Morphological and Molecular Identification of Obligatory Myiasis-Causing Species in Wild Cervids in Croatia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
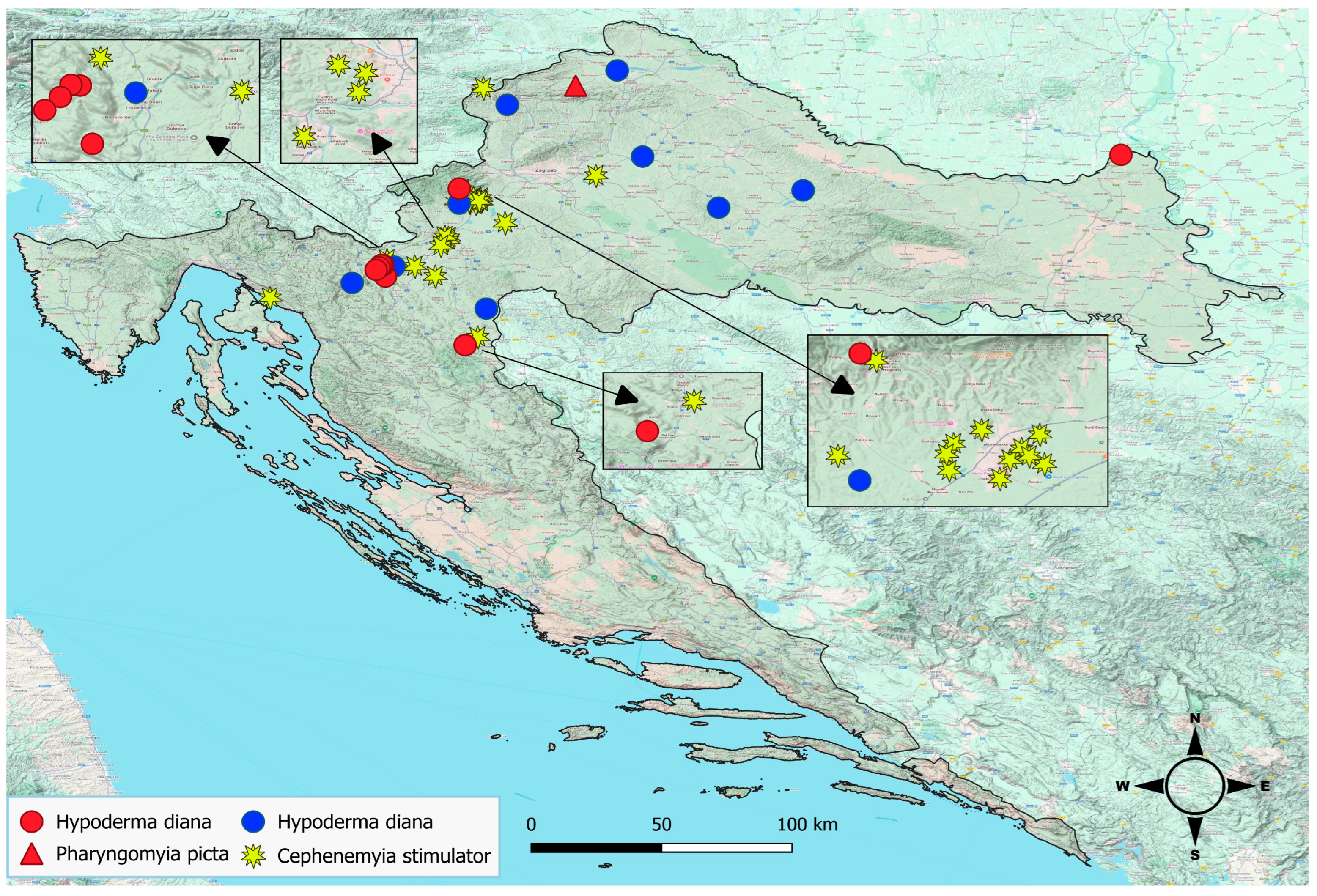
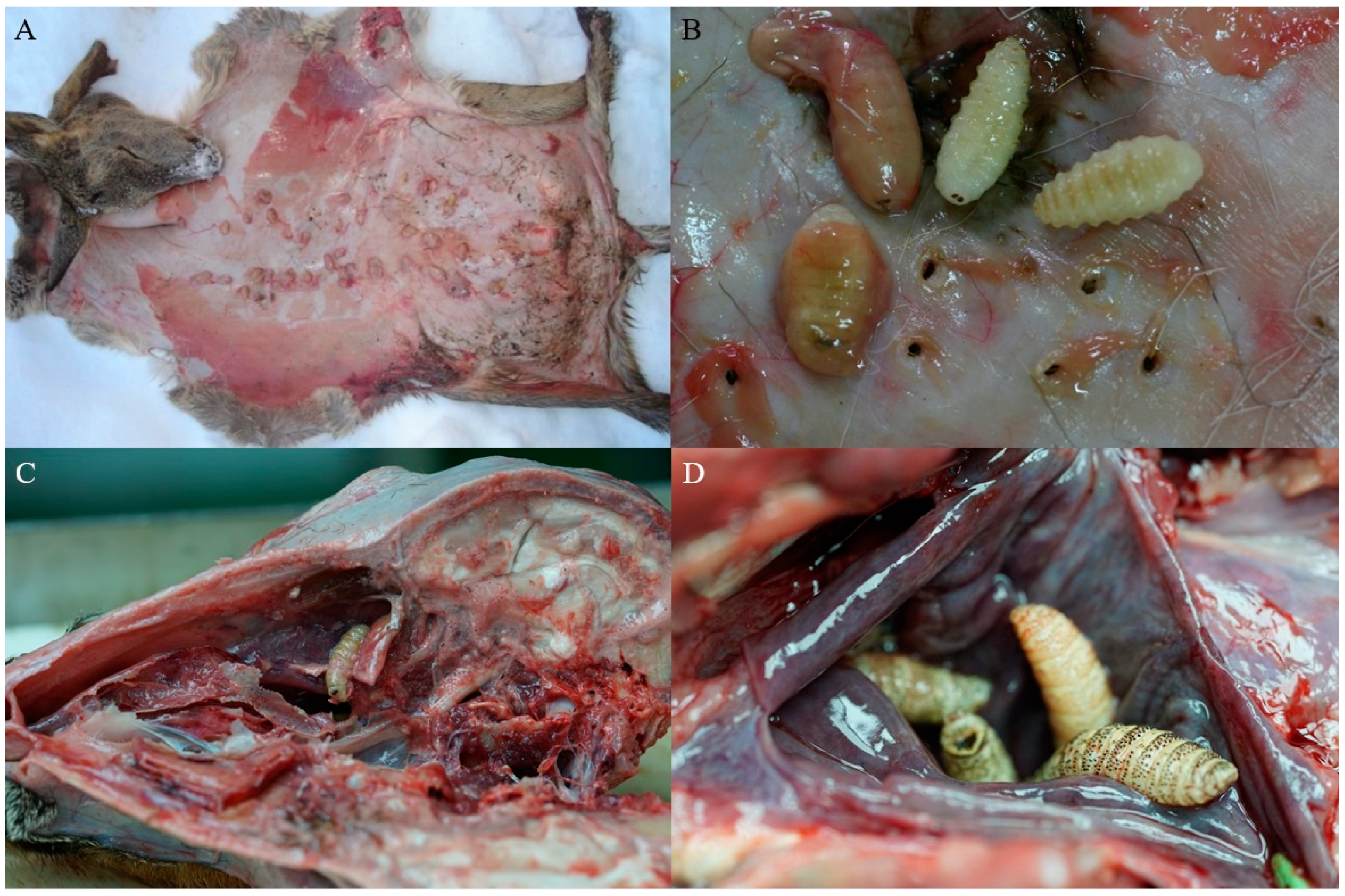
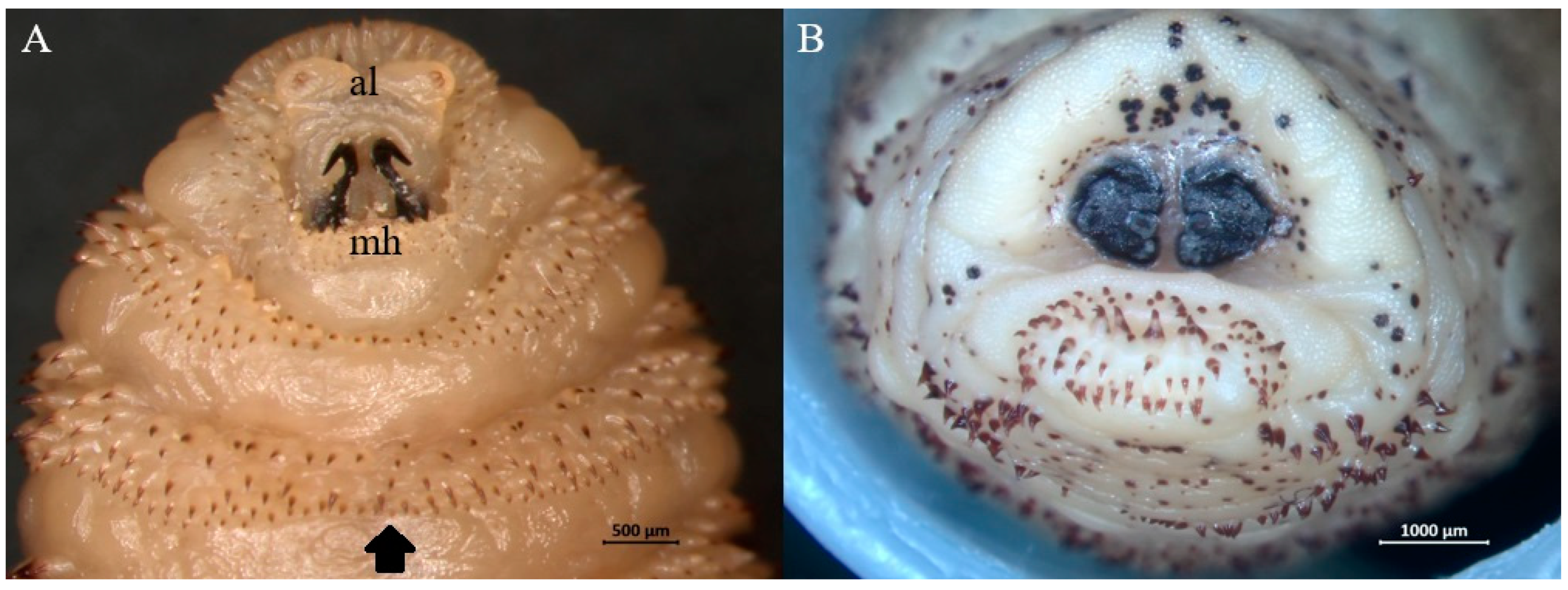
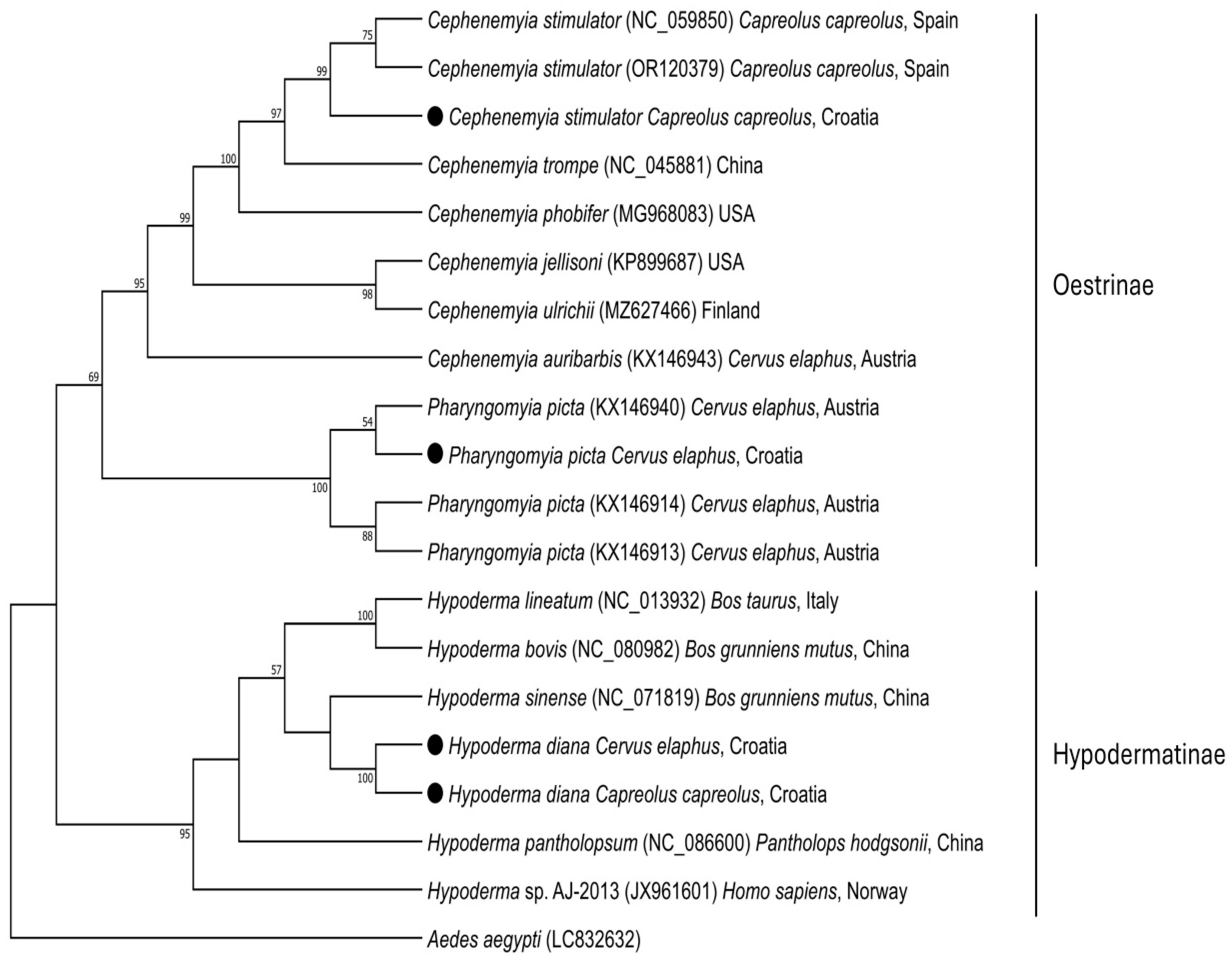
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colwell, D.D. Bot flies and warble flies (Order Diptera: Family Oestridae). In Parasitic Diseases of Wild Mammals, 2nd ed.; Samuel, W.M., Pybus, M.J., Kocan, A.A., Eds.; Iowa State University Press: Ames, IA, USA, 2001; Volume 2, pp. 46–71. [Google Scholar]
- Scholl, P.J.; Colwell, D.D.; Cepeda-Palacios, R. Myiasis (Muscoidea, Oestroidea). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 383–419. [Google Scholar]
- Hassan, M.-u.; Khan, M.N.; Abubakar, M.; Waheed, H.M.; Iqbal, Z.; Hussain, M. Bovine hypodermosis-a global aspect. Trop. Anim. Health Prod. 2010, 42, 1615–1625. [Google Scholar] [CrossRef]
- El-Tahawy, A.S. The prevalence of selected diseases and syndromes affecting Barki sheep with special emphasis on their economic impact. Small Ruminant Res. 2010, 90, 83–87. [Google Scholar] [CrossRef]
- Vicente, J.; Fierro, Y.; Martínez, M.; Gortázar, C. Long-term epidemiology, effect on body condition and interspecific interactions of concomitant infection by nasopharyngeal bot fly larvae (Cephenemyia auribarbis and Pharyngomyia picta, Oestridae) in a population of Iberian red deer (Cervus elaphus hispanicus). Parasitology 2004, 129, 349–361. [Google Scholar] [CrossRef]
- Ortiz-Leal, I.; Torres, M.V.; López-Beceiro, A.; Sanchez-Quinteiro, P.; Fidalgo, L. Dissecting the Effects of Cephenemyia stimulator on the Olfactory Turbinates and Nasopharynx of Roe Deers (Capreolus capreolus). Animals 2024, 14, 1297. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Colwell, D.D.; Traversa, D.; Stevens, J.R. Species identification of Hypoderma affecting domestic and wild ruminants by morphological and molecular characterization. Med. Vet. Entomol. 2003, 17, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Morrondo, P.; Pajares, G.; Arias, M.S.; Martínez-Calabuig, N.; Remesar, S.; García-Dios, D.; Díaz, P.; López, C.M.; Panadero, R.; Díez-Baños, P. An Update on Cephenemyiosis in the European Roe Deer: Emergent Myiasis in Spain. Animals 2021, 11, 3382. [Google Scholar] [CrossRef]
- Granados, J.E.; Forte-Gil, D.; Ramos, B.; Cano-Manuel, F.J.; Soriguer, R.C.; Fandos, P.; Pérez, J.M. First record of Pharyngomyia picta (Diptera: Oestridae) parasitizing Cervus elaphus in Sierra Nevada National Park. Parasitol. Res. 2021, 120, 3895–3898. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Calabuig, N.; Vieira-Pinto, M.; López, C.M.; Remesar, S.; Panadero, R. Cephenemyia stimulator (Diptera: Oestridae) myiasis in a roe deer (Capreolus capreolus) from Portugal. Vet. Parasitol. Reg. Stud. Rep. 2023, 41, 100883. [Google Scholar] [CrossRef]
- Singh, A.; Singh, Z. Incidence of myiasis among humans-a review. Parasitol. Res. 2015, 114, 3183–3199. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, V.; Finkelmeier, F.; Verhoff, M.A. Jens Amendt. Myiasis in humans-a global case report evaluation and literature analysis. Parasitol. Res. 2019, 118, 389–397. [Google Scholar] [CrossRef]
- Otranto, D.; Stevens, J.R.; Brianti, E.; Dorchies, P. Human and livestock migrations: A history of bot fly biodiversity in the Mediterranean region. Trends Parasitol. 2006, 22, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Kusak, J.; Krapinec, K. Ungulates and their management in Croatia. In European Ungulates and Their Management in the 21st Century, 1st ed.; Apollonio, M., Andersen, R., Putman, R., Eds.; Cambridge University Press: Cambridge, UK, 2010; Volume 1, pp. 527–539. [Google Scholar]
- Linnell, J.D.C.; Zachos, F.E. Status and distribution patterns of European ungulates: Genetics, population history and conservation. In Ungulate Management in Europe: Problems and Practices, 1st ed.; Putman, R., Apollonio, M., Andersen, R., Eds.; Cambridge University Press: Cambridge, UK, 2011; Volume 1, pp. 12–53. [Google Scholar]
- Konjević, D.; Janicki, Z.; Slavica, A.; Severin, K. Nosna štrkljivost u srna (Capreolus capreolus L.). Vet Stanica 2006, 37, 153–159. [Google Scholar]
- Batinjan, M. Morphological Characterization of Deer Botfly from the Airways of Roe Deer. Rector’s Award, FVMUZ, Heinzelova 55, 10000 Zagreb, 4.10.2022. Available online: https://apps.unizg.hr/rektorova-nagrada/javno/radovi/1545/preuzmi (accessed on 4 December 2024).
- Kusak, J.; Špičić, S.; Slijepčević, V.; Bosnić, S.; Rajković Janje, R.; Duvnjak, S.; Sindičić, M.; Majnarić, D.; Cvetnić, Ž.; Huber, Đ. Health status of red deer and roe deer in Gorski kotar, Croatia. Vet. Arhiv. 2012, 82, 59–73. Available online: https://hrcak.srce.hr/77399 (accessed on 4 December 2024).
- Radhakrishnan, S.; Ajithkumar, K.G.; Ravindran, R.; Rajagopal, K. First record in South Asia of deer throat bot fly larvae Pharyngomyia picta (Meigen, 1824) (Diptera: Oesteridae) from Sambar deer (Rusa unicolor), a new host record. Trop. Biomed. 2012, 29, 265–269. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Stevens, J.R. Molecular approaches to the study of myiasis-causing larvae. Int. J. Parasitol. 2002, 32, 1345–1360. [Google Scholar] [CrossRef] [PubMed]
- Richter, S. Parasitska fauna srne (Capreolus capreolus L.) u NR Hrvatskoj. Vet. Arh. Zagreb. 1959, 29, 34–45. [Google Scholar]
- Pupić-Bakrač, A.; Pupić-Bakrač, J.; Škara Kolega, M.; Beck, R. Human ophthalmomyiasis caused by Oestrus ovis—First report from Croatia and review on cases from Mediterranean countries. Parasitol. Res. 2020, 119, 783–793. [Google Scholar] [CrossRef]
- Martínez-Gómez, F.; Hernández-Rodríguez, S.; Ruiz-Sánchez, P.; Molina-Rodero, R.; Martínez-Moreno, A. Hypodermosis in the red deer Cervus elaphus in Cordoba, Spain. Med. Vet. Entomol. 1990, 4, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.S.; Imre, M.I.; Hotea, I.O.; Imre, K.; Sorescu, I.D.; Andrei, S.; Onita, P.; Oprescu, I.; Morariu, S.; Mihali, C.; et al. Prevalence of Hypoderma infestation in deer in Western Romania. Luc. Sti. Med. 2012, 19, 3. [Google Scholar]
- Borges, F.; Sybrecht, G.W.; von Samson-Himmelstjerna, G. First reported case of Hypoderma diana Brauer, 1985 (Diptera: Oestridae)-associated myiasis in a horse in Germany. Equine Vet. Educ. 2019, 31, 122–125. [Google Scholar] [CrossRef]
- Venjakob, P.L.; Vogel, C.; Clausen, P.H.; Nijhof, A.M. First report of a Hypoderma diana infestation in alpaca (Vicugna pacos) in Germany. Parasitol. Res. 2019, 118, 1963–1966. [Google Scholar] [CrossRef]
- Husvéth, B.; Egri, B. Retrospective study on the occurrence of warble fly infestation (Hypodermosis) of the red deer and roe deer in North-West Hungary (Szigetkoz, District of Ravazd and Tarjan). Int. J. Zool. Anim. Biol. 2021, 4, 1–7. [Google Scholar] [CrossRef]
- González, S.; del Rio, M.L.; Diez, M.N.; Hidalgo, M.R.; Martínez, A. Identification of Hypoderma actaeon (Diptera: Oestridae) in red deer (Cervus elaphus) from northern Spain: Microscopy study and molecular analysis. Microsc. Res. Tech. 2023, 86, 3–11. [Google Scholar] [CrossRef]
- Ahmed, H.; Ramalho Sousa, S.; Simsek, S.; Anastácio, S.; Gunyakti Kilind, S. First molecular characterization of Hypoderma actaeon in cattle and red deer (Cervus elaphus) in Portugal. Korean J. Parasitol. 2017, 55, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Panadero, R.; Varas, G.; Pajares, G.; Markina, F.; López, C.; Díaz, P.; Pérez-Creo, A.; Prieto, A.; Díez-Baños, P.; Morrondo, P. Hypoderma actaeon: An emerging myiasis in roe deer (Capreolus capreolus). Med. Vet. Entomol. 2017, 31, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Király, I.; Egri, B. Epidemiological characteristics of Cephenemyia stimulator (Clark, 1815) larval infestation in European roe deer (Capreolus capreolus) in Hungary. Acta Zool. Acad. Sci. Hung. 2007, 53, 271–279. [Google Scholar]
- Martinković, F.; Štimac, I.; Bujanić, M.; Konjević, D. First identification of Pharyngomyia picta in red deer in Republic of Croatia. In Proceedings of the 10th International Deer Biology Congress, Osijek, Hrvatska, 4–9 September 2022; p. 93. [Google Scholar]
- Draber-Mońko, A.; Bystrowski, C. Several new data from Poland on the occurrence of imagines of the hypodermatid and oestrid flies (Diptera: Hypodermatidae and Oestridae). Fragm. Faun. 2016, 59, 105–113. [Google Scholar] [CrossRef]
- Ruiz, I.; Soriguer, R.C.; Perez, J.M. Pharyngeal bot flies (Oestridae) from sympatric wild cervids in Southern Spain. J. Parasitol. 1993, 79, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Sugár, L. The occurrence of nasal throat bot flies (Oestridae) in wild ruminants in Hungary. Parasitol. Hung. 1974, 7, 181–189. [Google Scholar]
- Leitner, N.; Schwarzmann, L.; Zittra, C.; Palmieri, N.; Eigner, B.; Otranto, D.; Glawischnig, W.; Fuehrer, H.P. Morphological and molecular identification of nasopharyngeal bot fly larvae infesting red deer (Cervus elaphus) in Austria. Parasitol. Res. 2016, 115, 4417–4422. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.; Serejo, J.; Pérez, J.M.; Aranha, J.; Venâncio, C.; Vieira-Pinto, M. First Study of Pharingomyia picta and Cephanemyia auribarbis in Wild Populations of Red Deer (Cervus elaphus) in Portugal. Animals 2022, 12, 1896. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagović, E.; Jurković Žilić, D.; Pintur, K.; Hodžić, A.; Naletilić, Š.; Beck, R. Morphological and Molecular Identification of Obligatory Myiasis-Causing Species in Wild Cervids in Croatia. Animals 2025, 15, 208. https://doi.org/10.3390/ani15020208
Gagović E, Jurković Žilić D, Pintur K, Hodžić A, Naletilić Š, Beck R. Morphological and Molecular Identification of Obligatory Myiasis-Causing Species in Wild Cervids in Croatia. Animals. 2025; 15(2):208. https://doi.org/10.3390/ani15020208
Chicago/Turabian StyleGagović, Ema, Daria Jurković Žilić, Krunoslav Pintur, Adnan Hodžić, Šimun Naletilić, and Relja Beck. 2025. "Morphological and Molecular Identification of Obligatory Myiasis-Causing Species in Wild Cervids in Croatia" Animals 15, no. 2: 208. https://doi.org/10.3390/ani15020208
APA StyleGagović, E., Jurković Žilić, D., Pintur, K., Hodžić, A., Naletilić, Š., & Beck, R. (2025). Morphological and Molecular Identification of Obligatory Myiasis-Causing Species in Wild Cervids in Croatia. Animals, 15(2), 208. https://doi.org/10.3390/ani15020208






