Occurrence and Multi-Locus Genotyping of Giardia duodenalis in Black Goats from Fujian Province, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Areas and Sample Collection
2.2. Genomic DNA Extraction and PCR Amplifications
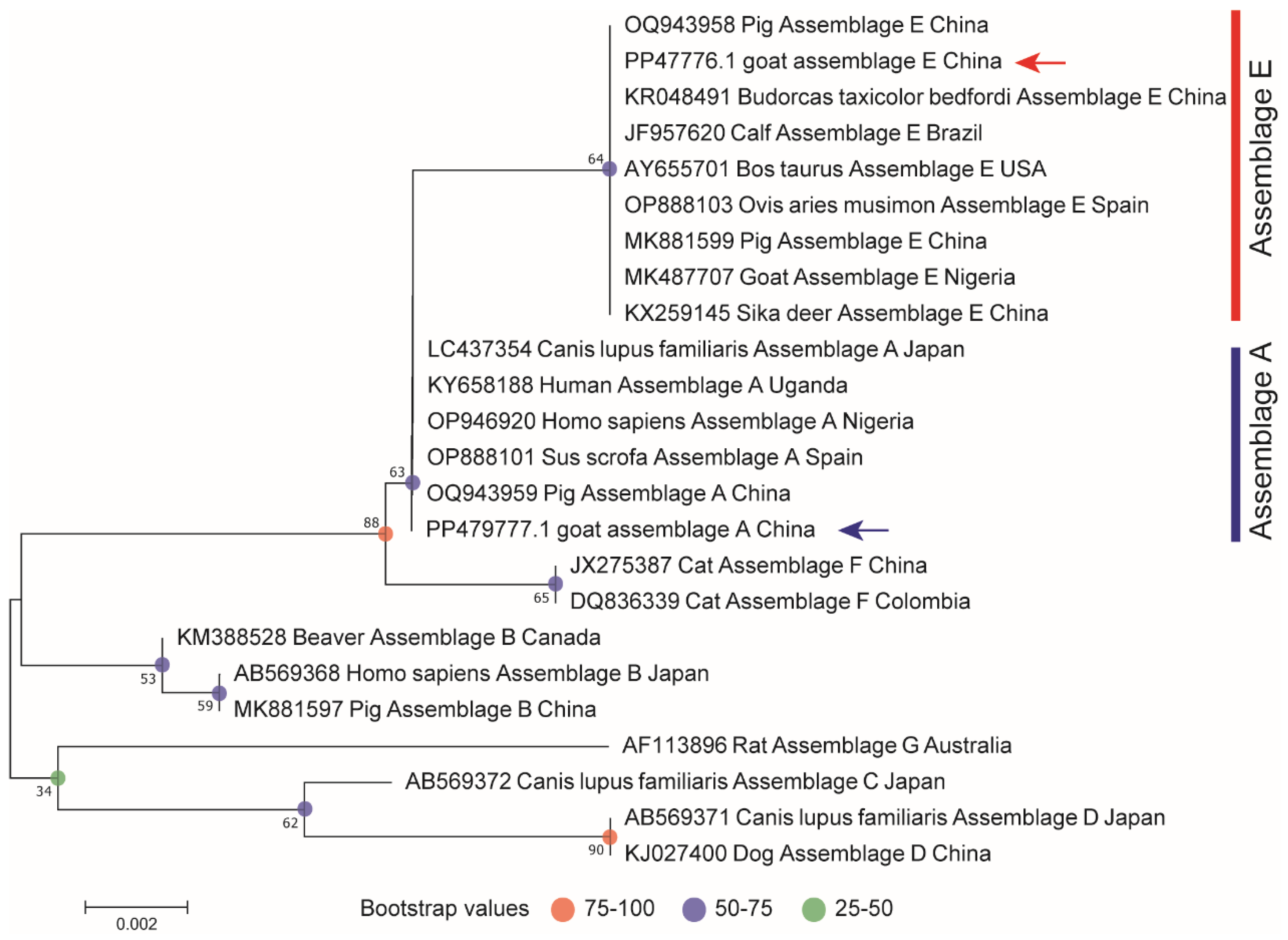
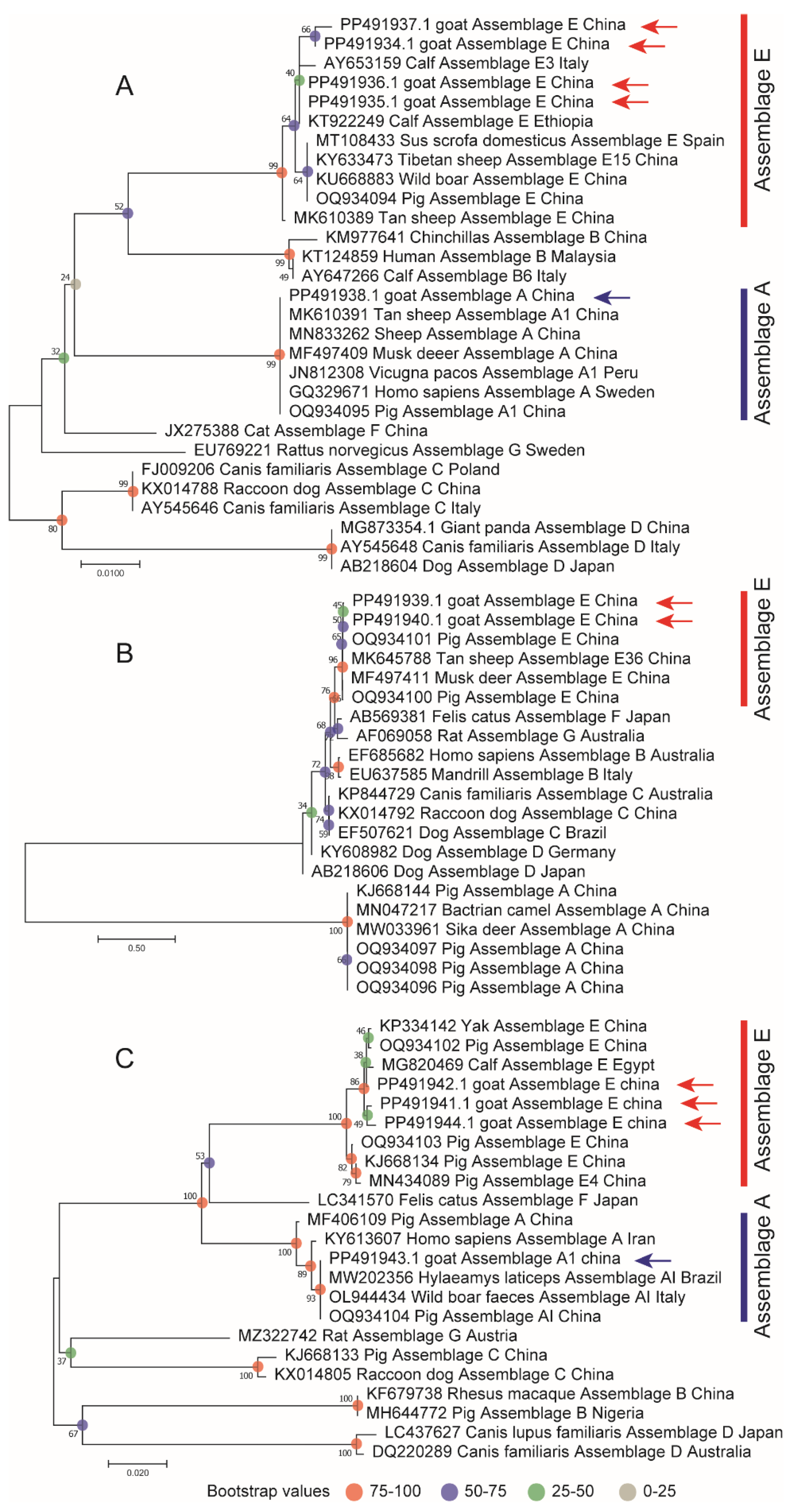
2.3. Genotype Identification
2.4. Sequencing and Phylogenetic Analysis
2.5. Statistical Analysis
3. Results
3.1. The Positivity Rate of G. duodenalis
3.2. Prevalence and Genotypic Diversity of G. duodenalis in Black Goats
3.3. Age-Elated Infection Rates of G. duodenalis
3.4. Prevalence of G. duodenalis Infection in Male and Female Black Goats
3.5. Genotyping of G. duodenalis
3.6. G. duodenalis MLST Typing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, R.C.; Monis, P.T. Variation in Giardia: Implications for taxonomy and epidemiology. Adv. Parasitol. 2004, 58, 69–137. [Google Scholar] [PubMed]
- Appelbee, A.J.; Thompson, R.C.; Olson, M.E. Giardia and Cryptosporidium in mammalian wildlife–current status and future needs. Trends Parasitol. 2005, 21, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef] [PubMed]
- Lux, L.; Ulrich, R.G.; Santos-Silva, S.; Queirós, J.; Imholt, C.; Klotz, C.; Paupério, J.; Pita, R.; Vale-Gonçalves, H.; Alves, P.C.; et al. Detection and Molecular Characterization of Giardia and Cryptosporidium spp. Circulating in Wild Small Mammals from Portugal. Animals 2023, 13, 515. [Google Scholar]
- Fan, Y.; Wang, X.; Yang, R.; Zhao, W.; Li, N.; Guo, Y.; Xiao, L.; Feng, Y. Molecular characterization of the waterborne pathogens Cryptosporidium spp., Giardia duodenalis, Enterocytozoon bieneusi, Cyclospora cayetanensis and Eimeria spp. in wastewater and sewage in Guangzhou, China. Parasites Vectors 2021, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, M.; Weible, L.J.; Champlain, J.D.; Jiang, R.Y.; Biondi, K.; Weil, A.A.; Van Voorhis, W.C.; Ojo, K.K. The Gut-Wrenching Effects of Cryptosporidiosis and Giardiasis in Children. Microorganisms 2023, 11, 2323. [Google Scholar] [CrossRef] [PubMed]
- Sprong, H.; Cacciò, S.M.; van der Giessen, J.W. Identification of zoonotic genotypes of Giardia duodenalis. PLoS Neglected Trop. Dis. 2009, 3, e558. [Google Scholar] [CrossRef]
- Thompson, R.C.; Monis, P. Giardia—From genome to proteome. Adv. Parasitol. 2012, 78, 57–95. [Google Scholar]
- Cacciò, S.M.; Thompson, R.C.; McLauchlin, J.; Smith, H.V. Unravelling Cryptosporidium and Giardia epidemiology. Trends Parasitol. 2005, 21, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, B.; Yin, J.; Yuan, Z.; Jiang, Y.; Zhang, J.; Cao, J.; Shen, Y.; Liu, H. Investigation of giardiasis in captive animals in zoological gardens with strain typing of assemblages in China. Parasitology 2021, 148, 1360–1365. [Google Scholar] [CrossRef]
- Cacciò, S.M.; Lalle, M.; Svärd, S.G. Host specificity in the Giardia duodenalis species complex, Infection, genetics and evolution. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2018, 66, 335–345. [Google Scholar] [CrossRef]
- Heyworth, M.F. Giardia duodenalis genetic assemblages and hosts. Parasite 2016, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- Kanski, S.; Weber, K.; Busch, K. Feline and canine giardiosis: An Update. Tierarztl. Prax. Ausg. K Kleintiere/Heimtiere 2023, 51, 411–421. [Google Scholar] [PubMed]
- Roxström-Lindquist, K.; Palm, D.; Reiner, D.; Ringqvist, E.; Svärd, S.G. Giardia immunity–an update. Trends Parasitol. 2006, 22, 26–31. [Google Scholar] [CrossRef]
- Amar, C.F.; East, C.L.; Gray, J.; Iturriza-Gomara, M.; Maclure, E.A.; McLauchlin, J. Detection by PCR of eight groups of enteric pathogens in 4,627 faecal samples: Re-examination of the English case-control Infectious Intestinal Disease Study (1993–1996). Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2007, 26, 311–323. [Google Scholar] [CrossRef]
- Zylberberg, H.M.; Green, P.H.; Turner, K.O.; Genta, R.M.; Lebwohl, B. Prevalence and Predictors of Giardia in the United States. Dig. Dis. Sci. 2017, 62, 432–440. [Google Scholar] [CrossRef]
- Bartolini, A.; Zorzi, G.; Besutti, V. Prevalence of intestinal parasitoses detected in Padua teaching hospital, Italy, March 2011–February 2013. Infez. Med. 2017, 25, 133–141. [Google Scholar]
- Iqbal, A.; Goldfarb, D.M.; Slinger, R.; Dixon, B.R. Prevalence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis in diarrhoeic patients in the Qikiqtani Region, Nunavut, Canada. Int. J. Circumpolar Health 2015, 74, 27713. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.; Roy, S.; Kabir, M.; Stroup, S.E.; Mondal, D.; Houpt, E.R. Giardia assemblage A infection and diarrhea in Bangladesh. J. Infect. Dis. 2005, 192, 2171–2173. [Google Scholar] [CrossRef] [PubMed]
- Volotão, A.C.; Costa-Macedo, L.M.; Haddad, F.S.; Brandão, A.; Peralta, J.M.; Fernandes, O. Genotyping of Giardia duodenalis from human and animal samples from Brazil using beta-giardin gene: A phylogenetic analysis. Acta Trop. 2007, 102, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Mahdy, A.K.; Surin, J.; Wan, K.L.; Mohd-Adnan, A.; Al-Mekhlafi, M.S.; Lim, Y.A. Giardia intestinalis genotypes: Risk factors and correlation with clinical symptoms. Acta Trop. 2009, 112, 67–70. [Google Scholar] [CrossRef]
- Traub, R.J.; Inpankaew, T.; Reid, S.A.; Sutthikornchai, C.; Sukthana, Y.; Robertson, I.D.; Thompson, R.C. Transmission cycles of Giardia duodenalis in dogs and humans in Temple communities in Bangkok—A critical evaluation of its prevalence using three diagnostic tests in the field in the absence of a gold standard. Acta Trop. 2009, 111, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, H.; Wang, R.; Zhang, L. Giardia duodenalis Infections in Humans and Other Animals in China. Front. Microbiol. 2017, 8, 2004. [Google Scholar] [CrossRef]
- Geurden, T.; Vandenhoute, E.; Pohle, H.; Casaert, S.; De Wilde, N.; Vercruysse, J.; Claerebout, E. The effect of a fenbendazole treatment on cyst excretion and weight gain in calves experimentally infected with Giardia duodenalis. Vet. Parasitol. 2010, 169, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.L.; Yan, W.L.; Wang, J.M.; Meng, J.X.; Zhang, M.; Zhao, J.X.; Shang, K.M.; Liu, J.; Liu, W.H. Meta-analysis of the prevalence of Giardia duodenalis in sheep and goats in China. Microb. Pathog. 2023, 179, 106097. [Google Scholar] [CrossRef]
- Xiao, H.D.; Su, N.; Zhang, Z.D.; Dai, L.L.; Luo, J.L.; Zhu, X.Q.; Xie, S.C.; Gao, W.W. Prevalence and Genetic Characterization of Giardia duodenalis and Blastocystis spp. in Black Goats in Shanxi Province, North China: From a Public Health Perspective. Animals 2024, 14, 1808. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, H.; Li, Y.; Mu, X.; Yuan, K.; Wu, A.; Guo, J.; Hong, Y.; Zhang, H. Occurrence and Genotypic Identification of Blastocystis spp., Enterocytozoon bieneusi, and Giardia duodenalis in Leizhou Black Goats in Zhanjiang City, Guangdong Province, China. Animals 2023, 13, 2777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, X.; Wang, R.; Liu, A.; Shen, Y.; Ling, H.; Cao, J.; Yang, F.; Zhang, X.; Zhang, L. Genetic characterizations of Giardia duodenalis in sheep and goats in Heilongjiang Province, China and possibility of zoonotic transmission. PLoS Neglected Trop. Dis. 2012, 6, e1826. [Google Scholar] [CrossRef]
- Jafari, H.; Jalali, M.H.; Shapouri, M.S.; Hajikolaii, M.R. Determination of Giardia duodenalis genotypes in sheep and goat from Iran. J. Parasit. Dis. Off. Organ Indian Soc. Parasitol. 2014, 38, 81–84. [Google Scholar] [CrossRef]
- Yin, Y.L.; Zhang, H.J.; Yuan, Y.J.; Tang, H.; Chen, D.; Jing, S.; Wu, H.X.; Wang, S.S.; Zhao, G.H. Prevalence and multi-locus genotyping of Giardia duodenalis from goats in Shaanxi province, northwestern China. Acta Trop. 2018, 182, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Wang, L.; Cao, L.; Sun, M.; Liang, N.; Wang, H.; Chang, Y.; Lin, X.; Yu, L.; Wang, R.; et al. Genetic characteristics and geographic segregation of Giardia duodenalis in dairy cattle from Guangdong Province, southern China, Infection, genetics and evolution. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2018, 66, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Santín, M.; Trout, J.M.; Fayer, R. A longitudinal study of Giardia duodenalis genotypes in dairy cows from birth to 2 years of age. Vet. Parasitol. 2009, 162, 40–45. [Google Scholar] [CrossRef]
- Erickson, M.C.; Ortega, Y.R. Inactivation of protozoan parasites in food, water, and environmental systems. J. Food Prot. 2006, 69, 2786–2808. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Klotz, C.; Wilking, H.; Krücken, J.; Nöckler, K.; Von Samson-Himmelstjerna, G.; Zessin, K.H.; Aebischer, T. Epidemiology of Giardia duodenalis infection in ruminant livestock and children in the Ismailia province of Egypt: Insights by genetic characterization. Parasites Vectors 2014, 7, 321. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Yuan, X.D.; Zhang, S.Y.; Zhang, H.Y.; Chen, X.Q. Molecular Detection and Characterization of Giardia duodenalis in Farmed Pigs in Three Provinces of Southern China. Pathogens 2021, 10, 1481. [Google Scholar] [CrossRef]
- Fantinatti, M.; Bello, A.R.; Fernandes, O.; Da-Cruz, A.M. Identification of Giardia lamblia Assemblage E in Humans Points to a New Anthropozoonotic Cycle. J. Infect. Dis. 2016, 214, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Field, D.; Ryan, U. Molecular typing of Giardia duodenalis in humans in Queensland-first report of Assemblage E. Parasitology 2017, 144, 1154–1161. [Google Scholar] [CrossRef]

| Region | Gender | Age | Summation | Total | ||||
|---|---|---|---|---|---|---|---|---|
| 1 Years | 2 Years | 3 Years | 4 Years | 5 Years | ||||
| Putian | Male | 9 | 0 | 7 | 2 | 62 | 539 | |
| Female | 10 | 7 | 8 | 16 | 3 | |||
| Sanming | Male | 6 | 10 | 2 | 73 | |||
| Female | 17 | 33 | 4 | 1 | ||||
| Quanzhou | Male | 5 | 1 | 1 | 39 | |||
| Female | 10 | 6 | 11 | 5 | ||||
| Zhang zhou | Male | 14 | 8 | 1 | 82 | |||
| Female | 13 | 32 | 13 | 1 | ||||
| Longyan | Male | 21 | 7 | 2 | 76 | |||
| Female | 19 | 16 | 11 | |||||
| Xiamen | Male | 7 | 3 | 16 | ||||
| Female | 1 | 6 | ||||||
| Nanping | Male | 12 | 11 | 1 | 70 | |||
| Female | 18 | 18 | 9 | 1 | ||||
| Ningde | Male | 17 | 2 | 57 | ||||
| Female | 27 | 9 | 1 | 1 | ||||
| Fuzhou | Male | 10 | 64 | |||||
| Female | 12 | 15 | 23 | 3 | 1 | |||
| District | Sample Number | Infection Rate | Assemblages |
|---|---|---|---|
| Sanming | 73 | 10 (13.7%) | E (10) |
| Putian | 62 | 23 (37.1%) | E (23) |
| Quanzhou | 39 | 3 (7.69%) | E (3) |
| Xiamen | 16 | 2 (12.5%) | A (1), E (1) |
| Zhang zhou | 82 | 32 (39%) | E (32) |
| Fuzhou | 64 | 2 (3.13%) | E (2) |
| longyan | 76 | 24 (31.58%) | E (24) |
| Ningde | 57 | 5 (8.78%) | E (5) |
| Nanping | 70 | 14 (20%) | E (14) |
| Total | 539 | 115 (21.34%) | A (1), E (114) |
| Type | No. Examined | No. Positive (%) | Genotypes | ||
|---|---|---|---|---|---|
| A | E | ||||
| Gender | Male | 161 | 40 (24.84) | 1 | 39 |
| Female | 378 | 75 (19.84) | - | 75 | |
| Age | ≤1 year | 228 | 56 (24.56) | 1 | 55 |
| 1–2 year | 181 | 27 (14.92) | - | 27 | |
| 2–3 year | 97 | 26 (26.8) | - | 26 | |
| ≥3 year | 33 | 6 (188) | - | 6 | |
| Total | 539 | 115 (21.34) | 1 | 114 | |
| District | Sample Number | Genotype | ||
|---|---|---|---|---|
| bg | gdh | tpi | ||
| Sanming | 73 | E (8) | E (4) | E (5) |
| Putian | 62 | E (7) | E (3) | E (5) |
| Quanzhou | 39 | E (2) | E (2) | E (2) |
| Xiamen | 16 | A (1), E (1) | E (2) | A1(1), E (1) |
| Zhang zhou | 82 | E (2) | E (1) | E (3) |
| Fuzhou | 64 | |||
| Longyan | 76 | E (9) | E (7) | E (6) |
| Ningde | 57 | E (3) | E (3) | E (2) |
| Nanping | 70 | E (2) | E (2) | E (2) |
| Total | 539 | A (1), E (34) | E (24) | A1(1), E (26) |
| Type | No. Examined | Genetic Loci | ||||||
|---|---|---|---|---|---|---|---|---|
| bg | gdh | tpi | ||||||
| A | E | A | E | A1 | E | |||
| Gender | Male | 161 | 1 | 16 | - | 12 | 1 | 12 |
| Female | 378 | - | 18 | - | 12 | - | 14 | |
| Age | ≤1 year | 228 | 1 | 24 | - | 17 | 1 | 19 |
| 1–2 year | 181 | - | 6 | - | 6 | - | 5 | |
| 2–3 year | 97 | - | 4 | - | 1 | - | 2 | |
| ≥3 year | 33 | - | - | - | - | - | - | |
| Total | 539 | 1 | 34 | - | 24 | 1 | 26 | |
| Regions | Sample Number | Genotype | MLGs | ||
|---|---|---|---|---|---|
| bg | gdh | tpi | |||
| Longyan | Ly (1, 32, 35, 36, 38, 52) | E | E | E | I |
| Nanping | Np (46, 52, 51, 57) | E | E | E | I |
| Putian | Pt (28) | E | E | E | I |
| Quanzhou | Qz (34, 36) | E | E | E | I |
| Sanming | Sm (44) | E | E | E | I |
| Zhangzhou | Zz (72) | E | E | E | I |
| Xiamen | Xm (14) | E | E | E | I |
| Xm (4) | A | E | A1 | II | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-X.; Hu, K.; Fu, P.-F.; Li, S.-A.; Liu, Y.; Niu, Z.; Zhou, D.-H. Occurrence and Multi-Locus Genotyping of Giardia duodenalis in Black Goats from Fujian Province, China. Animals 2025, 15, 199. https://doi.org/10.3390/ani15020199
Huang S-X, Hu K, Fu P-F, Li S-A, Liu Y, Niu Z, Zhou D-H. Occurrence and Multi-Locus Genotyping of Giardia duodenalis in Black Goats from Fujian Province, China. Animals. 2025; 15(2):199. https://doi.org/10.3390/ani15020199
Chicago/Turabian StyleHuang, Shou-Xiao, Kai Hu, Peng-Fei Fu, Si-Ang Li, Yang Liu, Zhipeng Niu, and Dong-Hui Zhou. 2025. "Occurrence and Multi-Locus Genotyping of Giardia duodenalis in Black Goats from Fujian Province, China" Animals 15, no. 2: 199. https://doi.org/10.3390/ani15020199
APA StyleHuang, S.-X., Hu, K., Fu, P.-F., Li, S.-A., Liu, Y., Niu, Z., & Zhou, D.-H. (2025). Occurrence and Multi-Locus Genotyping of Giardia duodenalis in Black Goats from Fujian Province, China. Animals, 15(2), 199. https://doi.org/10.3390/ani15020199






