Subterranean Biodiversity on the Brink: Urgent Framework for Conserving the Densest Cave Region in South America
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
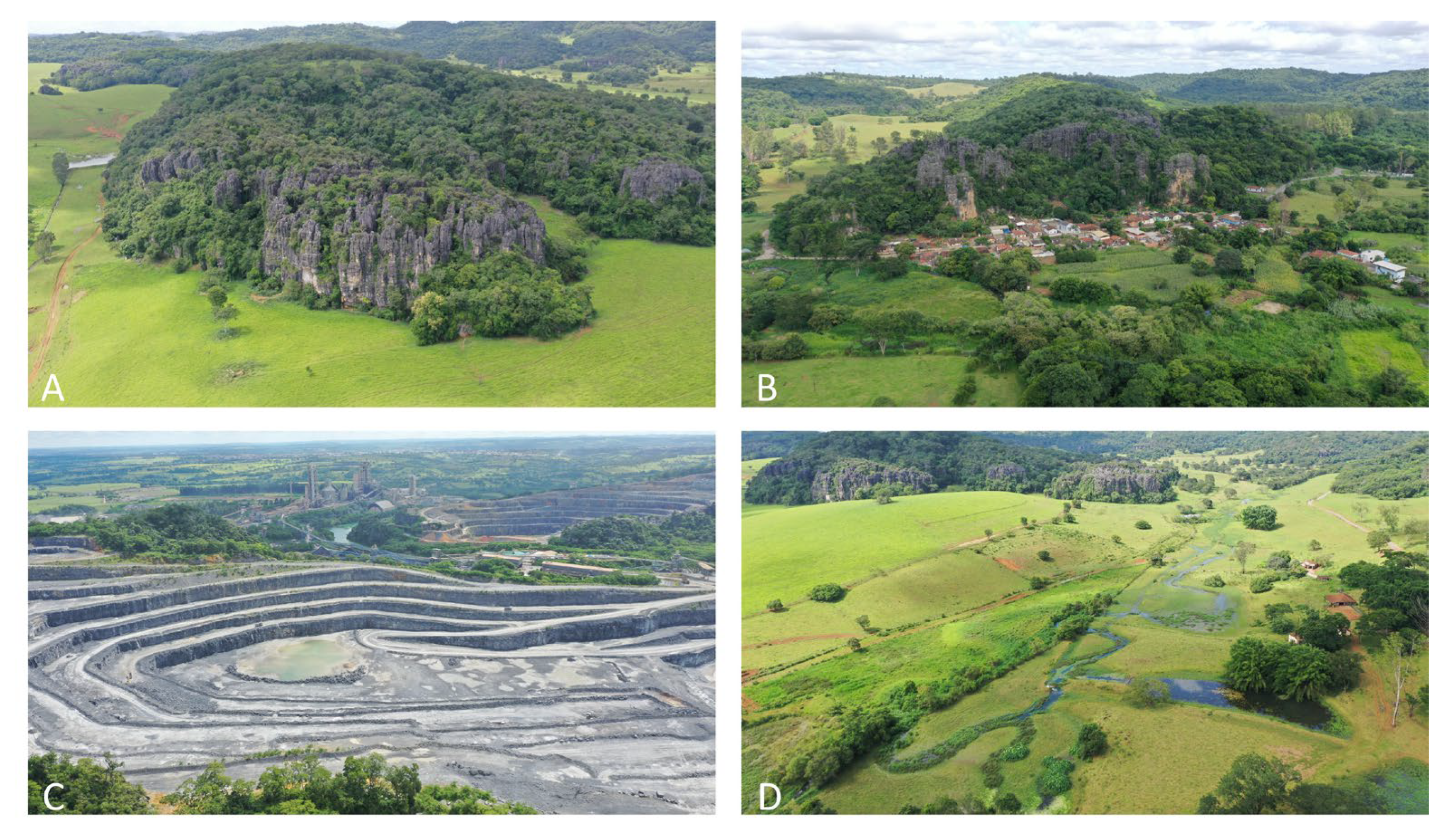
2.1. Study Area
2.2. Sampling Design
2.3. Inventory of the Cave Fauna
2.4. Determining Potential “Stygobionts” and “Troglobionts”
2.5. Data Analysis
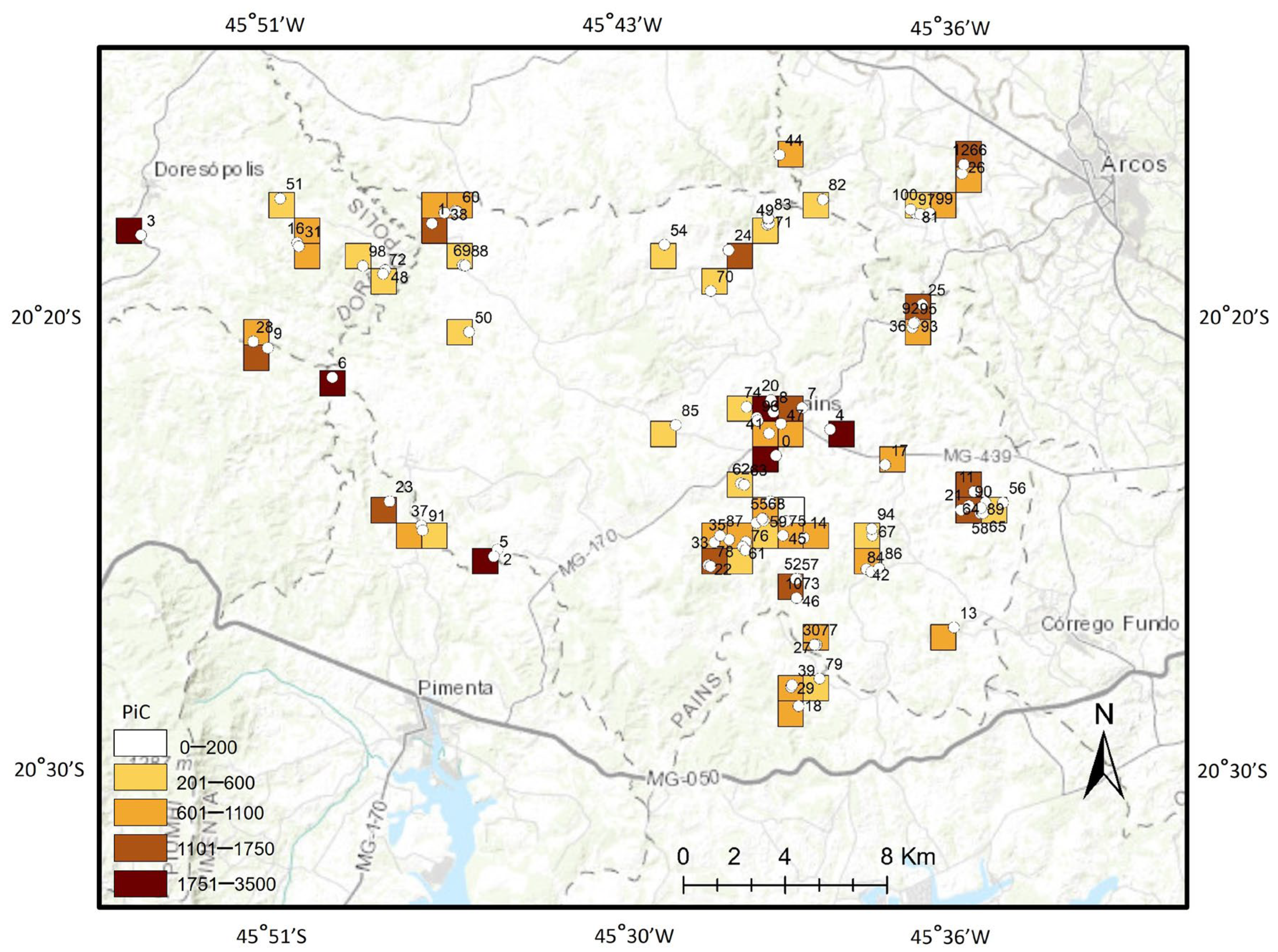
3. Results
4. Discussion
Indication of Priority Areas
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakalowicz, M. Karst groundwater: A challenge for new resources. Hydrogeol. J. 2005, 13, 148–160. [Google Scholar] [CrossRef]
- Goldscheider, N.; Chen, Z.; Auler, A.S.; Bakalowicz, M.; Broda, S.; Drew, D.; Veni, G. Global distribution of carbonate rocks and karst water resources. Hydrogeol. J. 2020, 28, 1661–1677. [Google Scholar] [CrossRef]
- Canedoli, C.; Ficetola, G.F.; Corengia, D.; Tognini, P.; Ferrario, A.; Padoa-Schioppa, E. Integrating landscape ecology and the assessment of ecosystem services in the study of karst areas. Landscape Ecol. 2022, 37, 347–365. [Google Scholar] [CrossRef]
- Santos, M.D.; Ruchkys, Ú.A.; Pereira, E.O. Quantification of Geodiversity Services in the São Francisco River Basin, Brazil (Minas Gerais portion), and Their Importance for the Management of Water Resources. Geoheritage 2023, 15, 111. [Google Scholar] [CrossRef]
- Brilha, J.; Gray, M.; Pereira, D.I.; Pereira, P. Geodiversity: An integrative review as a contribution to the sustainable management of the whole of nature. Environ. Sci. Policy 2018, 86, 19–28. [Google Scholar] [CrossRef]
- Vlaicu, M.; Munteanu, C.M. Karst groundwaters vulnerability assessment methods. Trav. Inst. Spéol. 2008, 40, 107–118. [Google Scholar]
- Gutiérrez, F.; Parise, M.; De Waele, J.; Jourde, H. A review on natural and human-induced geohazards and impacts in karst. Earth-Sci. Rev. 2014, 138, 61–88. [Google Scholar] [CrossRef]
- Lucon, T.N.; Costa, A.T.; Galvão, P.; Leite, M.G.P.; Madeira, T.; Nogueira, L.B. Recharge sources and hydraulic communication of karst aquifer, São Miguel watershed, MG, Brazil. J. S. Am. Earth Sci. 2020, 100, 102591. [Google Scholar] [CrossRef]
- Piló, L.B.; Auler, A. Introdução à espeleologia. In III Curso de Espeleologia e Licenciamento Ambiental; ICMBio/CECAV: Brasília, Brazil, 2011; pp. 7–23. Available online: https://www.researchgate.net/publication/336831313_Introducao_a_espeleologia (accessed on 28 July 2025).
- Edmonds, C.N. Karst and mining geohazards with particular reference to the Chalk outcrop, England. Q. J. Eng. Geol. Hydrogeol. 2008, 41, 261–278. [Google Scholar] [CrossRef]
- Colado, R.; Abellán, P.; Pallarés, S.; Mammola, S.; Milione, R.; Faille, A.; Sánchez-Fernández, D. A dark side of conservation biology: Protected areas fail in representing subterranean biodiversity. Insect Conserv. Divers. 2023, 16, 674–683. [Google Scholar] [CrossRef]
- Iannella, M.; Fiasca, B.; Di Lorenzo, T.; Biondi, M.; Di Cicco, M.; Galassi, D.M. Jumping into the grids: Mapping biodiversity hotspots in groundwater habitat types across Europe. Ecography 2020, 43, 1825–1841. [Google Scholar] [CrossRef]
- Wynne, J.J.; Howarth, F.G.; Sommer, S.; Dickson, B.G. Fifty years of cave arthropod sampling: Techniques and best practices. Int. J. Speleol. 2019, 48, 33–48. [Google Scholar] [CrossRef]
- Wynne, J.J.; Howarth, F.G.; Mammola, S.; Ferreira, R.L.; Cardoso, P.; Di Lorenzo, T.; Zhao, Y. A conservation roadmap for the subterranean biome. Conserv. Lett. 2021, 14, e12834. [Google Scholar] [CrossRef]
- Mammola, S.; Altermatt, F.; Alther, R.; Amorim, I.R.; Băncilă, R.I.; Borges, P.A.; Malard, F. Perspectives and pitfalls in preserving subterranean biodiversity through protected areas. npj Biodiversity 2024, 3, 2. [Google Scholar] [CrossRef]
- Mammola, S.; Lunghi, E.; Bilandžija, H.; Cardoso, P.; Grimm, V.; Schmidt, S.I.; Martínez, A. Collecting eco-evolutionary data in the dark: Impediments to subterranean research and how to overcome them. Ecol. Evol. 2021, 11, 5911–5926. [Google Scholar] [CrossRef]
- Gunn, J.; Hardwick, P.; Wood, P.J. The invertebrate community of the Peak-Speedwell Cave system, Derbyshire, England—Pressures and considerations for conservation management. Aquat. Conserv. 2000, 10, 353–369. [Google Scholar] [CrossRef]
- Mammola, S.; Goodacre, S.L.; Isaia, M. Climate change may drive cave spiders to extinction. Ecography 2018, 41, 233–243. [Google Scholar] [CrossRef]
- Hortal, J.; de Bello, F.; Diniz, J.A.F.; Lewinsohn, T.M.; Lobo, J.M.; Ladle, R.J. Seven shortfalls that beset large-scale knowledge of biodiversity. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 523–549. [Google Scholar] [CrossRef]
- Howarth, F.G. Delay in recognizing terrestrial obligate cave species in the tropics. Int. J. Speleol. 2022, 52, 23–43. [Google Scholar] [CrossRef]
- Mammola, S.; Cardoso, P.; Culver, D.C.; Deharveng, L.; Ferreira, R.L.; Fišer, C.; Zagmajster, M. Scientists’ warning on the conservation of subterranean ecosystems. BioScience 2019, 69, 641–650. [Google Scholar] [CrossRef]
- Vaccarelli, I.; Colado, R.; Pallares, S.; Galassi, D.M.; Sanchez-Fernandez, D.; Di Cicco, M.; Meierhofer, M.B.; Piano, E.; Di Lorenzo, T.; Mammola, S. A global meta-analysis reveals multilevel and context-dependent effects of climate change on subterranean ecosystems. One Earth 2023, 6, 1510–1522. [Google Scholar] [CrossRef]
- Mammola, S.; Amorim, I.R.; Bichuette, M.E.; Borges, P.A.; Cheeptham, N.; Cooper, S.J.; Culver, D.C.; Deharveng, L.; Eme, D.; Ferreira, R.L.; et al. Fundamental research questions in subterranean biology. Biol. Rev. 2020, 95, 1855–1872. [Google Scholar] [CrossRef]
- Mammola, S.; Meierhofer, M.B.; Borges, P.A.; Colado, R.; Culver, D.C.; Deharveng, L.; Delić, T.; Di Lorenzo, T.; Dražina, T.; Ferreira, R.L.; et al. Towards evidence-based conservation of subterranean ecosystems. Biol. Rev. 2022, 97, 1476–1510. [Google Scholar] [CrossRef] [PubMed]
- Auler, A. Histórico, ocorrência e potencial de cavernas no Brasil. In Cavernas: Atlas do Brasil Subterrâneo; Rubbioli, E., Auler, A.S., Menin, D., Brandi, R., Eds.; ICMBio: Brasília, Brazil, 2019; Available online: https://www.researchgate.net/publication/334307778_Cavernas_Atlas_do_Brasil_Subterraneo (accessed on 10 July 2024).
- Calux, A.; Cassimiro, R.; Salgado, A. Caves in iron formations in the Quadrilátero Ferrífero. Z. Geomorphol. 2019, 62, 125–144. [Google Scholar] [CrossRef]
- Cruz, J.B.; Costa Neto, J.F. Anuário Estatístico do Patrimônio Espeleológico Brasileiro. ICMBio 2022. Available online: https://www.gov.br/icmbio/pt-br/assuntos/centros-de-pesquisa/cavernas/anuario-estatistico-do-patrimonio-espeleologico-brasileiro/anuario-estatistico-do-patrimonio-espeleologico-brasileiro (accessed on 12 May 2023).
- Sugai, L.S.M.; Ochoa-Quintero, J.M.; Costa-Pereira, R.; Roque, F.O. Beyond aboveground. Biodivers. Conserv. 2015, 24, 2109–2112. [Google Scholar] [CrossRef]
- CECAV. Cadastro Nacional de Informações Espeleológicas—CANIE. ICMBio 2025. Available online: https://www.gov.br/icmbio/pt-br/assuntos/centros-de-pesquisa/cavernas/cadastro-nacional-de-informacoes-espeleologicas/canie (accessed on 20 July 2025).
- Pinheiro, R.O.; Gentilini, S.; Giardino, M. Geoconservation in mining landscapes. Resources 2023, 12, 20. [Google Scholar] [CrossRef]
- Martins, V.; Gomes, M.; Ferreira, R. A Buried Umbrella: Historical Changes in the Landscape Surrounding the Habitats of Threatened Troglobitic Isopods in Brazil. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4692363 (accessed on 29 September 2025).
- Myers, N.; Mittermeier, R.A.; Mittermeier, G.M.; Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Melo, P.H.A.; Lombardi, J.A.; Salino, A.; Carvalho, D.A. Composição florística de angiospermas no carste do Alto São Francisco. Rodriguésia 2013, 64, 29–36. [Google Scholar] [CrossRef]
- Zampaulo, R.A. Diversidade de invertebrados cavernícolas na província espeleológica de Arcos, Pains e Doresópolis. Master’s Thesis, UFLA, Lavras, Brazil, 19 March 2010. [Google Scholar]
- Timo, M.B.; Travassos, L.E.P. Geomorphological units in Arcos-pains karst region, Minas Gerais, Brazil. Acta Carsologica 2023, 52, 219–228. [Google Scholar] [CrossRef]
- Souza-Silva, M.; Martins, R.P.; Ferreira, R.L. Cave lithology and invertebrate communities. Biodivers. Conserv. 2011, 20, 1713–1729. [Google Scholar] [CrossRef]
- Sket, B. Ecological classification of subterranean animals. J. Nat. Hist. 2008, 42, 1549–1563. [Google Scholar] [CrossRef]
- Deharveng, L.; Bedos, A.; Pipan, T.; Culver, D.C. Global Subterranean Biodiversity: A Unique Pattern. Diversity 2024, 16, 157. [Google Scholar] [CrossRef]
- Souza-Silva, M.; Martins, R.P.; Ferreira, R.L. Cave conservation priority index to adopt a rapid protection strategy: A case study in Brazilian Atlantic rain forest. Environ. Manag. 2015, 55, 279–295. [Google Scholar] [CrossRef]
- Iannella, M.; Fiasca, B.; Di Lorenzo, T.; Di Cicco, M.; Biondi, M.; Mammola, S.; Galassi, D.M. Getting the ‘most out of the hotspot’ for practical conservation of groundwater biodiversity. Glob. Ecol. Conserv. 2021, 31, e01844. [Google Scholar] [CrossRef]
- Auler, A.S.; Piló, L.B. Caves and mining in Brazil: The dilemma of cave preservation within a mining context. In Hydrogeological and Environmental Investigations in Karst Systems; Springer: Berlin/Heidelberg, Germany, 2014; pp. 487–496. [Google Scholar]
- Tomlin, C.D. Geographic Information Systems and Cartographic Modeling; Prentice-Hall: Englewood Cliffs, NJ, USA, 1990; 249p. [Google Scholar]
- Jenks, G.F.; Caspall, F.C. Error on choroplethic maps: Definition, measurement, reduction. Ann. Assoc. Am. Geogr. 1971, 61, 217–244. [Google Scholar] [CrossRef]
- Cardoso, R.C.; Ferreira, R.L.; Souza-Silva, M. Caves’ environmental stability shaping subterranean biodiversity in the neotropics. Acta Oecol. 2024, 125, 104036. [Google Scholar] [CrossRef]
- Van Belle, G.; Fisher, L.D.; Heagerty, P.J.; Lumley, T. Biostatistics: A Methodology for the Health Sciences; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 0-471-03185-2. [Google Scholar]
- INMET—Instituto Nacional de Meteorologia. Available online: https://portal.inmet.gov.br/ (accessed on 10 August 2025).
- Simões, M.E.; Souza-Silva, M.; Ferreira, R.L. Cave invertebrates in northwestern Minas Gerais state, Brazil: Endemism, threats and conservation priorities. Acta Carsol. 2014, 43, 159–174. [Google Scholar] [CrossRef]
- Jaffé, R.; Prous, X.; Zampaulo, R.; Giannini, T.C.; Imperatriz-Fonseca, V.L.; Maurity, C.; Oliveira, G.; Brandi, I.V.; Siqueira, J.O. Reconciling mining with the conservation of cave biodiversity: A quantitative baseline to help establish conservation priorities. PLoS ONE 2016, 11, e0168348. [Google Scholar] [CrossRef]
- Rabelo, L.M.; Souza-Silva, M.; Ferreira, R.L. Priority caves for biodiversity conservation in a key karst area of Brazil: Comparing the applicability of cave conservation indices. Biodivers. Conserv. 2018, 27, 2097–2129. [Google Scholar] [CrossRef]
- Souza-Silva, M.; Ferreira, R.L. The first two hotspots of subterranean biodiversity in South America. Subterr. Biol. 2016, 19, 1–21. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Canedoli, C.; Stoch, F. The Racovitzan impediment and the hidden biodiversity of unexplored environments. Conserv. Biol. 2019, 33, 214–216. [Google Scholar] [CrossRef]
- Ferreira, R.L.; Souza-Silva, M.; Zampaulo, R.A. A vida subterrânea no carste de Pains: Biodiversidade, ameaças e conservação de fauna em uma notável paisagem cárstica tropical. In A Região Cárstica de Pains, 1st ed.; Piló, L.B., Cruz, J.B., Eds.; Instituto Chico Mendes de Conservação da Biodiversidade: Brasília, Brazil, 2022; Volume 1, pp. 150–177. [Google Scholar]
- Trevelin, L.C.; Gastauer, M.; Prous, X.; Nicácio, G.; Zampaulo, A.R.; Brandi, I.; Oliveira, G.; Siqueira, J.O.; Jaffé, R. Biodiversity surrogates in Amazonian iron cave ecosystems. Ecol. Indic. 2019, 101, 813–820. [Google Scholar] [CrossRef]
- Hoffmann, M.; Brooks, T.M.; Da Fonseca, G.A.B.; Gascon, C.; Hawkins, A.F.A.; James, R.E.; Silva, J.M.C. Conservation planning and the IUCN Red List. Endanger. Species Res. 2008, 6, 113–125. [Google Scholar] [CrossRef]
- Deharveng, L.; Bedos, A. Diversity of terrestrial invertebrates in subterranean habitats. In Cave Ecology; Moldovan, O., Kováč, Ľ., Halse, S., Eds.; Springer: Cham, Switzerland, 2018; pp. 107–172. [Google Scholar] [CrossRef]
- Manenti, R.; Barzaghi, B.; Lana, E.; Stocchino, G.A.; Manconi, R.; Lunghi, E. The stenoendemic cave-dwelling planarians of the Italian Alps and Apennines: Conservation issues. J. Nat. Conserv. 2018, 45, 90–97. [Google Scholar] [CrossRef]
- Zagmajster, M.; Culver, D.C.; Sket, B. Species richness patterns of obligate subterranean beetles in a global biodiversity hotspot: Effect of scale and sampling intensity. Divers. Distrib. 2008, 14, 95–105. [Google Scholar] [CrossRef]
- Bregović, P.; Zagmajster, M. Understanding hotspots within a global hotspot—Identifying the drivers of regional species richness patterns in terrestrial subterranean habitats. Insect Conserv. Divers. 2016, 9, 268–281. [Google Scholar] [CrossRef]
- Zagmajster, M.; Malard, F.; Eme, D.; Culver, D.C. Subterranean biodiversity patterns from global to regional scales. In Cave Ecology; Moldovan, O., Kováč, Ľ., Halse, S., Eds.; Springer: Cham, Switzerland, 2018; pp. 195–227. [Google Scholar] [CrossRef]
- Simões, M.H.; Souza-Silva, M.; Ferreira, R.L. Species-area relationship and richness persistence as a proxy of environmental carrying capacity: A case study in a neotropical show cave. Acta Oecol. 2022, 116, 10384860. [Google Scholar] [CrossRef]
- Souza-Silva, M.; Iniesta, L.F.M.; Ferreira, R.L. Cave lithology effect on subterranean biodiversity: A case study in quartzite and granitoid caves. Acta Oecol. 2020, 108, 103645. [Google Scholar] [CrossRef]
- Souza-Silva, M.; Iniesta, L.F.M.; Ferreira, R.L. Invertebrates diversity in mountain Neotropical quartzite caves: Which factors can influence the composition, richness, and distribution of the cave communities? Subterr. Biol. 2020, 33, 23–43. [Google Scholar] [CrossRef]
- Venarsky, M.P.; Huntsman, B.M. Food webs in caves. In Cave Ecology; Moldovan, O., Kováč, Ľ., Halse, S., Eds.; Springer: Cham, Switzerland, 2018; pp. 309–328. [Google Scholar] [CrossRef]
- Souza-Silva, M.; Júnior, A.S.; Ferreira, R.L. Food resource availability in a quartzite cave in the Brazilian montane Atlantic Forest. J. Cave Karst Stud. 2013, 75. [Google Scholar] [CrossRef]
- Mammola, S.; Piano, E.; Cardoso, P.; Vernon, P.; Domínguez-Villar, D.; Culver, D.C.; Pipan, T.; Isai, M. Climate change going deep: The effects of global climatic alterations on cave ecosystems. Anthr. Rev. 2019, 6, 98–116. [Google Scholar] [CrossRef]
- Ferreira, R.L.; Bernard, E.; da Cruz Júnior, F.W.; Piló, L.B.; Calux, A.; Souza-Silva, M.; Barlow, J.; Pompeu, P.S.; Cardoso, P.; Mammola, S.; et al. Brazilian cave heritage under siege. Science 2022, 375, 1238–1239. [Google Scholar] [CrossRef]


| Criterion | Description |
|---|---|
| Non-troglobite Species Richness | Total number of non-troglobite species observed per cave. This value reflects the ecological connectivity and the cave’s contribution to the broader subterranean network [39]. |
| Troglobite Species Richness | Number of species exclusively adapted to subterranean environments (troglobites). Represents the taxonomic and evolutionary significance of the cave habitat [12,39,40]. |
| Endemic Troglobite Species | The number of troglobite species found in only one cave (stenoendemic) [12,40]. These species are highly endemic and extremely vulnerable to extinction due to environmental impacts, such as surface alteration or direct cave destruction (e.g., from mining activities). |
| Vulnerability | A 250 m buffer was established around each cave to evaluate the presence or absence of human impacts. Caves were assessed for vulnerability to anthropogenic disturbances (pollution, land use change, groundwater contamination). This buffer is based on Brazilian Decree No. 6640 (2008) [41]. |
| CT | SC | nTS | TbS | EnD | VuL | PiC |
|---|---|---|---|---|---|---|
| Low | 100 | 17–36 | 1–2 | 0 | 1 | 201–600 |
| Average | 250 | 37–55 | 3–4 | 1 | 2 | 601–110 |
| High | 500 | 56–74 | 5–6 | 2 | 3 | 1101–176 |
| Extreme | 1000 | 75–93 | 6–7 | 3 | 4, 5 | 1751–350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zampaulo, R.d.A.; Souza-Silva, M.; Ferreira, R.L. Subterranean Biodiversity on the Brink: Urgent Framework for Conserving the Densest Cave Region in South America. Animals 2025, 15, 2899. https://doi.org/10.3390/ani15192899
Zampaulo RdA, Souza-Silva M, Ferreira RL. Subterranean Biodiversity on the Brink: Urgent Framework for Conserving the Densest Cave Region in South America. Animals. 2025; 15(19):2899. https://doi.org/10.3390/ani15192899
Chicago/Turabian StyleZampaulo, Robson de Almeida, Marconi Souza-Silva, and Rodrigo Lopes Ferreira. 2025. "Subterranean Biodiversity on the Brink: Urgent Framework for Conserving the Densest Cave Region in South America" Animals 15, no. 19: 2899. https://doi.org/10.3390/ani15192899
APA StyleZampaulo, R. d. A., Souza-Silva, M., & Ferreira, R. L. (2025). Subterranean Biodiversity on the Brink: Urgent Framework for Conserving the Densest Cave Region in South America. Animals, 15(19), 2899. https://doi.org/10.3390/ani15192899








