnol. 2018, 6, 135. [Google Scholar] [CrossRef]
- Vidal, L.; Lebrun, E.; Park, Y.K.; Mottet, G.; Nicaud, J.M. Bidirectional hybrid erythritol-inducible promoter for synthetic biology in Yarrowia lipolytica. Microb. Cell Factories 2023, 22, 7. [Google Scholar] [CrossRef]
- Wray, G.A. The evolutionary significance of cis-regulatory mutations. Nat. Rev. Genet. 2007, 8, 206–216. [Google Scholar] [CrossRef]
- Bell, R.J.; Rube, H.T.; Xavier-Magalhães, A.; Costa, B.M.; Mancini, A.; Song, J.S.; Costello, J.F. Understanding TERT Promoter Mutations: A Common Path to Immortality. Mol. Cancer Res. MCR 2016, 14, 315–323. [Google Scholar] [CrossRef]
- Otto, J.R.; Pewan, S.B.; Edmunds, R.C.; Mwangi, F.W.; Kinobe, R.T.; Adegboye, O.A.; Malau-Aduli, A.E.O. Differential expressions of FASN, SCD, and FABP4 genes in the ribeye muscle of omega-3 oil-supplemented Tattykeel Australian White lambs. BMC Genom. 2023, 24, 666. [Google Scholar] [CrossRef]
- Jia, H.J.; Zhu, J.H.; Yi, L.L. Research progress on the physiological function of Lysine and its application in pig production. Feed Res. 2007, 47, 154–159. [Google Scholar] [CrossRef]
- Tornesello, M.L.; Cerasuolo, A.; Starita, N.; Amiranda, S.; Bonelli, P.; Tuccillo, F.M.; Buonaguro, F.M.; Buonaguro, L.; Tornesello, A.L. Reactivation of telomerase reverse transcriptase expression in cancer: The role of TERT promoter mutations. Front. Cell Dev. Biol. 2023, 11, 1286683. [Google Scholar] [CrossRef]
- Mayr, C. What Are 3′ UTRs Doing? Cold Spring Harb. Perspect. Biol. 2019, 11, a034728. [Google Scholar] [CrossRef]
- Chen, L.; Heikkinen, L.; Wang, C.; Yang, Y.; Sun, H.; Wong, G. Trends in the development of miRNA bioinformatics tools. Brief. Bioinform. 2019, 20, 1836–1852. [Google Scholar] [CrossRef] [PubMed]
- Rykova, E.; Ershov, N.; Damarov, I.; Merkulova, T. SNPs in 3′UTR miRNA Target Sequences Associated with Individual Drug Susceptibility. Int. J. Mol. Sci. 2022, 23, 13725. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, P.; Yu, D.; Du, F.; Zhu, A.; Li, Q.; Zhang, P.; Lin, D.; Xu, B. A single-nucleotide polymorphism in the 3′UTR region of the adipocyte fatty acid binding protein 4 gene is associated with prognosis of triple-negative breast cancer. Oncotarget 2016, 7, 18984–18998. [Google Scholar] [CrossRef][Green Version]
- Apaya, M.K.; Hsiao, P.W.; Yang, Y.C.; Shyur, L.F. Deregulating the CYP2C19/Epoxy-Eicosatrienoic Acid-Associated FABP4/FABP5 Signaling Network as a Therapeutic Approach for Metastatic Triple-Negative Breast Cancer. Cancers 2020, 12, 199. [Google Scholar] [CrossRef]
- Zhou, L.; Raza, S.H.A.; Ma, B.; Shater, A.F.; Mohammedsaleh, Z.M.; Jahejo, A.R.; Li, J.; Gui, L. Mutations in FGFR1 were associated with growth traits in sheep (Ovis aries). Anim. Biotechnol. 2023, 34, 1–7. [Google Scholar] [CrossRef]
- Lunesu, M.F.; Battacone, G.; Mellino, M.R.; Carta, S.; Pulina, G.; Nudda, A. The heavy suckling lamb of Sarda dairy sheep and its crossbreed with Dorper rams: Performance, meat quality and consumer perceptions. Meat Sci. 2023, 204, 109234. [Google Scholar] [CrossRef]
- Rule, D.C.; MacNeil, M.D.; Short, R.E. Influence of sire growth potential, time on feed, and growing-finishing strategy on cholesterol and fatty acids of the ground carcass and longissimus muscle of beef steers. J. Anim. Sci. 1997, 75, 1525–1533. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Butler, K.L.; Jacob, R.H.; Pethick, D.W.; Ball, A.J.; Edwards, J.E.; Geesink, G.; Hopkins, D.L. Health beneficial long chain omega-3 fatty acid levels in Australian lamb managed under extensive finishing systems. Meat Sci. 2014, 96, 1104–1110. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, X.; Zhang, J.; Zhang, Q.; Tana. Comparison of Three Feeding Regimens on Blood Fatty Acids Metabolites of Wujumqin Sheep in Inner Mongolia. Animals 2021, 11, 1080. [Google Scholar] [CrossRef]
| Gene | Primer Sequence (5′-3′) | Product Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| GAPDH | F: TTCCACGGCACAGTCAAGG | 114 | 60.5 |
| R: CTCAGCACCAGCATCACCC | |||
| FABP4 | F: AATACTGAGATGTCCTTC | 140 | 53.8 |
| R: TTTATGGTGGTTGATTTC |
| Fatty Acid Composition (mg/100 g) | g.57765038C>T | g.57765008A>G-LD1 | g.57764667T>C | g.57764436T>G | g.57764242G>A | g.57757988A>G | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Genotype | Genotype | Genotype | Genotype | Genotype | ||||||||
| CT (51) 1 | TT (216) | AG (49) | GG (218) | TT (245) | TC (25) | TT (253) | TG (18) | GG (89) | GA (133) | AA (49) | AA (247) | AG (23) | |
| C16:1 | 0.20 ± 0.02 a | 0.25 ± 0.01 b | 0.20 ± 0.02 a | 0.25 ± 0.01 b | 0.24 ± 0.01 | 0.25 ± 0.04 | 0.24 ± 0.01 | 0.30 ± 0.04 | 0.25 ± 0.02 | 0.24 ± 0.01 | 0.22 ± 0.02 | 0.24 ± 0.01 | 0.25 ± 0.04 |
| C18:1n9t | 1.02 ± 0.29 | 1.04 ± 0.15 | 1.20 ± 0.30 | 1.43 ± 0.15 | 1.37 ± 0.14 | 1.61 ± 0.45 | 1.46 ± 0.14 a | 0.32 ± 0.04 b | 1.18 ± 0.21 | 1.48 ± 0.20 | 1.48 ± 0.34 | 1.38 ± 0.14 | 1.48 ± 0.45 |
| C18:1n9c | 0.56 ± 0.21 a | 1.04 ± 0.18 b | 0.58 ± 0.22 a | 1.40 ± 0.18 b | 1.20 ± 0.16 | 1.65 ± 0.64 | 1.20 ± 0.16 | 1.71 ± 0.61 | 1.71 ± 0.32 a | 0.90 ± 0.18 b | 1.30 ± 0.37 ab | 1.19 ± 0.16 | 1.78 ± 0.69 |
| C18:2n6c | 0.76 ± 0.03 | 0.76 ± 0.02 | 0.75 ± 0.03 | 0.76 ± 0.02 | 0.75 ± 0.01 | 0.83 ± 0.06 | 0.75 ± 0.01 a | 0.90 ± 0.07 b | 0.80 ± 0.03 a | 0.73 ± 0.02 b | 0.76 ± 0.03 ab | 0.75 ± 0.01 | 0.84 ± 0.06 |
| C18:3n3 | 0.21 ± 0.01 | 0.22 ± 0.00 | 0.21 ± 0.01 | 0.22 ± 0.00 | 0.21 ± 0.00 | 0.24 ± 0.02 | 0.21 ± 0.00 | 0.23 ± 0.01 | 0.23 ± 0.01 a | 0.21 ± 0.01 b | 0.21 ± 0.01 ab | 0.21 ± 0.00 | 0.24 ± 0.02 |
| C20:3n3 | 0.33 ± 0.03 | 0.28 ± 0.01 | 0.33 ± 0.03 | 0.28 ± 0.01 | 0.28 ± 0.01 | 0.37 ± 0.04 | 0.31 ± 0.01 a | 0.07 ± 0.04 b | 0.28 ± 0.02 | 0.29 ± 0.02 | 0.30 ± 0.03 | 0.28 ± 0.01 | 0.37 ± 0.04 |
| C22:6n3 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 a | 0.03 ± 0.00 b | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 a | 0.03 ± 0.00 b |
| Chemical Composition | g.57764436T>G | g.57764242G>A | g.57758026G>A | |||||
|---|---|---|---|---|---|---|---|---|
| Genotype | Genotype | Genotype | ||||||
| TT (92) 1 | TG (19) | GG (18) | GA (55) | AA (38) | GG (96) | GA (15) | ||
| Crude protein (%) | 21.16 ± 0.13 | 20.66 ± 0.33 | 20.88 ± 0.22 a | 21.13 ± 0.17 ab | 21.6 ± 0.27 b | 21.15 ± 0.12 | 20.27 ± 0.57 | |
| Crude fat (g/100 g) | 6.96 ± 0.12 | 6.94 ± 0.31 | 7.43 ± 0.21 a | 6.75 ± 0.15 b | 6.59 ± 0.27 b | 6.93 ± 0.12 | 7.75 ± 0.28 | |
| Free amino acid (ng/0.1 g) | Asp | 49.66 ± 2.67 a | 95.74 ± 58.72 b | 83.34 ± 34.68 a | 47.08 ± 3.71 b | 50.90 ± 4.27 ab | 54.33 ± 5.98 | 42.39 ± 15.99 |
| Ala | 2042.17 ± 35.20 | 2204.71 ± 279.04 | 2118.71 ± 162.87 | 2027.49 ± 48.53 | 2073.87 ± 61.66 | 2075.41 ± 40.78 a | 1696.45 ± 131.76 b | |
| Lys | 1245.22 ± 28.23 a | 1464.63 ± 128.07 b | 1348.55 ± 85.35 | 1235.97 ± 45.24 | 1268.45 ± 30.58 | 1255.31 ± 28.99 | 1404.62 ± 129.27 | |
| Carcass Trait 1 | g.57764667T>C | g.57764242G>A | g.57757988A>G | ||||
|---|---|---|---|---|---|---|---|
| Genotype | Genotype | Genotype | |||||
| TT (95) 2 | TC (16) | GG (18) | GA (55) | AA (38) | AA (95) | AG (16) | |
| L* (lightness) | 23.01 ± 0.31 | 24.28 ± 0.73 | 23.22 ± 0.49 | 23.12 ± 0.39 | 23.13 ± 0.85 | 23.01 ± 0.31 | 24.28 ± 0.73 |
| a* (redness) | 13.98 ± 0.27 | 13.80 ± 0.67 | 13.75 ± 0.63 | 14.03 ± 0.27 | 14.13 ± 0.48 | 13.98 ± 0.27 | 13.80 ± 0.67 |
| b* (yellowness) | 5.07 ± 0.14 | 4.93 ± 0.35 | 5.20 ± 0.22 | 5.10 ± 0.19 | 4.69 ± 0.24 | 5.07 ± 0.14 | 4.93 ± 0.35 |
| pH1 (at 45 min) | 6.56 ± 0.03 | 6.56 ± 0.09 | 6.55 ± 0.05 | 6.53 ± 0.03 | 6.66 ± 0.06 | 6.56 ± 0.03 | 6.56 ± 0.09 |
| pH2 (at 24 h) | 5.41 ± 0.01 | 5.46 ± 0.05 | 5.41 ± 0.01 | 5.43 ± 0.02 | 5.39 ± 0.02 | 5.41 ± 0.01 | 5.46 ± 0.05 |
| Carcass weight (kg) | 11.10 ± 0.24 a | 9.33 ± 0.80 b | 11.84 ± 0.58 a | 10.87 ± 0.31 ab | 9.89 ± 0.41 b | 11.10 ± 0.24 a | 9.33 ± 0.80 b |
| Live weight (kg) | 25.67 ± 0.45 A | 21.14 ± 1.50 B | 26.53 ± 0.93 a | 25.15 ± 0.61 ab | 23.56 ± 0.97 b | 25.67 ± 0.45 A | 21.14 ± 1.50 B |
| Dressing percentage | 0.43 ± 0.01 | 0.44 ± 0.03 | 0.45 ± 0.02 | 0.43 ± 0.01 | 0.42 ± 0.01 | 0.43 ± 0.01 | 0.44 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, J.; Li, H.; Guo, Z.; Li, T.; Yamada, T.; Li, X.; Bao, S.; Da, L.; Borjigin, G.; Cang, M.; et al. Effect of FABP4 Gene Polymorphisms on Fatty Acid Composition, Chemical Composition, and Carcass Traits in Sonid Sheep. Animals 2025, 15, 226. https://doi.org/10.3390/ani15020226
Xiang J, Li H, Guo Z, Li T, Yamada T, Li X, Bao S, Da L, Borjigin G, Cang M, et al. Effect of FABP4 Gene Polymorphisms on Fatty Acid Composition, Chemical Composition, and Carcass Traits in Sonid Sheep. Animals. 2025; 15(2):226. https://doi.org/10.3390/ani15020226
Chicago/Turabian StyleXiang, Jiada, Haofan Li, Zhaoxin Guo, Terigele Li, Takahisa Yamada, Xihe Li, Siqin Bao, Lai Da, Gerelt Borjigin, Ming Cang, and et al. 2025. "Effect of FABP4 Gene Polymorphisms on Fatty Acid Composition, Chemical Composition, and Carcass Traits in Sonid Sheep" Animals 15, no. 2: 226. https://doi.org/10.3390/ani15020226
APA StyleXiang, J., Li, H., Guo, Z., Li, T., Yamada, T., Li, X., Bao, S., Da, L., Borjigin, G., Cang, M., & Tong, B. (2025). Effect of FABP4 Gene Polymorphisms on Fatty Acid Composition, Chemical Composition, and Carcass Traits in Sonid Sheep. Animals, 15(2), 226. https://doi.org/10.3390/ani15020226





