Effects of Vitamin D3 and 25(OH)D3 Supplementation on Growth Performance, Bone Parameters and Gut Microbiota of Broiler Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Animal Care
2.2. Animals, Experimental Design, and Husbandry
2.3. Experimental Diets
- -
- Negative control (NC): Negative control (NC): Diet without vitamin D3 supplementation in the premix, used as a biological baseline rather than a commercial model, with welfare carefully maintained.
- -
- Positive control (PC): Diet supplemented with vitamin D3 according to Ross 308 recommendations.
- -
- PC + 25(OH)D3_1X: Diet supplemented with vitamin D3 and 25(OH)D3 (commercial product: Bio D®, supported by Huvepharma, Bangkok, Thailand) was supplemented at levels of 1394 IU/kg diet.
- -
- PC + 25(OH)D3_2X: Diet supplemented with vitamin D3 and 25(OH)D3 from the same source at 2788 IU/kg.
2.4. Growth Performance
2.5. Bone Parameters
2.5.1. Tibia Ash Analysis
2.5.2. Tibia Breaking Strength
2.6. Gut Microbiota Analysis
2.6.1. Sample Collection
2.6.2. DNA Extraction and 16S rRNA Gene Amplicon Sequencing
2.6.3. Bioinformatics and Statistical Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Growth Performance
3.2. Bone Mineralization and Strength
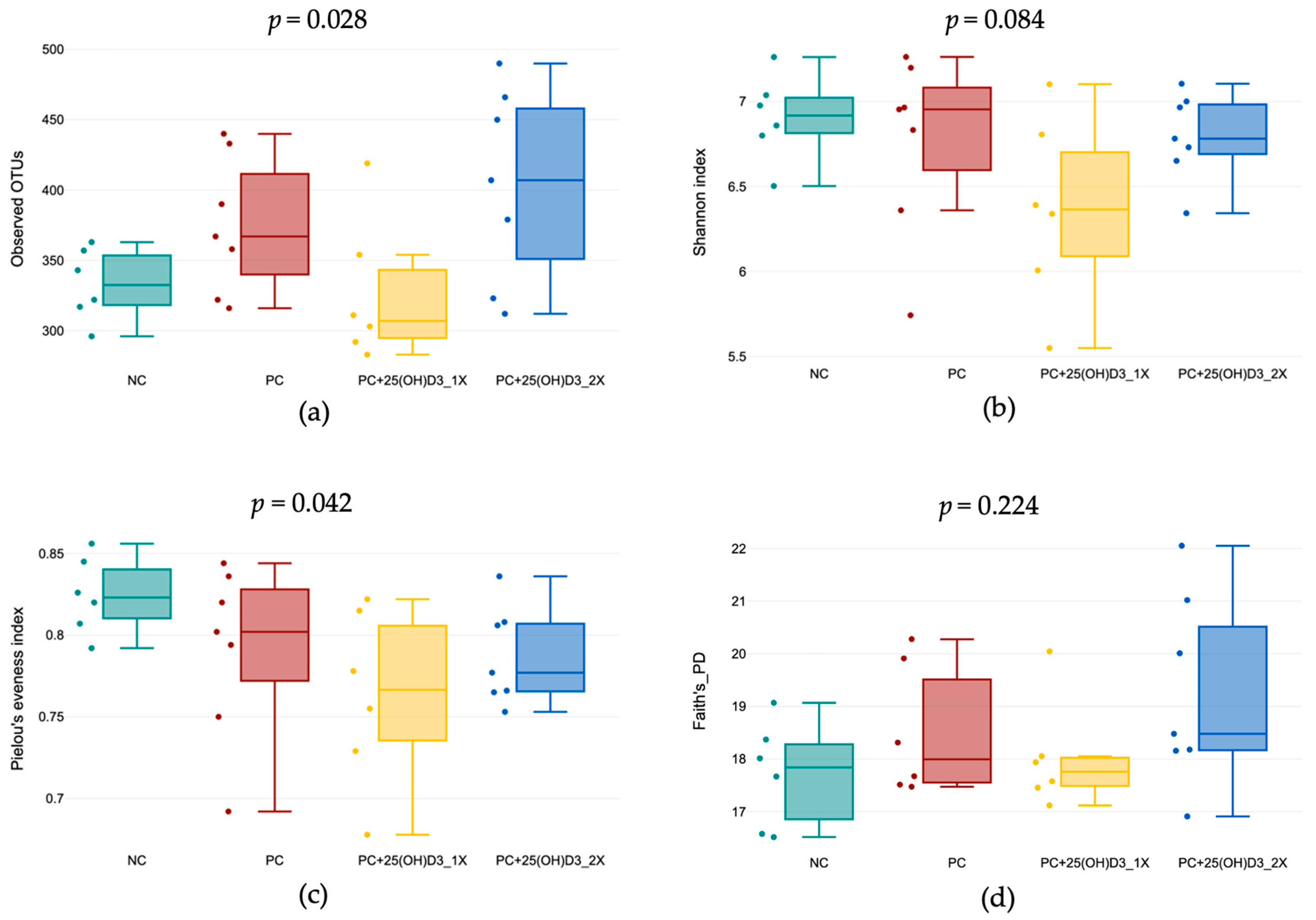
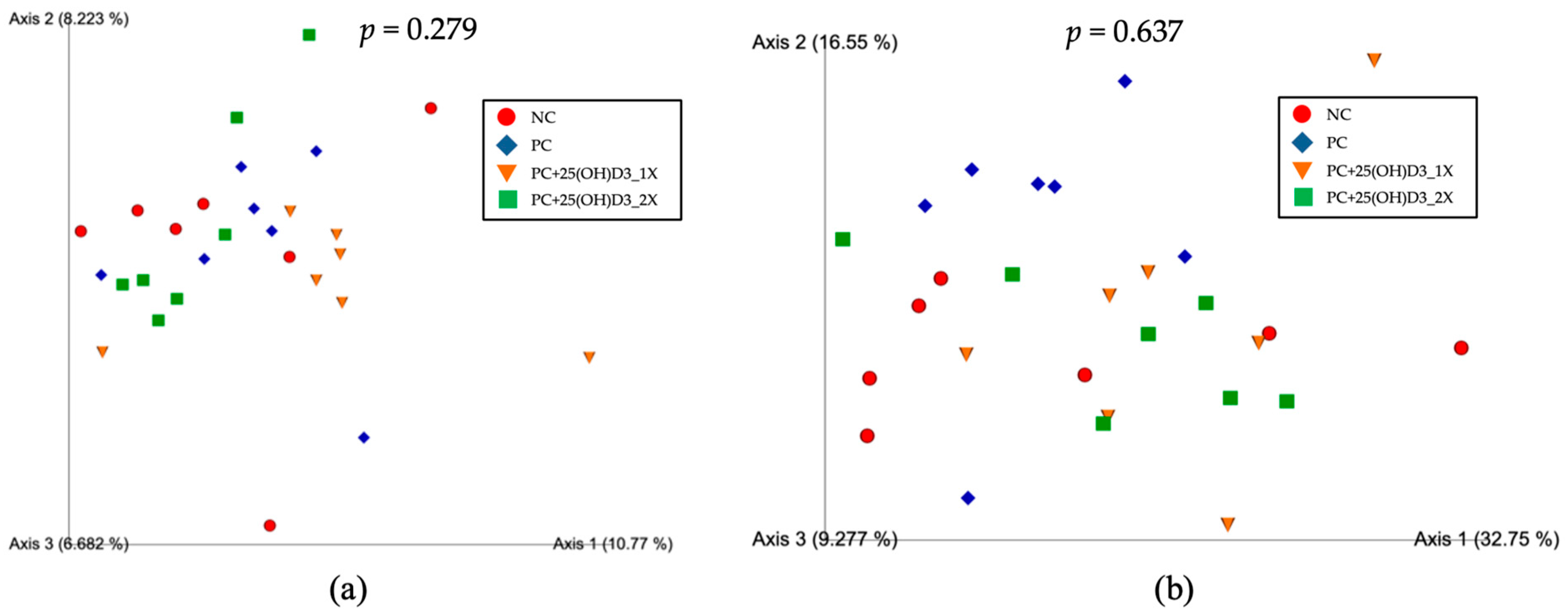
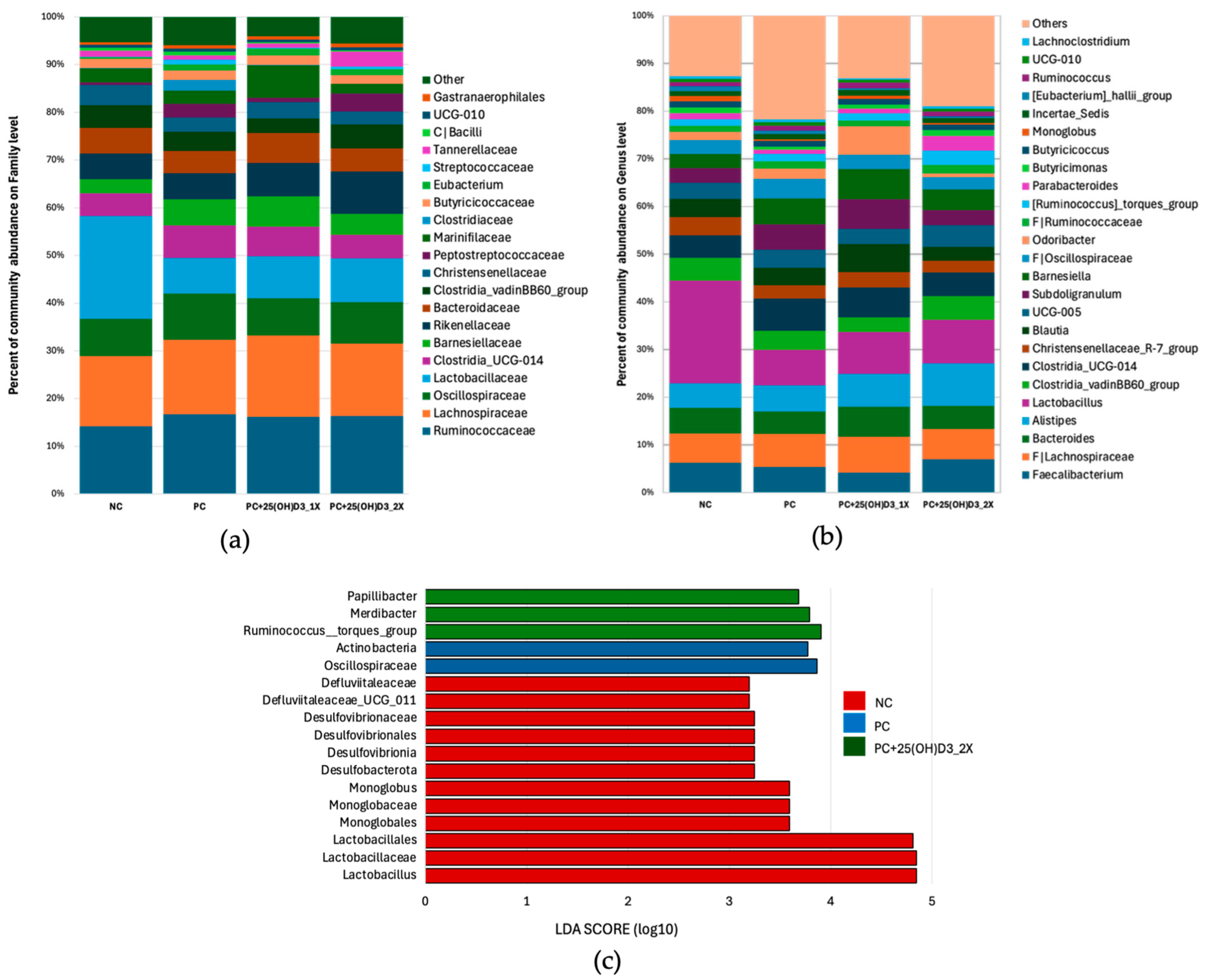
3.3. Microbiota Structure in the Cecal Contents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitehead, C.C. Overview of bone biology in the egg-laying hen. Poult. Sci. 2004, 83, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.Y.; Karnuah, A.B.; Mitchell, A.D.; Anthony, N.B.; Pesti, G.M.; Aggrey, S.E. The effects of growth rate on leg morphology and tibia breaking strength, mineral density, mineral content, and bone ash in broilers. Poult. Sci. 2012, 91, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, C.C.; McCormack, H.A.; McTeir, L.; Fleming, R.H. High vitamin D3 requirements in broilers for bone quality and prevention of tibial dyschondroplasia and interactions with dietary calcium, available phosphorus and vitamin A. Br. Poult. Sci. 2004, 45, 425–436. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, S1689–S1696. [Google Scholar] [CrossRef]
- Świątkiewicz, S.; Arczewska-Włosek, A.; Bederska-Łojewska, D. Efficacy of dietary vitamin D and its metabolites in poultry—Review and implications of the recent studies. World’s Poult. Sci. J. 2017, 73, 57–68. [Google Scholar] [CrossRef]
- Plum, L.A.; DeLuca, H.F. Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug Discov. 2010, 9, 941–955. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, S1080–S1086. [Google Scholar] [CrossRef]
- Fritts, C.A.; Waldroup, P.W. Effect of source and level of vitamin D on live performance and bone development in growing broilers. J. Appl. Poult. Res. 2003, 12, 45–52. [Google Scholar] [CrossRef]
- Leyva-Jimenez, H.; Khan, M.; Gardner, K.; Abdaljaleel, R.A.; Yansoon, A.J.; Alsadwi, A.M.; Bailey, C.A. Developing a novel oral vitamin D3 intake bioassay to re-evaluate the vitamin D3 requirement for modern broiler chickens. Poult. Sci. 2019, 98, 3770–3776. [Google Scholar] [CrossRef]
- Bar, A.; Sharvit, M.; Noff, D.; Edelstein, S.; Hurwitz, S. Absorption and excretion of cholecalciferol and of 25-hydroxycholecalciferol and metabolites in birds. J. Nutr. 1980, 110, 1930–1934. [Google Scholar] [CrossRef]
- Guo, F.; Geng, Y.; Abbas, W.; Zhen, W.; Wang, S.; Huang, Y.; Guo, Y.; Ma, Q.; Wang, Z. Vitamin D3 nutritional status affects gut health of Salmonella-challenged laying hens. Front. Nutr. 2022, 9, 888580. [Google Scholar] [CrossRef] [PubMed]
- FASS. Guide for the Care and Use of Agricultural Animals in Research and Teaching, 4th ed.; Federation of Animal Science Societies: Champaign, IL, USA, 2020. [Google Scholar]
- Agricultural Commodity and Food Standards (ACFS). Guidance on the Application of Thai Agricultural Standard (TAS 6901-2017). ACFS: Bangkok, Thailand, 2017. Available online: https://acfs-backend.acfs.go.th/storage/ProductStandards/Files//20190614120531_637937.pdf (accessed on 15 August 2025).
- Aviagen. Ross 308 Broiler: Nutrition Specifications; Aviagen Group: Huntsville, AL, USA, 2019; Available online: https://en.aviagen.com/ (accessed on 24 August 2025).
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Kechin, A.; Boyarskikh, U.; Kel, A.; Filipenko, M. cutPrimers: A New Tool for Accurate Cutting of Primers from Reads of Targeted Next Generation Sequencing. J. Comput. Biol. 2017, 24, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach, 2nd ed.; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Fleet, J.C. The role of vitamin D in the endocrinology controlling calcium homeostasis. Mol. Cell Endocrinol. 2017, 453, 36–45. [Google Scholar] [CrossRef]
- Wongdee, K.; Charoenphandhu, N. Vitamin D-enhanced duodenal calcium transport. Vitam. Horm. 2015, 98, 407–440. [Google Scholar] [CrossRef]
- Hutton, K.; Vaughn, M.; Litta, G.; Turner, B.; Starkey, J. Effect of vitamin D status improvement with 25-hydroxycholecalciferol on skeletal muscle growth characteristics and satellite cell activity in broiler chickens. J. Anim. Sci. 2014, 92, 411–420. [Google Scholar] [CrossRef]
- Yarger, J.G.; Saunders, C.A.; McNaughton, J.L.; Quarles, C.L.; Hollis, B.W.; Gray, R.W. Comparison of dietary 25-hydroxycholecalciferol and cholecalciferol in broiler chickens. Poult. Sci. 1995, 74, 1159–1167. [Google Scholar] [CrossRef]
- Wei, J.; Li, L.; Peng, Y.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y.; Sun, J. The Effects of Optimal Dietary Vitamin D3 on Growth and Carcass Performance, Tibia Traits, Meat Quality, and Intestinal Morphology of Chinese Yellow-Feathered Broiler Chickens. Animals 2024, 14, 920. [Google Scholar] [CrossRef]
- Norman, A.W. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. Am. J. Clin. Nutr. 2008, 88, S491–S499. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Porta, A.; Mady, L.J.; Seth, T. Vitamin D and intestinal calcium absorption. Mol. Cell Endocrinol. 2011, 347, 25–29. [Google Scholar] [CrossRef]
- van de Peppel, J.; van Leeuwen, J.P.T.M. Vitamin D and gene networks in human osteoblasts. Front. Physiol. 2014, 5, 137. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.H.; Turner, A.G.; Morris, H.A. Vitamin D actions to regulate calcium and skeletal homeostasis. Clin. Biochem. 2012, 45, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Turner, B.; Applegate, T.J.; Litta, G.; Kim, W.K. Role of long-term supplementation of 25-hydroxyvitamin D3 on laying hen bone 3-dimensional structural development. Poult. Sci. 2020, 99, 5771–5782. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ding, X.; Bai, S.; Wang, J.; Zeng, Q.; Peng, H.; Xuan, Y.; Zhang, K. Effects of Supplementation of 25-Hydroxyvitamin D3 as a Vitamin D3 Substitute on Performance, Bone Traits, and Egg Quality of Laying Hens from 1 Day to 72 Weeks of Age. Agriculture 2023, 13, 383. [Google Scholar] [CrossRef]
- Gombart, A.F. The Vitamin D–Antimicrobial Peptide Pathway and Its Role in Protection Against Infection. Future Microbiol. 2009, 4, 1151–1165. [Google Scholar] [CrossRef]
- Sun, J. Vitamin D and mucosal immune function. Curr. Opin. Gastroenterol. 2010, 26, 591–595. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, G.; Shuler, F.D.; Wang, C.H.; Xie, J. Local sustained delivery of 25-hydroxyvitamin D3 for production of antimicrobial peptides. Pharm. Res. 2015, 32, 2851–2862. [Google Scholar] [CrossRef]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Lutfor, A.B.; Razzaque, M.S. Vitamin D and the Host-Gut Microbiome: A Brief Overview. Acta Histochem. Cytochem. 2020, 53, 33–42. [Google Scholar] [CrossRef]
- Battistini, C.; Ballan, R.; Herkenhoff, M.E.; Saad, S.M.I.; Sun, J. Vitamin D Modulates Intestinal Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 362. [Google Scholar] [CrossRef]
- He, L.; Liu, T.; Shi, Y.; Tian, F.; Hu, H.; Deb, D.K.; Chen, Y.; Bissonnette, M.; Li, Y.C. Gut epithelial vitamin D receptor regulates microbiota-dependent mucosal inflammation by suppressing intestinal epithelial cell apoptosis. Endocrinology 2018, 159, 967–979. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D–mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Sun, B.; Hou, L.; Yang, Y. The development of the gut microbiota and short-chain fatty acids of layer chickens in different growth periods. Front. Vet. Sci. 2021, 8, 666535. [Google Scholar] [CrossRef]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira—A candidate for the next-generation probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef]
- Fuentes, S.; den Hartog, G.; Nanlohy, N.M.; Wijnands, L.; Ferreira, J.A.; Nicolaie, M.A.; Pennings, J.L.A.; Jacobi, R.; de Wit, J.; van Beek, J.; et al. Associations of faecal microbiota with influenza-like illness in participants aged 60 years or older: An observational study. Lancet Healthy Longev. 2021, 2, e436–e445. [Google Scholar] [CrossRef]
- Zong, X.; Zhang, H.; Zhu, L.; Deehan, E.C.; Fu, J.; Wang, Y.; Jin, M. Auricularia auricula polysaccharides attenuate obesity in mice through gut commensal Papillibacter cinnamivorans. J. Adv. Res. 2023, 52, 203–218. [Google Scholar] [CrossRef] [PubMed]
| Ingredients (kg) | Starter | Grower | Finisher |
|---|---|---|---|
| Corn | 33.11 | 49.13 | 43.15 |
| Broken rice | 10.00 | 10.00 | - |
| Wheat | 10.00 | - | 20.00 |
| Wheat bran | 2.00 | - | - |
| Full-fat soybean | 10.00 | 10.00 | 12.00 |
| Soybean meal 44% | 31.46 | 27.63 | 20.16 |
| Acid oil | - | 0.25 | 2.15 |
| Monodicalcium phosphate | 0.95 | 0.85 | 0.52 |
| Limestone | 1.20 | 1.05 | 0.70 |
| Sodium bicarbonate | 0.20 | 0.20 | 0.30 |
| Choline | 0.03 | 0.03 | 0.00 |
| DL-methionine | 0.36 | 0.30 | 0.26 |
| L-Lysine | 0.18 | 0.13 | 0.14 |
| L-threonine | 0.10 | 0.07 | 0.06 |
| Phytase | 0.01 | 0.01 | 0.01 |
| NSP Enzyme | 0.01 | 0.01 | 0.01 |
| Salt | 0.25 | 0.20 | 0.20 |
| Mineral Premix 1 | 0.10 | 0.10 | 0.10 |
| Vitamin Premix 2 | 0.04 | 0.04 | 0.04 |
| Total (kg) | 100.00 | 100.00 | 100.00 |
| Nutrients by calculation | |||
| AME, Kcal/kg | 3000.00 | 3100.00 | 3200.00 |
| Crude protein, % | 23.50 | 21.50 | 19.50 |
| Fiber, % | 3.10 | 2.92 | 3.05 |
| Fat, % | 4.13 | 4.65 | 6.95 |
| Methionine, % | 0.56 | 0.51 | 0.47 |
| Methionine + Cystine, % | 1.08 | 0.99 | 0.90 |
| Lysine, % | 1.44 | 1.29 | 1.15 |
| Threonine, % | 0.97 | 0.88 | 0.78 |
| Valine, % | 1.11 | 1.00 | 0.89 |
| Isoleucine, % | 0.97 | 0.89 | 0.80 |
| Arginine, % | 1.52 | 1.37 | 1.21 |
| Tryptophan, % | 0.23 | 0.21 | 0.18 |
| Calcium, % | 0.96 | 0.88 | 0.78 |
| Total phosphorus, % | 0.72 | 0.67 | 0.62 |
| Available phosphorus, % | 0.48 | 0.44 | 0.39 |
| Na, % | 0.23 | 0.23 | 0.20 |
| DEB, mEq/kg | 292.63 | 266.10 | 233.49 |
| Treatments | Starter | Grower | Finisher |
|---|---|---|---|
| NC | N | N | N |
| PC | Vit D3 5000 IU | Vit D3 4500 IU | Vit D3 4000 IU |
| PC+25(OH)D3_1X | Vit D3 5000 IU +25(OH)D3 1394 IU | Vit D3 4500 IU +25(OH)D3 1394 IU | Vit D3 4000 IU +25(OH)D3 1394 IU |
| PC+25(OH)D3_2X | Vit D3 5000 IU +25(OH)D3 2788 IU | Vit D3 4500 IU +25(OH)D3 2788 IU | Vit D3 4000 IU +25(OH)D3 2788 IU |
| Item | NC | PC | PC+ | PC+ | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| 25(OH)D3_1X | 25(OH)D3_2X | ANOVA | L | Q | ||||
| Starter phase (0–11 days) | ||||||||
| BW (g/b) | 294.45 b ± 19.34 | 306.98 a ± 14.47 | 310.13 a ± 8.41 | 314.91 a ± 10.24 | 2.06 | <0.01 | <0.01 | 0.30 |
| BWG (g/b) | 251.55 b ± 19.34 | 264.09 a ± 14.47 | 267.23 a ± 8.41 | 272.01 a ± 10.24 | 2.06 | <0.01 | <0.01 | 0.30 |
| ADG (g/b) | 22.87 b ± 1.76 | 24.01 a ± 1.32 | 24.29 a ± 0.76 | 24.73 a ± 0.93 | 0.19 | <0.01 | <0.01 | 0.30 |
| FI (g/b) | 332.69 ± 15.19 | 348.03 ± 21.34 | 346.41 ± 14.82 | 346.12 ± 23.95 | 2.63 | 0.14 | 0.10 | 0.13 |
| FCR | 1.33 ± 0.06 | 1.32 ± 0.06 | 1.30 ± 0.04 | 1.27 ± 0.08 | 0.01 | 0.11 | 0.02 | 0.65 |
| Mortality (%) | 0.84 ± 2.14 | 0.42 ± 1.57 | 0.00 ± 0.00 | 0.42 ± 1.57 | 0.20 | 0.56 | 0.37 | 0.31 |
| Grower phase (12–30 days) | ||||||||
| BW (g/b) | 1719.05 b ± 50.49 | 1784.05 a ± 42.57 | 1764.88 a ± 64.56 | 1803.95 a ± 74.06 | 8.78 | <0.01 | <0.01 | 0.42 |
| BWG (g/b) | 1408.93 b ± 47.24 | 1469.14 a ± 42.05 | 1470.43 a ± 52.91 | 1496.96 a ± 66.70 | 8.15 | <0.01 | <0.01 | 0.24 |
| ADG (g/b) | 74.15 b ± 2.49 | 77.32 a ± 2.21 | 77.39 a ± 2.78 | 78.79 a ± 3.51 | 0.43 | <0.01 | <0.01 | 0.24 |
| FI (g/b) | 2120.73 ± 86.19 | 2168.44 ± 59.97 | 2116.09 ± 84.31 | 2151.33 ± 88.31 | 10.86 | 0.27 | 0.68 | 0.77 |
| FCR | 1.51 a ± 0.05 | 1.48 ab ± 0.05 | 1.44 b ± 0.05 | 1.44 b ± 0.07 | 0.01 | <0.01 | <0.01 | 0.36 |
| Mortality (%) | 3.78 ± 2.92 | 3.78 ± 4.95 | 4.20 ± 4.86 | 2.94 ± 4.47 | 0.57 | 0.89 | 0.69 | 0.59 |
| Finisher phase (31–40 days) | ||||||||
| BW (g/b) | 2793.03 b ± 122.26 | 2913.80 a ± 83.91 | 2870.30 a ± 72.81 | 2934.38 a ± 78.82 | 13.96 | <0.01 | <0.01 | 0.25 |
| BWG (g/b) | 1073.98 ± 103.45 | 1122.61 ± 63.48 | 1105.43 ± 56.11 | 1130.43 ± 79.72 | 10.53 | 0.24 | 0.11 | 0.57 |
| ADG (g/b) | 107.40 ± 10.35 | 112.26 ± 6.35 | 110.54 ± 5.61 | 113.04 ± 7.97 | 1.05 | 0.24 | 0.11 | 0.57 |
| FI (g/b) | 1864.01 ± 83.20 | 1863.97 ± 118.42 | 1900.74 ± 87.47 | 1894.34 ± 64.09 | 11.97 | 0.58 | 0.24 | 0.90 |
| FCR | 1.75 ± 0.14 | 1.66 ± 0.11 | 1.72 ± 0.11 | 1.68 ± 0.12 | 0.02 | 0.24 | 0.34 | 0.49 |
| Mortality (%) | 2.52 ± 3.80 | 2.10 ± 2.92 | 3.36 ± 5.01 | 2.10 ± 2.92 | 0.49 | 0.79 | 1.00 | 0.68 |
| Overall (0–40 days) | ||||||||
| BW (g/b) | 2793.03 b ± 122.26 | 2913.80 a ± 83.91 | 2870.30 a ± 72.81 | 2934.38 a ± 78.82 | 13.96 | <0.01 | <0.01 | 0.25 |
| BWG (g/b) | 2750.14 b ± 122.26 | 2870.91 a ± 83.91 | 2827.41 a ± 72.81 | 2891.48 a ± 78.82 | 13.96 | <0.01 | <0.01 | 0.25 |
| ADG (g/b) | 68.75 b ± 3.06 | 71.77 a ± 2.10 | 70.69 a ± 1.82 | 72.29 a ± 1.97 | 0.35 | <0.01 | <0.01 | 0.25 |
| FI (g/b) | 4317.42 ± 133.62 | 4380.43 ± 122.00 | 4363.24 ± 117.04 | 4391.79 ± 105.75 | 16.06 | 0.38 | 0.16 | 0.59 |
| FCR | 1.57 a ± 0.04 | 1.53 b ± 0.03 | 1.54 b ± 0.03 | 1.52 b ± 0.03 | 0.01 | <0.01 | <0.01 | 0.27 |
| Mortality (%) | 7.56 ± 6.29 | 5.58 ± 6.10 | 7.56 ± 6.70 | 5.46 ± 4.87 | 0.79 | 0.70 | 0.52 | 0.90 |
| Item | NC | PC | PC+ | PC+ | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| 25(OH)D3_1X | 25(OH)D3_2X | ANOVA | L | Q | ||||
| Tibia ash (%) | 41.30 ± 1.70 | 42.08 ± 2.40 | 42.44 ± 1.38 | 42.52 ± 1.51 | 0.20 | 0.51 | 0.08 | 0.49 |
| Bone-breaking strength (N) | 30.26 ± 5.23 | 32.03 ± 6.63 | 33.49 ± 4.87 | 35.86 ± 7.04 | 0.93 | 0.21 | 0.06 | 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phutthaphol, R.; Bunchasak, C.; Loongyai, W.; Rakangthong, C. Effects of Vitamin D3 and 25(OH)D3 Supplementation on Growth Performance, Bone Parameters and Gut Microbiota of Broiler Chickens. Animals 2025, 15, 2900. https://doi.org/10.3390/ani15192900
Phutthaphol R, Bunchasak C, Loongyai W, Rakangthong C. Effects of Vitamin D3 and 25(OH)D3 Supplementation on Growth Performance, Bone Parameters and Gut Microbiota of Broiler Chickens. Animals. 2025; 15(19):2900. https://doi.org/10.3390/ani15192900
Chicago/Turabian StylePhutthaphol, Rakchanok, Chaiyapoom Bunchasak, Wiriya Loongyai, and Choawit Rakangthong. 2025. "Effects of Vitamin D3 and 25(OH)D3 Supplementation on Growth Performance, Bone Parameters and Gut Microbiota of Broiler Chickens" Animals 15, no. 19: 2900. https://doi.org/10.3390/ani15192900
APA StylePhutthaphol, R., Bunchasak, C., Loongyai, W., & Rakangthong, C. (2025). Effects of Vitamin D3 and 25(OH)D3 Supplementation on Growth Performance, Bone Parameters and Gut Microbiota of Broiler Chickens. Animals, 15(19), 2900. https://doi.org/10.3390/ani15192900





