Integrating Metabolomics and Genomics to Uncover the Impact of Fermented Total Mixed Ration on Heifer Growth Performance Through Host-Dependent Metabolic Pathways
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Animals, Experimental Design, and Diets
2.3. Measurement of Dry Matter Intake and Body Weight
2.4. Sample Collection
2.5. Biochemical Index Measurements
2.6. GC-TOF-MS-Based Untargeted Metabolomics Analysis
2.6.1. Metabolites Extraction
2.6.2. GC–TOF/MS Analysis
2.6.3. Data Analysis
2.7. Correlation Analysis and Random Forest
2.8. Genome-Wide Association Analysis
2.9. Statistical Analysis
3. Results
3.1. Feed Intake and Growth Performance
3.2. Plasma and Urine Parameters
3.3. Urine Metabolome
3.4. Individual Variability of Urine Metabolites Affecting ADG
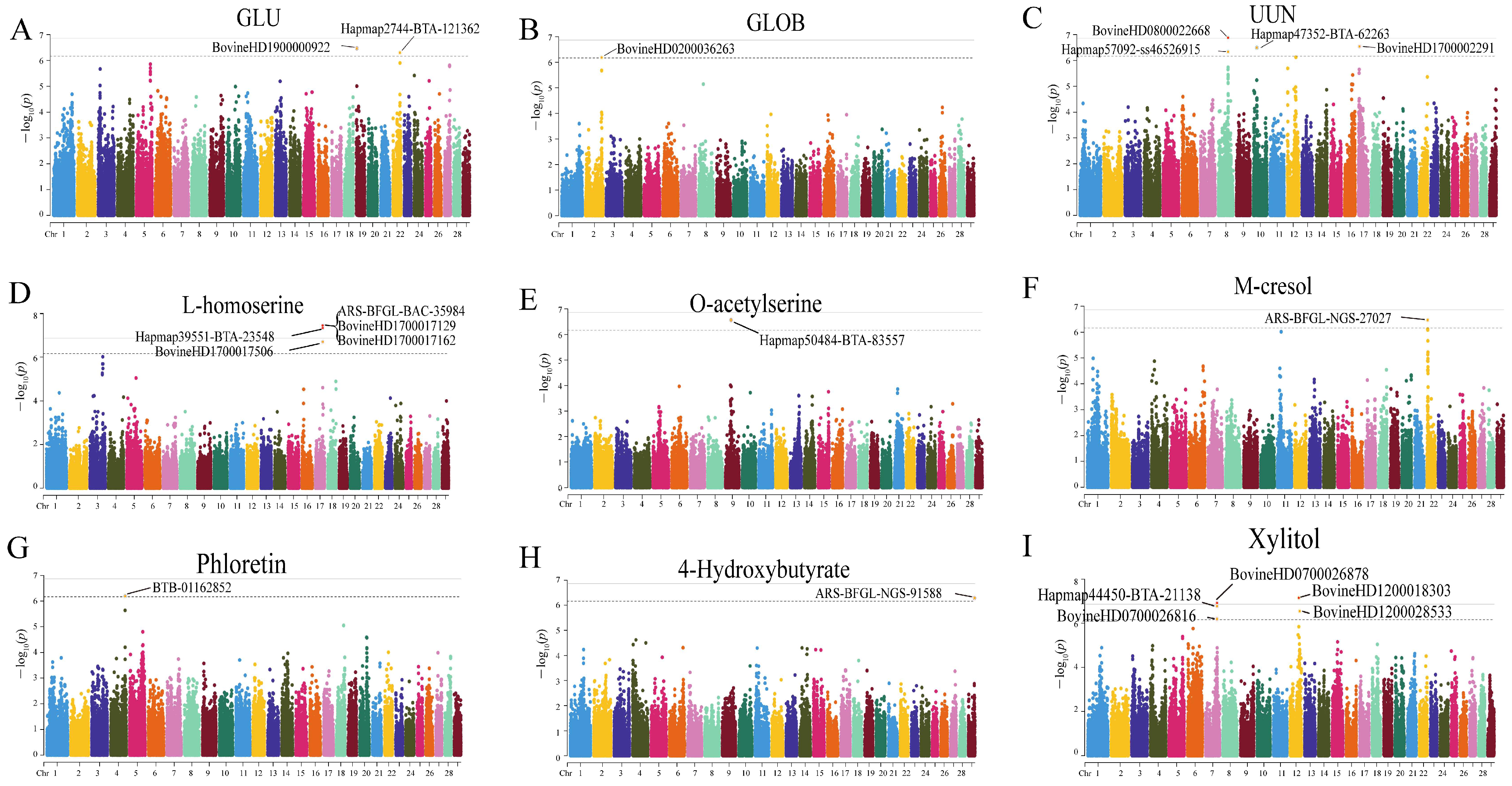
3.5. Genetic Affection on Host Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, S.; Ma, L.; Zhang, Y.; Wang, J.; Loor, J.; Bu, D. Hepatic transcriptome perturbations in dairy cows fed different forage resources. BMC Genom. 2021, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Gunun, N.; Khejornsart, P.; Polyorach, S.; Kaewpila, C.; Kimprasit, T.; Sanjun, I.; Cherdthong, A.; Wanapat, M.; Gunun, P. Utilization of Mao (Antidesma thwaitesianum Muell. Arg.) Pomace Meal to Substitute Rice Bran on Feed Utilization and Rumen Fermentation in Tropical Beef Cattle. Vet. Sci. 2022, 9, 585. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Khalid, A.; Khalid, F.; Ye, M.; Li, Y.; Zhan, K.; Li, Y.; Liu, W.; Wang, Z. Effect of fermented corn by-products on production performance, blood biochemistry, and egg quality indices of laying hens. J. Anim. Sci. 2022, 100, skac130. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zeng, X.; Qiao, S. Advances in research on solid-state fermented feed and its utilization: The pioneer of private customization for intestinal microorganisms. Anim. Nutr. 2021, 7, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Du, M.; Tu, Y.; You, W.; Chen, W.; Liu, G.; Li, J.; Wang, Y.; Lu, Z.; Wang, T.; et al. Fermented mixed feed alters growth performance, carcass traits, meat quality and muscle fatty acid and amino acid profiles in finishing pigs. Anim. Nutr. 2023, 12, 87–95. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, Z.; Li, J.; Chen, L.; Bai, Y.; Jia, Y.; Shao, T. Effects of lactic acid bacteria and molasses on fermentation dynamics, structural and nonstructural carbohydrate composition and in vitro ruminal fermentation of rice straw silage. Asian-Australas. J. Anim. Sci. 2019, 32, 783–791. [Google Scholar] [CrossRef]
- Mohd Azlan, P.; Jahromi, M.F.; Ariff, M.; Ebrahimi, M.; Candyrine, S.C.L.; Liang, J.B. Aspergillus terreus treated rice straw suppresses methane production and enhances feed digestibility in goats. Trop. Anim. Health Prod. 2018, 50, 565–571. [Google Scholar] [CrossRef]
- Abo-Donia, F.M.; Elsheikh, H.A.; Esh, A.M.H.; El-Shora, M.A.H.; Eldiahy, Y.M.M. Co-ensiled rice straw with whole sugar beet and its effect on the performance of lactating cows. Trop. Anim. Health Prod. 2024, 56, 173. [Google Scholar] [CrossRef]
- Sun, H.; Shi, K.; Wu, X.; Xue, M.; Wei, Z.; Liu, J.; Liu, H. Lactation-related metabolic mechanism investigated based on mammary gland metabolomics and 4 biofluids’ metabolomics relationships in dairy cows. BMC Genom. 2017, 18, 936. [Google Scholar] [CrossRef]
- Su, Z.; Bai, X.; Wang, H.; Wang, S.; Chen, C.; Xiao, F.; Guo, H.; Gao, H.; Leng, L.; Li, H. Identification of biomarkers associated with the feed efficiency by metabolomics profiling: Results from the broiler lines divergent for high or low abdominal fat content. J. Anim. Sci. Biotechnol. 2022, 13, 122. [Google Scholar] [CrossRef]
- Keel, B.N.; Zarek, C.M.; Keele, J.W.; Kuehn, L.A.; Snelling, W.M.; Oliver, W.T.; Freetly, H.C.; Lindholm-Perry, A.K. RNA-Seq Meta-analysis identifies genes in skeletal muscle associated with gain and intake across a multi-season study of crossbred beef steers. BMC Genom. 2018, 19, 430. [Google Scholar] [CrossRef] [PubMed]
- Neary, M.T.; Neary, J.M.; Lund, G.K.; Garry, F.B.; Holt, T.N.; Mohun, T.J.; Breckenridge, R.A. Technical note: A comparison of DNA collection methods in cattle and yaks. J. Anim. Sci. 2014, 92, 3811–3815. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Huang, C.; Luosang, D.; Ma, X.; La, Y.; Wu, X.; Guo, X.; Pingcuo, Z.; Liang, C. Serum Metabolomic Analysis of Synchronous Estrus in Yaks Based on UPLC-Q-TOF MS Technology. Animals 2024, 14, 1399. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Fukumori, R.; Osada, T.; Shimada, K.; Oikawa, S.; Izumi, K. Effects of starch content of calf starter on feed intake, growth performance, and fecal properties in dairy calves under a high plane of milk replacer feeding. Anim. Sci. J. 2023, 94, e13911. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, H.; Gu, F.; He, J.; Zhao, F.; Liu, J. Blood neutrophil extracellular traps: A novel target for the assessment of mammary health in transition dairy cows. J. Anim. Sci. Biotechnol. 2022, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; He, Y.; Gao, S.; Liao, Z.; Lai, T.; Zhou, H.; Chen, Q.; Li, L.; Gao, H.; Lu, W. The effect of a diet based on rice straw co-fermented with probiotics and enzymes versus a fresh corn Stover-based diet on the rumen bacterial community and metabolites of beef cattle. Sci. Rep. 2020, 10, 10721. [Google Scholar] [CrossRef]
- Wang, C.; Shi, C.; Zhang, Y.; Song, D.; Lu, Z.; Wang, Y. Microbiota in fermented feed and swine gut. Appl. Microbiol. Biotechnol. 2018, 102, 2941–2948. [Google Scholar] [CrossRef]
- McCann, J.C.; Sawyer, J.E.; Wickersham, T.A. Effect of source and level of protein supplementation on rice straw utilization by Brahman steers. J. Anim. Sci. 2017, 95, 387–394. [Google Scholar] [CrossRef]
- Kaeokliang, O.; Kawashima, T.; Narmseelee, R.; Butcha, P.; Sunato, S.; Thinowong, A.; Jindatajak, Y. Effects of physically effective fiber in diets based on rice straw and cassava pulp on chewing activity, ruminal fermentation, milk production, and digestibility in dairy cows. Anim. Sci. J. 2019, 90, 1193–1199. [Google Scholar] [CrossRef]
- Khonkhaeng, B.; Cherdthong, A. Improving Nutritive Value of Purple Field Corn Residue and Rice Straw by Culturing with White-Rot Fungi. J. Fungi 2020, 6, 69. [Google Scholar] [CrossRef]
- Pena-Torres, E.F.; Davila-Ramirez, J.L.; Pena-Ramos, E.A.; Valenzuela-Melendres, M.; Pinelli-Saavedra, A.; Avendano-Reyes, L.; González-Ríos, H. Effects of dietary ferulic acid on growth performance, carcass traits and meat quality of heifers. J. Sci. Food Agric. 2021, 101, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Devant, M.; Ferret, A.; Gasa, J.; Calsamiglia, S.; Casals, R. Effects of protein concentration and degradability on performance, ruminal fermentation, and nitrogen metabolism in rapidly growing heifers fed high-concentrate diets from 100 to 230 kg body weight. J. Anim. Sci. 2000, 78, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Xie, Y.; Zang, X.; Zhong, Y.; Ma, X.; Sun, H.; Liu, J. Deciphering functional groups of rumen microbiome and their underlying potentially causal relationships in shaping host traits. Imeta 2024, 3, e225. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.L.; Amaral, R.C.; Goulart, R.S.; Zopollatto, M.; Santos, V.P.; Toledo Filho, S.G.; Cabezas-Garcia, E.H.; Lima, J.R.; Santos, M.C.; Nussio, L.G. Short-term effects of silage volatile compounds on feed intake and digestion in beef cattle. J. Anim. Sci. 2013, 91, 2321–2331. [Google Scholar] [CrossRef]
- Carneiro de Souza, V.; Duarte Messana, J.; Darlisson Batista, E.; Larissa Gomes Carvalho Alves, K.; Titgemeyer, E.C.; Vaz Pires, A.; Vinícius Ferraz Junior, M.; Galoro Silva, L.; Alberto Negrão, J.; Eliodoro Costa, V.; et al. Effects of protein sources and inclusion levels on nitrogen metabolism and urea kinetics of Nellore feedlot steers fed concentrate-based diets. J. Anim. Sci. 2021, 99, skab185. [Google Scholar] [CrossRef]
- Pang, R.; Xiao, X.; Mao, T.; Yu, J.; Huang, L.; Xu, W.; Li, Y.; Zhu, W. The molecular mechanism of propionate-regulating gluconeogenesis in bovine hepatocytes. Anim. Biosci. 2023, 36, 1693–1699. [Google Scholar] [CrossRef]
- Scheerer, U.; Haensch, R.; Mendel, R.R.; Kopriva, S.; Rennenberg, H.; Herschbach, C. Sulphur flux through the sulphate assimilation pathway is differently controlled by adenosine 5′-phosphosulphate reductase under stress and in transgenic poplar plants overexpressing gamma-ECS, SO, or APR. J. Exp. Bot. 2010, 61, 609–622. [Google Scholar] [CrossRef]
- Lapenna, D. Glutathione and glutathione-dependent enzymes: From biochemistry to gerontology and successful aging. Ageing Res. Rev. 2023, 92, 102066. [Google Scholar] [CrossRef]
- Zhitkovich, A. Ascorbate: Antioxidant and biochemical activities and their importance for in vitro models. Arch. Toxicol. 2021, 95, 3623–3631. [Google Scholar] [CrossRef]
- Kou, T.; Wu, J.; Chen, X.; Chen, Z.; Zheng, J.; Peng, B. Exogenous glycine promotes oxidation of glutathione and restores sensitivity of bacterial pathogens to serum-induced cell death. Redox Biol. 2022, 58, 102512. [Google Scholar] [CrossRef]
- Salyha, N.; Salyha, Y. Protective role of l-glutamic acid and l-cysteine in mitigation the chlorpyrifos-induced oxidative stress in rats. Environ. Toxicol. Pharmacol. 2018, 64, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Zhang, S.; Mao, X.; Tao, Y.; Yu, B. Highly efficient production of L-homoserine in Escherichia coli by engineering a redox balance route. Metab. Eng. 2021, 67, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Taleb, F.; Ammar, M.; Mosbah, M.B.; Salem, R.B.; Moussaoui, Y. Chemical modification of lignin derived from spent coffee grounds for methylene blue adsorption. Sci. Rep. 2020, 10, 11048. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Gao, X.; Gao, Q.; Bao, J. Mechanism of Tolerance to the Lignin-Derived Inhibitor p-Benzoquinone and Metabolic Modification of Biorefinery Fermentation Strains. Appl. Environ. Microbiol. 2019, 85, e01443-19. [Google Scholar] [CrossRef]
- Wang, B.; Sun, H.; Wang, D.; Liu, H.; Liu, J. Constraints on the utilization of cereal straw in lactating dairy cows: A review from the perspective of systems biology. Anim. Nutr. 2022, 9, 240–248. [Google Scholar] [CrossRef]
- Wu, X.; Sun, H.; Xue, M.; Wang, D.; Guan, L.L.; Liu, J. Serum metabolome profiling revealed potential biomarkers for milk protein yield in dairy cows. J. Proteom. 2018, 184, 54–61. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, M.; Wang, O.; Chen, Y.; Liu, J.; Guan, L.L. Multi-omics reveals functional genomic and metabolic mechanisms of milk production and quality in dairy cows. Bioinformatics 2020, 36, 2530–2537. [Google Scholar] [CrossRef]
- Guinguina, A.; Yan, T.; Lund, P.; Bayat, A.R.; Hellwing, A.L.F.; Huhtanen, P. Between-cow variation in the components of feed efficiency. J. Dairy Sci. 2020, 103, 7968–7982. [Google Scholar] [CrossRef]
- Ji, J.; Zhu, P.; Blazenovic, I.; Cui, F.; Gholami, M.; Sun, J.; Habimana, J.; Zhang, Y.; Sun, X. Explaining combinatorial effects of mycotoxins Deoxynivalenol and Zearalenone in mice with urinary metabolomic profiling. Sci. Rep. 2018, 8, 3762. [Google Scholar] [CrossRef]
- Wang, J.; Qu, Q.; Liu, X.; Cui, W.; Yu, F.; Chen, X.; Xing, X.; Zhou, Y.; Yang, Y.; Bello-Onaghise, G.; et al. 1-Hydroxyanthraquinone exhibited antibacterial activity by regulating glutamine synthetase of Staphylococcus xylosus as a virulence factor. Biomed. Pharmacother. 2020, 123, 109779. [Google Scholar] [CrossRef]
- Alhamdow, A.; Essig, Y.J.; Krais, A.M.; Gustavsson, P.; Tinnerberg, H.; Lindh, C.H.; Hagberg, J.; Graff, P.; Albin, M.; Broberg, K. Fluorene exposure among PAH-exposed workers is associated with epigenetic markers related to lung cancer. Occup. Environ. Med. 2020, 77, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Rath, P.; Chauhan, A.; Ramniwas, S.; Vashishth, K.; Varol, M.; Jaswal, V.S.; Haque, S.; Sak, K. Phloretin, as a Potent Anticancer Compound: From Chemistry to Cellular Interactions. Molecules. 2022, 27, 8819. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Nemet, I.; Li, X.S.; Wilcox, J.; Ferrell, M.; Alamri, H.; Gupta, N.; Wang, Z.; Tang, W.H.W.; Hazen, S.L. Xylitol is prothrombotic and associated with cardiovascular risk. Eur. Heart J. 2024, 45, 2439–2452. [Google Scholar] [CrossRef]
- Tifft, C.J. N-Acetyl-l-Leucine and Neurodegenerative Disease. N. Engl. J. Med. 2024, 390, 467–470. [Google Scholar] [CrossRef]
- Wu, X.; Eiteman, M.A. Synthesis of citramalic acid from glycerol by metabolically engineered Escherichia coli. J. Ind. Microbiol. Biotechnol. 2017, 44, 1483–1490. [Google Scholar] [CrossRef]
- Brikis, C.J.; Zarei, A.; Chiu, G.Z.; Deyman, K.L.; Liu, J.; Trobacher, C.P.; Hoover, G.J.; Subedi, S.; DeEll, J.R.; Bozzo, G.G.; et al. Targeted quantitative profiling of metabolites and gene transcripts associated with 4-aminobutyrate (GABA) in apple fruit stored under multiple abiotic stresses. Hortic. Res. 2018, 5, 61. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Wan, M.; Yang, D.; Liu, F.; Li, K.; Hu, M.; Tang, Y.; Lu, H.; Zhang, S.; et al. m-Cresol, a pesticide intermediate, induces hepatotoxicity and behavioral abnormalities in zebrafish larvae through oxidative stress, apoptosis. Toxicol. Vitr. 2024, 94, 105723. [Google Scholar] [CrossRef]
- Kolker, S.; Okun, J.G.; Horster, F.; Assmann, B.; Ahlemeyer, B.; Kohlmuller, D.; Exner-Camps, S.; Mayatepek, E.; Krieglstein, J.; Hoffmann, G.F. 3-Ureidopropionate contributes to the neuropathology of 3-ureidopropionase deficiency and severe propionic aciduria: A hypothesis. J. Neurosci. Res. 2001, 66, 666–673. [Google Scholar] [CrossRef]






| Items | Treatment 1 | |||
|---|---|---|---|---|
| LSF | MSF | HSF | ZF | |
| TMR, % of DM 2 | 46.87 | 46.47 | 46.19 | 46.0 |
| Dietary ingredient, % of DM | ||||
| Corn | 2.4 | 5.3 | 9.3 | 12.4 |
| Soybean meal | 6.7 | 3.8 | 6.8 | 3.04 |
| Sprayed corn bran | 16.0 | 16.0 | 16.0 | 21.3 |
| Distiller grain | 4.6 | 4.6 | 4.6 | 9.37 |
| Whole corn silage | 17.0 | 12.0 | 7.0 | 0 |
| Alfalfa hay | 10.0 | 18.0 | 6.0 | 0 |
| Oat hay | 13.0 | 0.0 | 0.0 | 22.7 |
| TP 3 | 30.0 | 40.0 | 50.0 | 30.8 |
| Stone powder | 0.18 | 0.18 | 0.18 | 0.18 |
| Salt | 0.18 | 0.18 | 0.18 | 0.18 |
| Chemical composition, % of DM | ||||
| Crude protein | 14.2 | 14.2 | 14.2 | 14.2 |
| Neutral detergent fiber | 49.71 | 47.53 | 46.09 | 52.3 |
| Acid detergent fiber | 27.32 | 26.86 | 25.46 | - |
| Starch | 9.88 | 10.72 | 12.83 | 15.4 |
| Crude ash | 7.62 | 8.34 | 8.51 | 6.22 |
| NEL 4, Mcal/kg | 1.45 | 1.44 | 1.43 | 1.43 |
| Diet price, ¥/t | 2152.6 | 2084.9 | 2018.2 | 1749.2 |
| Items | Treatment 1 | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| LSF | MSF | HSF | ZF | |||
| Dry matter intake, kg/d | 8.0 | 7.7 | 7.9 | 8.3 | ||
| Average daily gain of body weight, g/d | 572.5 | 633.6 | 565.7 | 591.6 | 35.4 | 0.349 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Zuo, M.; Ding, S.; Zhong, Y.; Xue, M.; Zheng, H. Integrating Metabolomics and Genomics to Uncover the Impact of Fermented Total Mixed Ration on Heifer Growth Performance Through Host-Dependent Metabolic Pathways. Animals 2025, 15, 173. https://doi.org/10.3390/ani15020173
Hu Z, Zuo M, Ding S, Zhong Y, Xue M, Zheng H. Integrating Metabolomics and Genomics to Uncover the Impact of Fermented Total Mixed Ration on Heifer Growth Performance Through Host-Dependent Metabolic Pathways. Animals. 2025; 15(2):173. https://doi.org/10.3390/ani15020173
Chicago/Turabian StyleHu, Zhenzhen, Minyu Zuo, Shixuan Ding, Yifan Zhong, Mingyuan Xue, and Huichao Zheng. 2025. "Integrating Metabolomics and Genomics to Uncover the Impact of Fermented Total Mixed Ration on Heifer Growth Performance Through Host-Dependent Metabolic Pathways" Animals 15, no. 2: 173. https://doi.org/10.3390/ani15020173
APA StyleHu, Z., Zuo, M., Ding, S., Zhong, Y., Xue, M., & Zheng, H. (2025). Integrating Metabolomics and Genomics to Uncover the Impact of Fermented Total Mixed Ration on Heifer Growth Performance Through Host-Dependent Metabolic Pathways. Animals, 15(2), 173. https://doi.org/10.3390/ani15020173





