Current Enzooticity of Dirofilaria immitis and Angiostrongylus vasorum in Central and Southern Italy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
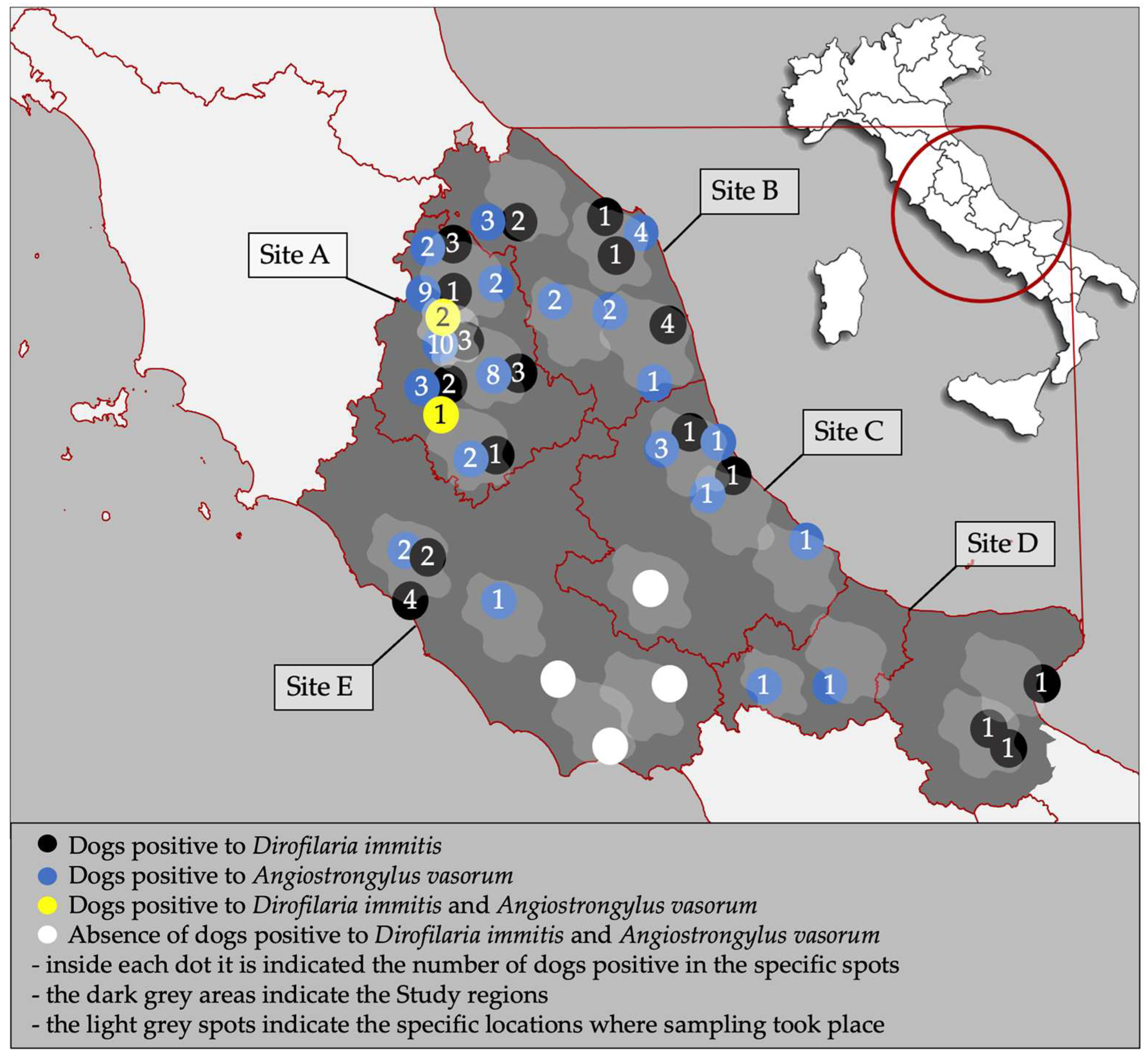
2.1. Study Design
2.2. Laboratory Techniques
2.3. Statistical Analysis
3. Results
Statistical Analysis
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, E.R.; Modry, D.; Paredes-Esquivel, C.; Foronda, P.; Traversa, D. Angiostrongylosis in Animals and Humans I Europe. Pathogens 2021, 10, 1236. [Google Scholar] [CrossRef]
- Noack, S.; Harrington, J.; Carithers, D.S.; Kaminsky, R.; Selzer, P.M. Heartworm Disease—Overview, Intervention, and Industry Perspective. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 65–89. [Google Scholar] [CrossRef] [PubMed]
- Ames, M.K.; Atkins, C.E. Treatment of Dogs with Severe Heartworm Disease. Vet. Parasitol. 2020, 283, 109131. [Google Scholar] [CrossRef] [PubMed]
- Simón, F.; Siles-Lucas, M.; Morchón, R.; González-Miguel, J.; Mellado, I.; Carretón, E.; Montoya-Alonso, J.A. Human and Animal Dirofilariasis: The Emergence of a Zoonotic Mosaic. Clin. Microbiol. Rev. 2012, 25, 507–544. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Shaw, S. Angiostrongylus vasorum Infection in Dogs: Continuing Spread and Developments in Diagnosis and Treatment. J. Small. Anim. Pract. 2010, 51, 616–621. [Google Scholar] [CrossRef]
- Paradies, P.; Schnyder, M.; Capogna, A.; Lia, R.P.; Sasanelli, M. Canine angiostrongylosis in naturally infected dogs: Clinical approach and monitoring of infection after treatment. Sci. World J. 2013, 2013, 702056. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, A.S.; Petersen, M.P.; Willesen, J.L.; Bach, M.B.T.; Kieler, I.N.; Kristensen, A.T.; Koch, J.; Nielsen, L.N. Clinical Bleeding Diathesis, Laboratory Haemostatic Aberrations and Survival in Dogs Infected with Angiostrongylus vasorum: 180 cases (2005–2019). J. Small. Anim. Pract. 2024, 65, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Fuehrer, H.P.; Morelli, S.; Unterköfler, M.S.; Bajer, A.; Bakran-Lebl, K.; Dwużnik-Szarek, D.; Farkas, R.; Grandi, G.; Heddergott, M.; Jokelainen, P.; et al. Dirofilaria spp. and Angiostrongylus vasorum: Current Risk of Spreading in Central and Northern Europe. Pathogens 2021, 10, 1268. [Google Scholar] [CrossRef]
- Mendoza-Roldan, J.; Benelli, G.; Panarese, R.; Iatta, R.; Furlanello, T.; Beugnet, F.; Zatelli, A.; Otranto, D. Leishmania infantum and Dirofilaria immitis Infections in Italy, 2009-2019: Changing Distribution Patterns. Parasites Vectors 2020, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Napoli, E.; De Benedetto, G.; Ciuca, L.; Bosco, A.; Lia, R.P.; Veneziano, V.; Bezerra Santos, M.A.; Otranto, D.; Rinaldi, L.; Brianti, E. New Distribution Patterns of Dirofilaria immitis in Italy. Front. Vet. Sci. 2023, 10, 1162403. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, E.; Zanzani, S.A.; Gazzonis, A.L.; Giudice, C.; Brambilla, P.; Alberti, I.; Romussi, S.; Lombardo, R.; Mortellaro, C.M.; Banco, B.; et al. Angiostrongylus vasorum Infection in Dogs from a Cardiopulmonary Dirofilariosis Endemic Area of Northwestern Italy: A Case Study and a Retrospective Data Analysis. BMC Vet. Res. 2017, 13, 165. [Google Scholar] [CrossRef]
- Di Cesare, A.; Traversa, D.; Manzocchi, S.; Meloni, S.; Grillotti, E.; Auriemma, E.; Pampurini, F.; Garofani, C.; Ibba, F.; Venco, L. Elusive Angiostrongylus vasorum Infections. Parasites Vectors 2015, 8, 438. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, L.; Maurelli, M.P.; Pennacchio, S.; Bosco, A.; Musella, V.; Ciuca, L.; Cringoli, G.; Rinaldi, L. Dirofilaria immitis and Angiostrongylus vasorum: The Contemporaneous Detection in Kennels. BMC Vet. Res. 2015, 11, 305. [Google Scholar] [CrossRef]
- Sloss, M.W.; Kemp, R.L.; Zajac, A.M. Veterinary Clinical Parasitology, 6th ed.; Iowa State University Press: Ames, IA, USA, 1994; ISBN 0813817331. [Google Scholar]
- De Liberato, C.; Berrilli, F.; Odorizi, L.; Scarcella, R.; Barni, M.; Amoruso, C.; Scarito, A.; Filippo, M.M.D.; Carvelli, A.; Iacoponi, F.; et al. Parasites in Stray Dogs from Italy: Prevalence, Risk Factors and Management Concerns. Acta. Parasitol. 2018, 63, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Traversa, D.; Morelli, S.; Cassini, R.; Crisi, P.E.; Russi, I.; Grillotti, E.; Manzocchi, S.; Simonato, G.; Beraldo, P.; Viglietti, A.; et al. Occurrence of Canine and Feline Extra-intestinal Nematodes in Key Endemic Regions of Italy. Acta. Trop. 2019, 193, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Morelli, S.; Simonato, G.; Di Cesare, A.; Veronesi, F.; Frangipane di Regalbono, A.; Grassi, L.; Russi, I.; Tiscar, P.G.; Morganti, G.; et al. Exposure to Major Vector-Borne Diseases in Dogs Subjected to Different Preventative Regimens in Endemic Areas of Italy. Pathogens 2021, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Gori, F.; Colombo, M.; Traversa, D.; Sarrocco, G.; Simonato, G.; Nespeca, C.; Di Cesare, A.; Frangipane di Regalbono, A.; Veronesi, F.; et al. Simultaneous Exposure to Angiostrongylus vasorum and Vector-Borne Pathogens in Dogs from Italy. Pathogens 2021, 10, 1200. [Google Scholar] [CrossRef] [PubMed]
- Drake, J. Dog Relocation and Rapidly Changing Parasite Threats. Biol. Life Sci. Forum 2021, 5, 4. [Google Scholar] [CrossRef]
- Available online: https://www.heartwormsociety.org/images/A-News/SKO_Transport_Guidelines_for_Web_G.pdf (accessed on 23 November 2024).
- Genchi, C.; Kramer, L.H. The Prevalence of Dirofilaria immitis and Dirofilaria repens in the Old World. Vet. Parasitol. 2020, 280, 108995. [Google Scholar] [CrossRef] [PubMed]
- Széll, Z.; Bacsadi, Á.; Szeredi, L.; Nemes, C.; Fézer, B.; Bakcsa, E.; Kalla, H.; Tolnai, Z.; Sréter, T. Rapid Spread and Emergence of Heartworm Resulting from Climate and Climate-driven Ecological Changes in Hungary. Vet. Parasitol. 2020, 280, 109067. [Google Scholar] [CrossRef]
- Gizzarelli, M.; Foglia Manzillo, V.; Ciuca, L.; Morgoglione, M.E.; El Houda Ben Fayala, N.; Cringoli, G.; Oliva, G.; Rinaldi, L.; Maurelli, M.P. Simultaneous Detection of Parasitic Vector Borne Diseases: A Robust Cross-Sectional Survey in Hunting, Stray and Sheep Dogs in a Mediterranean Area. Front. Vet. Sci. 2019, 6, 288. [Google Scholar] [CrossRef] [PubMed]
- Gillis-Germitsch, N.; Tritten, L.; Hegglin, D.; Deplazes, P.; Schnyder, M. Conquering Switzerland: The emergence of Angiostrongylus vasorum in Foxes over Three Decades and its Rapid Regional Increase in Prevalence Contrast with the Stable Occurrence of Lungworms. Parasitology 2020, 147, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.S.; Boag, A.K.; Guitian, J.; Boswood, A. Angiostrongylus vasorum Infection in 23 Dogs (1999–2002). J. Small. Anim. Pract. 2004, 45, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Bolt, G.; Monrad, J.; Frandsen, F.; Henriksen, P.; Dietz, H.H. The Common Frog (Rana temporaria) as a Potential Paratenic and Intermediate Host for Angiostrongylus vasorum. Parasitol. Res. 1993, 79, 428–430. [Google Scholar] [CrossRef]
- Mozzer, L.R.; Lima, W.S. Gallus gallus domesticus: Paratenic Host of Angiostrongylus vasorum. Vet. Parasitol. 2015, 207, 81–84. [Google Scholar] [CrossRef]
- Robbins, W.; Conboy, G.; Greenwood, S.; Schaper, R. Infectivity of Gastropod-shed Third-stage Larvae of Angiostrongylus vasorum and Crenosoma vulpis to Dogs. Parasites Vectors 2021, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Capelli, G.; Genchi, C.; Baneth, G.; Bourdeau, P.; Brianti, E.; Cardoso, L.; Danesi, P.; Fuehrer, H.P.; Giannelli, A.; Ionică, A.M.; et al. Recent Advances on Dirofilaria repens in Dogs and Humans in Europe. Parasites Vectors 2018, 11, 663. [Google Scholar] [CrossRef]
- ESCCAP, European Scientific Counsel Companion Animal Parasites. Available online: https://www.esccap.org/guidelines/ (accessed on 23 November 2024).
- Lorenzo-Rebenaque, L.; López-Fernández, S.; Marco-Jiménez, F.; Montoro-Dasi, L.; Marin, C.; Vega, S.; Martínez-Manzanares, E.; Fariñas, F. Zoonotic Parasites in Playgrounds in Southern Spain: A One Health Approach. Microorganisms 2023, 11, 721. [Google Scholar] [CrossRef]
- Traversa, D.; Diakou, A.; Colombo, M.; Kumar, S.; Long, T.; Chaintoutis, S.C.; Venco, L.; Betti Miller, G.; Prichard, R. First Case of Macrocyclic Lactone-resistant Dirofilaria immitis in Europe—Cause for Concern. Int. J. Parasitol. Drugs Drug Resist. 2024, 25, 100549. [Google Scholar] [CrossRef] [PubMed]
| Site A | Site B | Site C | Site D | Site E | Tot | |
|---|---|---|---|---|---|---|
| Owned | 387 | 400 | 383 | 313 | 374 | 1857 |
| Kenneled | 13 | 0 | 17 | 53 | 60 | 143 |
| Males | 231 | 228 | 196 | 180 | 234 | 1069 |
| Females | 169 | 172 | 204 | 186 | 173 | 931 |
| >4 years old | 150 | 137 | 185 | 194 | 187 | 853 |
| ≤4 years old | 250 | 263 | 215 | 172 | 247 | 1147 |
| Gastropod ingestion | 109 | 58 | 27 | 26 | 64 | 284 |
| History of travel | 50 | 96 | 62 | 44 | 13 | 265 |
| Hunting | 136 | 295 | 212 | 44 | 197 | 883 |
| Contact/Cohabitation with other dogs | 251 | 331 | 326 | 266 | 391 | 1565 |
| Permanent outdoor housing | 149 | 202 | 252 | 151 | 303 | 1057 |
| Test | Parasite | Site A n/400 (%) | Site B n/400 (%) | Site C n/400 (%) | Site D n/366 (%) | Site E n/434 (%) | Tot n/2000 (%) |
|---|---|---|---|---|---|---|---|
| Knott | Dirofilaria immitis | 16 (4) | 8 (2) | 2 (0.6) | 3 (0.8) | 6 (1.7) | 35 (1.7) |
| Dirofilaria repens | 69 (17.2) | 52 (13) | 10 (2.5) | 3 (0.8) | 14 (3.4) | 148 (7.4) | |
| Acanthocheilonema reconditum | - | 3 (0.8) | - | - | 3 (0.7) | 6 (0.3) | |
| Baermann | Angiostrongylus vasorum | 39 (10.2) | 12 (3) | 6 (1.5) | 2 (0.5) | 3 (0.7) | 62 (3.1) |
| Strongyloides stercoralis | 13 (3.2) | 11 (2.7) | - | - | - | 24 (1.2) |
| Parasite | Site A n/400 (%) | Site B n/400 (%) | Site C n/400 (%) | Site D n/366 (%) | Site E n/434 (%) | Tot n/2000 (%) |
|---|---|---|---|---|---|---|
| Ascarids | 23 (5.7) | 64 (16) | 28 (7) | 12 (3.3) | 45 (10.4) | 172 (8.6) |
| Ancylostomatidae | 92 (23) | 90 (22.5) | 59 (14.7) | 17 (4.6) | 65 (15) | 323 (16.5) |
| Trichuris vulpis | 99 (24.7) | 83 (20.7) | 20 (5) | 14 (3.8) | 33 (7.6) | 249 (12.4) |
| Taeniidae | 2 (0.5) | 1 (0.2) | 4 (1) | - | - | 7 (0.3) |
| Capillaria aerophila | 62 (15.5) | 63 (15.7) | 20 (5) | 7 (1.9) | 65 (15) | 217 (10.8) |
| Capillaria boehmi | 21 (5.2) | 4 (1) | 6 (1.5) | - | 13 (3.2) | 44 (2.2) |
| Dipylidium caninum | - | 2 (0.5) | - | - | - | 2 (0.1) |
| Cystoisospora spp. | - | 5 (1.2) | 5 (1.2) | 2 (0.5) | 7 (1.6) | 19 (0.9) |
| Giardia spp. | 20 (5) | 14 (3.5) | 2 (0.5) | - | 2 (0.5) | 38 (1.9) |
| Strongyloides stercoralis | - | 1 (0.25) | - | - | - | 1 (0.05) |
| Dirofilaria immitis | |||
| Category of Clinical Signs | n (%) | Clinical Sign | n (%) |
| Cardiorespiratory signs | 10 (28.6) | Pale mucous membranes | 10 (28.6) |
| Dyspnea | 2 (5.7) | ||
| Cough | 2 (5.7) | ||
| Ocular signs | 1 (2.85) | Uveitis | 1 (2.85) |
| Non-specific signs | 10 (28.6) | Fatigue | 5 (14.3) |
| Weight loss | 5 (14.3) | ||
| Vomiting | 1 (2.9) | ||
| Diarrhea | 1 (2.9) | ||
| No clinical signs | 21 (60) | - | - |
| Angiostrongylus vasorum | |||
| Category of Clinical Signs | n (%) | Clinical Sign | n (%) |
| Neurological | 2 (3.2) | Seizures | 2 (3.2) |
| Skin lesions | 4 (6.4) | Dermatitis | 3 (4.8) |
| Nodules | 1 (1.6) | ||
| Cardiorespiratory signs | 18 (29) | Dyspnea | 5 (8.1) |
| Pale mucous membranes | 8 (12.9) | ||
| Cough | 12 (19.3) | ||
| Signs possibly related to coagulation disorders | 1 (4.3) | Hemoptysis | 1 (4.3) |
| Melena | 1 (4.3) | ||
| Hematuria | 1 (4.3) | ||
| Non-specific signs | 14 (22.6) | Weight loss | 7 (11.3) |
| Fatigue | 2 (3.2) | ||
| Diarrhea | 5 (8.1) | ||
| No clinical signs | 36 (58.1) | - | |
| Dirofilaria immitis | Angiostrongylus vasorum | |||||
|---|---|---|---|---|---|---|
| Factor | p | OR | 95% CI | p | OR | 95% CI |
| Neurological signs | 0.994 | 2.87 | 0–infinity | 0.147 | 3.67 | 0.63–21.30 |
| Skin lesions | 0.058 | 1.95 | 1.32–10.15 | 0.524 | 1.44 | 0.47–4.39 |
| Cardiorespiratory signs | <0.001 * | 4.93 | 2.00–12.20 | <0.001 * | 3.51 | 1.79–6.90 |
| Ocular signs | 0.989 | 2.02 | 0–infinity | 0.984 | 5.79 | 0–infinity |
| Signs possibly related to coagulation disorders | 0.993 | 8.43 | 0–infinity | 0.799 | 1.35 | 0.13–13.90 |
| Non-specific signs | 0.044 * | 2.41 | 1.03–5.67 | 0.145 | 1.68 | 0.84–3.39 |
| Male sex | 0.057 | 2.10 | 1.00–4.53 | 0.395 | 1.27 | 0.74–2.18 |
| Gastropod ingestion | 0.451 | 1.42 | 0.57–3.57 | <0.001 * | 4.74 | 2.72–8.26 |
| Animal movements (within Italy) | 0.003 * | 3.47 | 1.55–7.79 | 0.092 | 0.29 | 0.07–1.22 |
| Hunting | 0.975 | 1.01 | 0.45–2.30 | 0.331 | 1.37 | 0.73–2.56 |
| Contact/cohabitation with other dogs | 0.877 | 1.07 | 0.43–2.67 | 0.439 | 0.77 | 0.39–1.50 |
| Permanently outdoor housing | 0.527 | 0.77 | 0.35–1.72 | 0.029 * | 0.50 | 0.27–0.93 |
| >4 years old | 0.041 * | 2.15 | 1.03–4.47 | 0.994 | 1.00 | 0.58–1.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traversa, D.; Morelli, S.; Di Cesare, A.; Astuti, C.; Barlaam, A.; Colombo, M.; Veronesi, F.; Paoletti, B.; Iorio, R.; Maggi, R.; et al. Current Enzooticity of Dirofilaria immitis and Angiostrongylus vasorum in Central and Southern Italy. Animals 2025, 15, 172. https://doi.org/10.3390/ani15020172
Traversa D, Morelli S, Di Cesare A, Astuti C, Barlaam A, Colombo M, Veronesi F, Paoletti B, Iorio R, Maggi R, et al. Current Enzooticity of Dirofilaria immitis and Angiostrongylus vasorum in Central and Southern Italy. Animals. 2025; 15(2):172. https://doi.org/10.3390/ani15020172
Chicago/Turabian StyleTraversa, Donato, Simone Morelli, Angela Di Cesare, Chiara Astuti, Alessandra Barlaam, Mariasole Colombo, Fabrizia Veronesi, Barbara Paoletti, Raffaella Iorio, Raffaella Maggi, and et al. 2025. "Current Enzooticity of Dirofilaria immitis and Angiostrongylus vasorum in Central and Southern Italy" Animals 15, no. 2: 172. https://doi.org/10.3390/ani15020172
APA StyleTraversa, D., Morelli, S., Di Cesare, A., Astuti, C., Barlaam, A., Colombo, M., Veronesi, F., Paoletti, B., Iorio, R., Maggi, R., Passarelli, A., Pede, A., Rossi, L., & Diaferia, M. (2025). Current Enzooticity of Dirofilaria immitis and Angiostrongylus vasorum in Central and Southern Italy. Animals, 15(2), 172. https://doi.org/10.3390/ani15020172












