Integrating Habitat Prediction and Risk Assessment to Prioritize Conservation Areas for the Long-Tailed Goral (Naemorhedus caudatus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Study Site
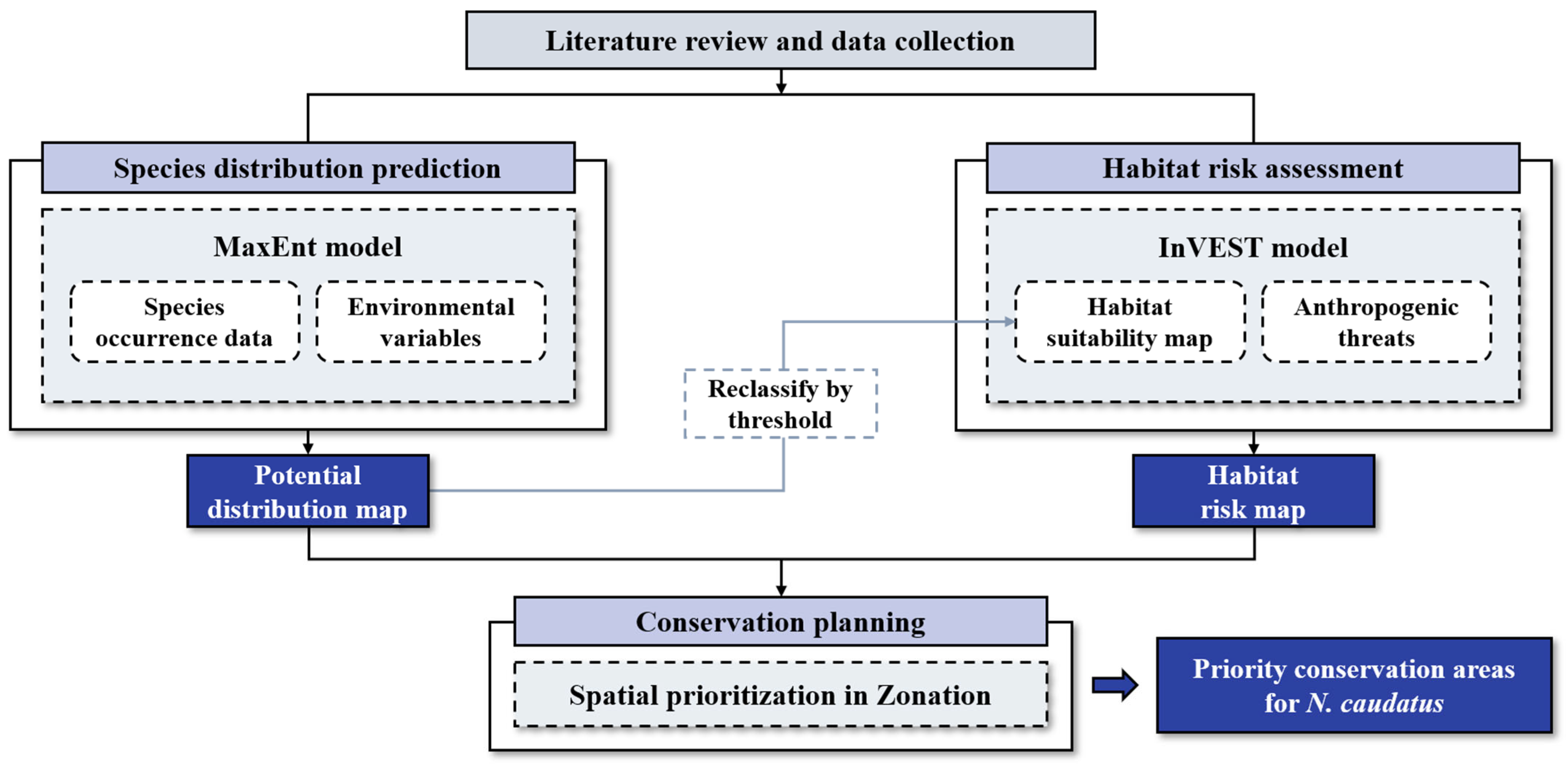
2.3. Study Flow
2.4. Potential Habitat Prediction
2.5. Habitat Risk Assessment
2.6. Spatial Prioritization and Core Conservation Areas
3. Results and Discussion
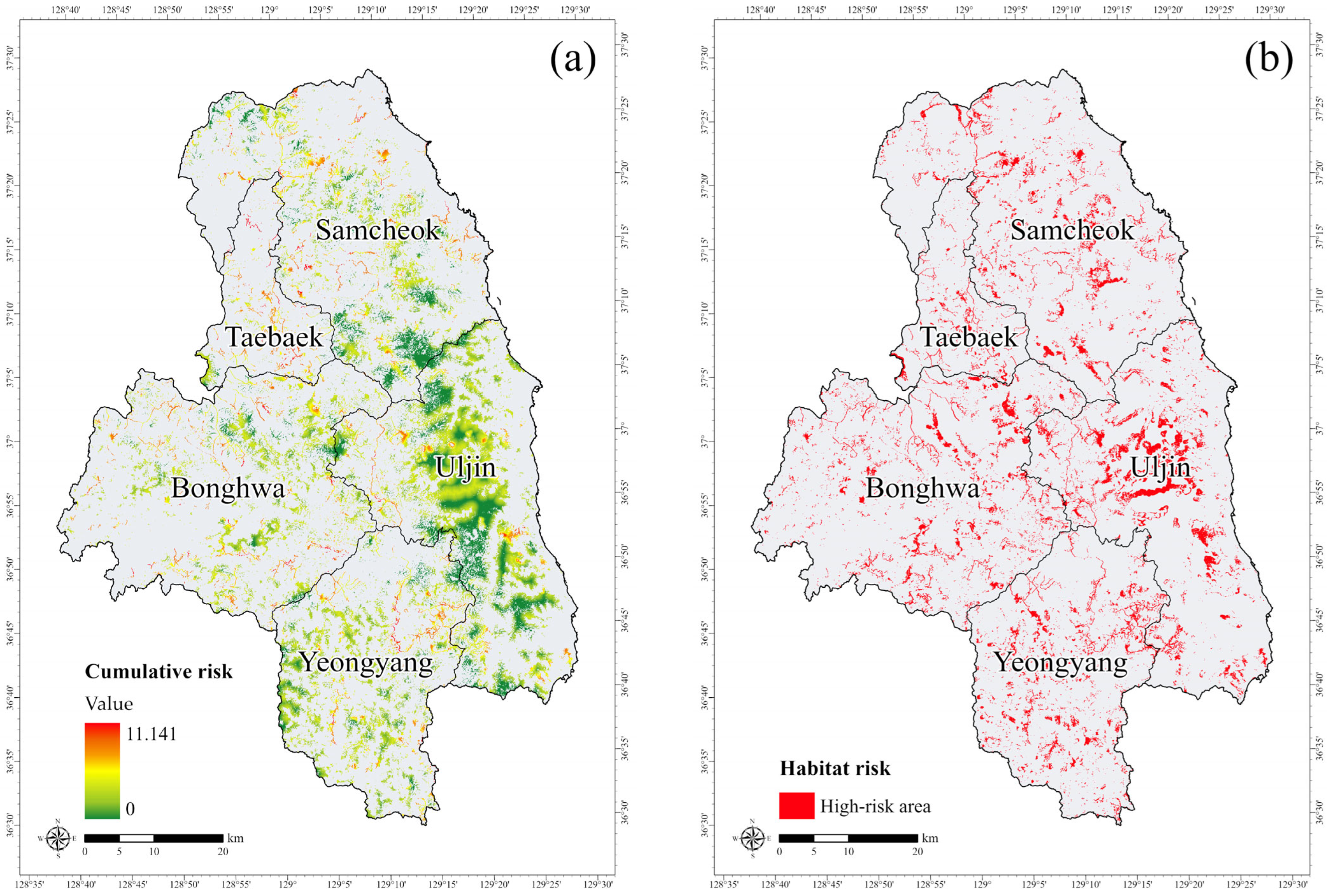
3.1. Potential Habitat for the Long-Tailed Goral
3.2. Risk Areas in the Habitat of the Long-Tailed Goral
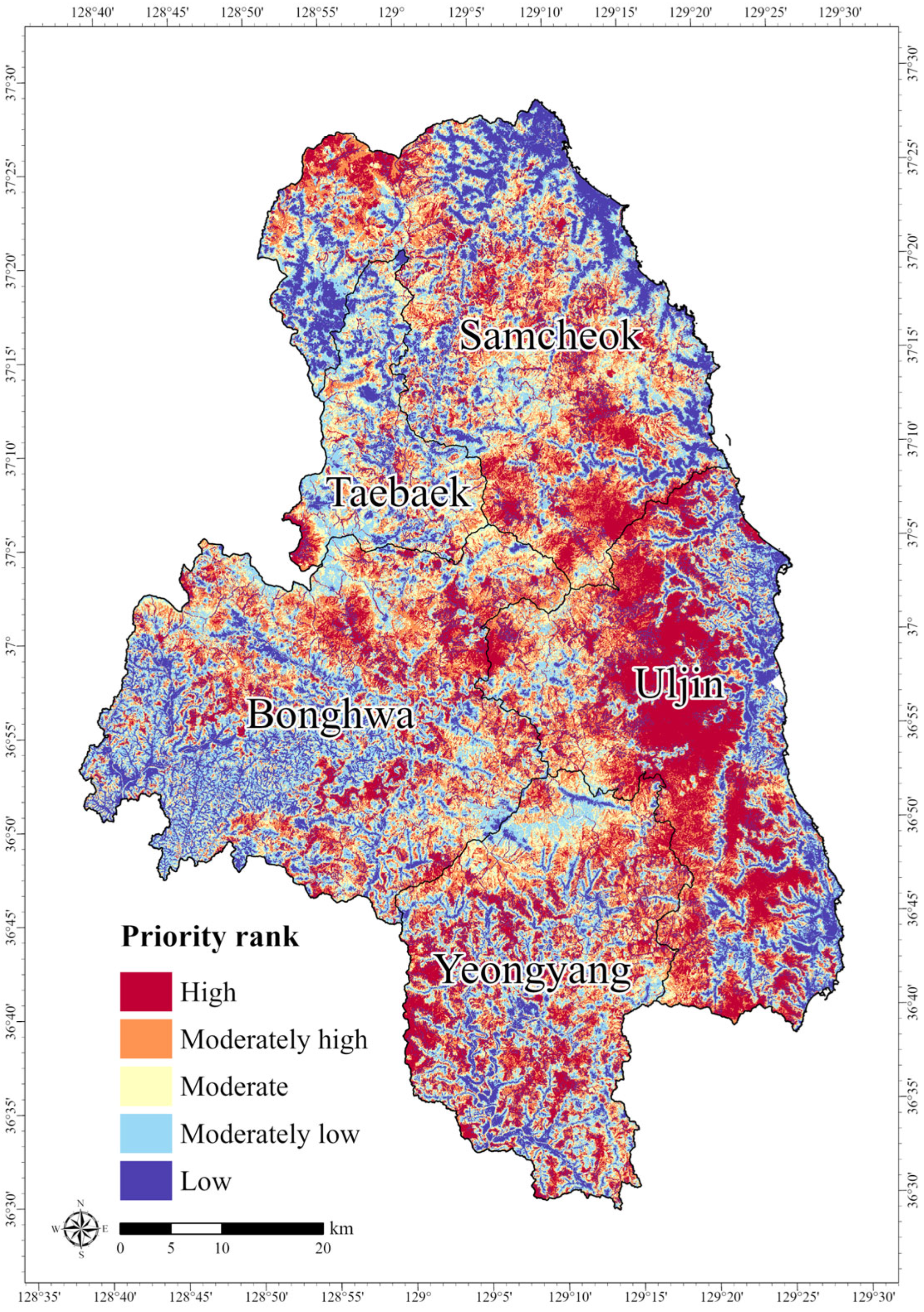
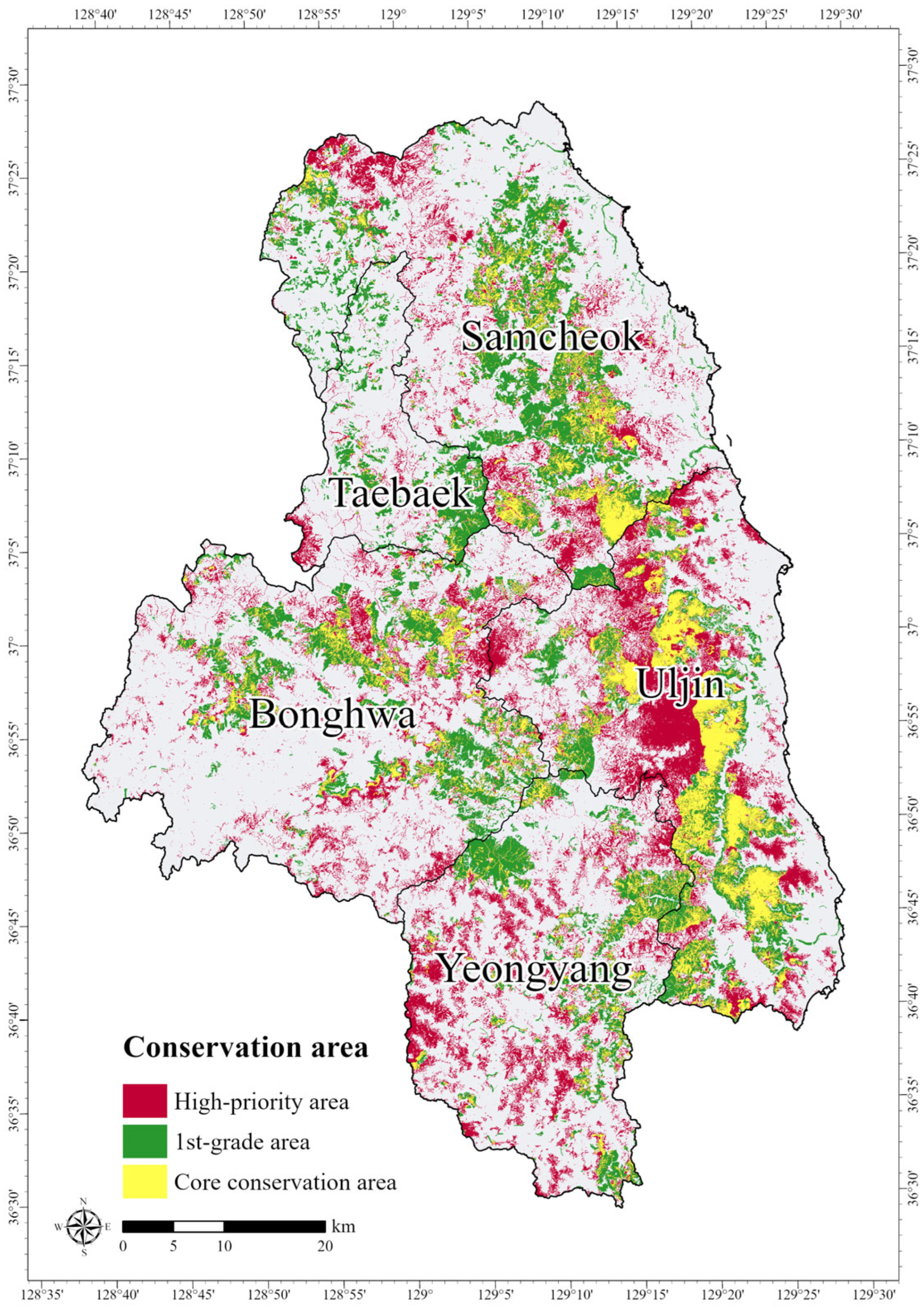
3.3. Prioritizing Conservation Areas
3.4. Ecological Value of the Core Conservation Areas
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilcove, D.S.; Rothstein, D.; Dubow, J.; Phillips, A.; Losos, E. Quantifying Threats to Imperiled Species in the United States. BioScience 1998, 48, 607–615. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Sala, O.E.; Hobbie, S.E.; et al. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef]
- Singh, S.K.; Sharma, M.; Pandey, A. Biodiversity-Threats and Conservation. In Environmental Science and Engineering; Gurjar, B.R., Kumar, A., Eds.; Studium Press LLC: Houston, TX, USA, 2017; pp. 282–316. [Google Scholar]
- Sher, A.A.; Primack, R.B.; Lee, S.D.; Kim, Y.H.; Kim, J.G.; Nam, D.H.; Park, J.H.; Bae, Y.J.; Seo, J.E.; Lee, G.S. Conservation Biology; Worldscience: Seoul, Republic of Korea, 2021; pp. 182–187. [Google Scholar]
- Duffy, J.E. Why biodiversity is important to the functioning of real-world ecosystems. Front. Ecol. Environ. 2009, 7, 437–444. [Google Scholar] [CrossRef]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.; Petchey, O.L.; et al. Biodiversity and Resilience of Ecosystem Functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef]
- Spiers, J.A.; Oatham, M.P.; Rostant, L.V.; Farrell, A.D. Applying species distribution modelling to improving conservation based decisions: A gap analysis of Trinidad and Tobago’s endemic vascular plants. Biodivers. Conserv. 2018, 27, 2931–2949. [Google Scholar] [CrossRef]
- Yang, B.G. Systematics, Ecology and Current Population Status of the Goral, Naemorhedus caudatus, in Korea. Ph.D. Thesis, Chungbuk National University, Cheongju, Republic of Korea, 2002. [Google Scholar]
- Choi, S.K.; Chun, S.W.; An, J.H.; Lee, M.Y.; Kim, H.J.; Min, M.S.; Kwon, S.W.; Choi, T.Y.; Lee, H.; Kim, K.S. Genetic diversity and population structure of the long-tailed goral, Naemorhedus caudatus, in South Korea. Genes Genet. Syst. 2015, 90, 31–41. [Google Scholar] [CrossRef]
- NIBR (National Institute of Biological Resources). Red Data Book of Endangered Mammals in Korea; National Institute of Biological Resources: Incheon, Republic of Korea, 2012; pp. 48–49.
- NIE (National Institute of Ecology). 2023. Available online: https://www.nie.re.kr (accessed on 24 July 2024).
- Lee, B.G.; Lee, Y.U.; Jo, J.U.; Kim, Y.M.; Bae, C.H.; Gwon, G.H.; Lee, A.N. Analysis for behavioral characteristics of Common Goral (Naemorhedus caudatus) in the Woraksan National Park. Proc. Korean Soc. Environ. Ecol. Con. 2011, 21, 69–71. [Google Scholar]
- Margules, C.R.; Pressey, R.L. Systematic conservation planning. Nature 2000, 405, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.W. Spatial Conservation Prioritization Considering Development Impacts and Habitat Suitability of Endangered Species. Korean J. Environ. Ecol. 2021, 35, 193–203. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Miller, J. Species Distribution Modeling. Geogr. Compass 2010, 4, 490–509. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, H.M.; Lee, S.D. Potential Habitat and Priority Conservation Areas for Endangered Species in South Korea. Animals 2025, 15, 1158. [Google Scholar] [CrossRef]
- Suter, G.W., II. Ecological Risk Assessment; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Duggan, J.M.; Eichelberger, B.A.; Ma, S.; Lawler, J.J.; Ziv, G. Informing management of rare species with an approach combining scenario modeling and spatially explicit risk assessment. Ecosyst. Health Sustain. 2015, 1, 11878994. [Google Scholar] [CrossRef]
- Kim, M.J. Risk Assessment of Major Artificially Planted Coniferous Forests in Korea in response to Climate Change Using the Habitat Risk Map. Master’s Thesis, Korea University, Seoul, Republic of Korea, 2020. [Google Scholar]
- Moilanen, A.; Lehtinen, P.; Kohonen, I.; Jalkanen, J.; Kivistö, I.; Virtanen, E.; Kujala, H. Zonation 5 v2.0 User Manual. 2024. Available online: https://zonationteam.github.io/Zonation5 (accessed on 6 March 2024).
- Wilson, D.E.; Reeder, D.M. Mammal Species of the World: A Taxonomic and Geographic Reference; JHU Press: Baltimore, MD, USA, 2005; pp. 705–706. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Choi, T.Y.; Park, C.H. Establishing a Korean Goral (Naemorhedus caudatus raddeanus Heude) Reserve in Soraksan National Park, Korea: Based on Habitat Suitability Model, Habitat Capability Model, and the Concept of Minimum Viable Population. J. Korean Inst. Landsc. Archit. 2005, 32, 23–35. [Google Scholar]
- Seo, C.W.; Choi, T.Y.; Choi, Y.S.; Kim, D.Y. A Study on Wildlife Habitat Suitability Modeling for Goral (Naemorhedus caudatus raddeanus) in Seoraksan National Park. J. Korean Environ. Res. Technol. 2008, 11, 28–38. [Google Scholar]
- Kim, J.Y.; Seo, C.W.; Kwon, H.S.; Ryu, J.E.; Kim, M.J. A Study on the Species Distribution Modeling using National Ecosystem Survey Data. J. Environ. Impact Assess. 2012, 21, 593–607. [Google Scholar] [CrossRef]
- Jeon, S.W.; Kim, J.E.; Jung, H.C.; Lee, W.K.; Kim, J.S. Species Distribution Modeling of Endangered Mammals for Ecosystem Services Valuation-Focused on National Ecosystem Survey Data. J. Korean Environ. Restor. Technol. 2014, 17, 111–122. [Google Scholar] [CrossRef]
- KHS (Korea Heritage Service). Habitat Survey of Naemorhedus caudatus in the Seoraksan Mountain Natural Reserve-Osaek·Jangsudae Area; Korea Heritage Service: Daejeon, Republic of Korea, 2016.
- Lee, M.J. Predicted the Distribution of Goral Habitat in Korea Using Maxent Model-Seoraksan and Uljin-Samcheok Area. Master’s Thesis, Ewha Womans University, Seoul, Republic of Korea, 2017. [Google Scholar]
- Natural Capital Project. InVEST, Version 3.14.2. Stanford University: Stanford, CA, USA. 2024. Available online: https://naturalcapitalproject.stanford.edu/software/invest (accessed on 14 August 2024).
- Arkema, K.K.; Verutes, G.; Bernhardt, J.R.; Clarke, C.; Rosado, S.; Canto, M.; Wood, S.A.; Ruckelshaus, M.; Rosenthal, A.; McField, M.; et al. Assessing habitat risk from human activities to inform coastal and marine spatial planning: A demonstration in Belize. Environ. Res. Lett. 2014, 9, 114016. [Google Scholar] [CrossRef]
- Cho, C.U.; Gyun, G.H.; Yang, J.J.; Lim, S.J.; Lee, A.N.; Park, H.B.; Lee, B.K. Home Range and Behavioral Characteristics of the Endangered Korea Gorals (Naemorhedus caudatus) with GPS Collar. Korean J. Environ. Ecol. 2014, 28, 1–9. [Google Scholar] [CrossRef]
- Choi, T.Y.; Park, C.H. Korean Groal Potential Habitat Suitability Model at Soraksan National Park Using Fuzzy Set and Multi-Criteria Evaluation. J. Korean Inst. Landsc. Archit. 2004, 32, 28–38. [Google Scholar]
- KHS (Korea Heritage Service). Habitat Survey and Management Plan of the Natural Monument Naemorhedus caudatus; Korea Heritage Service: Daejeon, Republic of Korea, 2012. Available online: https://khs.go.kr (accessed on 1 March 2025).
- Helldin, J.O.; Jung, J.; Neumann, W.; Olsson, M.; Skarin, A.; Widemo, F. The Impacts of Wind Power on Terrestrial Mammals; Swedish Environmental Protection Agency: Stockholm, Sweden, 2012. [Google Scholar]
- Gullison, R.E.; Hardner, J.J. The effects of road design and harvest intensity on forest damage caused by selective logging: Empirical results and a simulation model from the Bosque Chimanes, Bolivia. For. Ecol. Manag. 1993, 59, 1–14. [Google Scholar] [CrossRef]
- Lugo, A.E.; Gucinski, H. Function, effects, and management of forest roads. For. Ecol. Manag. 2000, 133, 249–262. [Google Scholar] [CrossRef]
- Trombulak, S.C.; Frissell, C.A. Review of Ecological Effects of Roads on Terrestrial and Aquatic Communities. Conserv. Biol. 2000, 14, 18–30. [Google Scholar] [CrossRef]
- Song, J.S.; Lee, K.J.; Ki, K.S.; Jun, I.Y. The Efficiency and Improvement of the Highway Wild-Life Fences for Decrease of Mammals Road-kill-In Case of Manjong-Hongchun Section on Jungang Highway. Korean J. Environ. Ecol. 2011, 25, 649–657. [Google Scholar]
- Kang, W.M. Network Analyses of Habitat Connectivity for Biodiversity of Urban Forest Birds, Forest Mammals, and a Threatened Tree Species. Ph.D. Thesis, Seoul National University, Seoul, Republic of Korea, 2013. [Google Scholar]
- Park, H.M.; Kim, M.K.; Lee, S.D. Spatial Characteristics of Wildlife-Vehicle Collisions of Water Deer in Korea Expressway. Sustainability 2021, 13, 13523. [Google Scholar] [CrossRef]
- Kwon, T.H.; Kim, D.W.; Lee, J.W. Trail Deterioration in Woraksan National Park. Korean. J. Environ. Ecol. 2005, 19, 130–138. [Google Scholar]
- Cho, W. Deterioration Status of Closed-Trail of National Parks on the Baekdudaegan Mountains, South Korea. Korean J. Environ. Ecol. 2012, 26, 827–834. [Google Scholar]
- Jeong, W.O.; Kwon, H.G. Trail Deteriorations Characteristics and Stability Evaluation in Sobaeksan National Park. J. Korean Inst. For. Recreat. 2008, 12, 51–57. [Google Scholar]
- KNPS (Korea National Park Service). 2025. Available online: https://www.knps.or.kr (accessed on 1 March 2025).
- Ash, A.N. Effects of Clear-Cutting on Litter Parameters in the Southern Blue Ridge Mountains. Castanea 1995, 60, 89–97. [Google Scholar]
- Potvin, F.; Courtois, R.; Bélanger, L. Short-term response of wildlife to clear-cutting in Quebec boreal forest: Multiscale effects and management implications. Can. J. For. Res. 1999, 29, 1120–1127. [Google Scholar] [CrossRef]
- Rab, M.A. Measures and operating standards for assessing Montreal process soil sustainability indicators with reference to Victorian Central Highlands forest, southeastern Australia. For. Ecol. Manag. 1999, 117, 53–73. [Google Scholar] [CrossRef]
- McRae, D.J.; Duchesne, L.C.; Freedman, B.; Lynham, T.J.; Woodley, S. Comparisons between wildfire and forest harvesting and their implications in forest management. Environ. Rev. 2001, 9, 223–260. [Google Scholar] [CrossRef]
- Nelson, C.R.; Halpern, C.B. Edge-related responses of understory plants to aggregated retention harvest in the Pacific Northwest. Ecol. Appl. 2005, 15, 196–209. [Google Scholar] [CrossRef]
- Chazdon, R.L. Beyond Deforestation: Restoring Forests and Ecosystem Services on Degraded Lands. Science 2008, 320, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, E.S.; Dey, D.C.; Stanturf, J.A.; Lockhart, B.R. Approaches to restoration of oak forests on farmed lowlands of the Mississippi River and its tributaries. Colombia For. 2010, 13, 223–236. [Google Scholar] [CrossRef]
- Choi, B.K. Soil Physical and Hydrological Properties Affected by Forest Harvesting within Piparian Areas of Forested Headwaters. J. Korean For. Soc. 2012, 101, 538–545. [Google Scholar]
- Park, Y.S.; Park, Y.K.; Yang, H.M. Effects of Clear-cutting on Forest Arthropod Communities at Two Different Vertical Levels (Crown and Ground Surface). Korean J. Ecol. Environ. 2016, 49, 271–278. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, J.W.; Park, C.M. Analysis of Consciousness on Land for Another Use After Quarrying. J. Korean Environ. Res. Technol. 2010, 13, 143–151. [Google Scholar]
- White, K.S.; Gregovich, D.P. Mountain goat resource selection in relation to mining-related disturbance. Wildl. Biol. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, Y.J.; Lee, W.S.; Lee, B.K. Analysis System for Regional Environmental Status to Support Environmental Assessment: Analysis of the Status and Development Adequacy of Earth & Rock Extraction Project; Korea Environment Institute: Sejong, Republic of Korea, 2018; pp. 1–70. Available online: https://www.kei.re.kr (accessed on 1 October 2024).
- Cho, S.Y.; Yim, G.J.; Lee, J.Y.; Ji, S.W. A Study on Mixed-use Development Cases Using Closed Quarry Site of Overseas: The UK and Australia. Econ. Environ. Geol. 2021, 54, 505–513. [Google Scholar] [CrossRef]
- Arnett, E.B.; Inkley, D.B.; Johnson, D.H.; Larkin, R.P.; Manes, S.; Manville, A.M.; Mason, R.; Morrison, M.; Strickland, M.D.; Thresher, R. Impacts of Wind Energy Facilities on Wildlife and Wildlife habitat. In Wildlife Society Technical Review; The Wildlife Society: Bethesda, MD, USA, 2007; pp. 1–51. [Google Scholar]
- Crawford, R.H. Life cycle energy and greenhouse emissions analysis of wind turbines and the effect of size on energy yield. Renew. Sustain. Energy Rev. 2009, 13, 2653–2660. [Google Scholar] [CrossRef]
- Lee, S.B.; Sa, G.H.; Ju, Y.J.; Shim, S.Y.; Seo, Y.H.; Kwon, Y.H.; Kim, J.Y.; Im, Y.S. A Study on the Environmental Assessment of Wind Farm: 1. Onshore 2. Offshore; Korea Environment Institute: Seoul, Republic of Korea, 2011; pp. 1–96. [Google Scholar]
- Park, J.Y.; Lee, Y.J.; Chun, D.J.; Lee, M.J.; Eun, J. Analysis System for Regional Environmental Status to Support Environmental Assessment: The Status and Potential of 1. Onshore Wind Power Generation and 2. Floating Photovoltaic Power Generation; Korea Environment Institute: Sejong, Republic of Korea, 2017; pp. 1–116. Available online: https://www.kei.re.kr (accessed on 1 October 2024).
- Zhu, Y.Y.; Sung, H.C.; Kim, Y.J.; Cha, S.H.; Jeon, S.W. Study on Location and Ecological Environmental Characteristics of Onshore Wind and Solar Generation Projects. J. Clim. Change Res. 2020, 11, 145–153. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, H.G.; Park, W.S.; Park, S.W.; Park, J.Y.; Kang, Y.J. Direction for the Mid- and Long-Term Development for Expanding Renewable Energy and Responding to Future Environmental Changes: Current Status and Direction of Onshore Wind Power; Korea Environment Institute: Sejong, Republic of Korea, 2020; pp. 1–160. Available online: https://www.kei.re.kr (accessed on 1 October 2024).
- Schöll, E.M.; Nopp-Mayr, U. Impact of wind power plants on mammalian and avian wildlife species in shrub-and woodlands. Biol. Conserv. 2021, 256, 109037. [Google Scholar] [CrossRef]
- Teff-Seker, Y.; Berger-Tal, O.; Lehnardt, Y.; Teschner, N. Noise pollution from wind turbines and its effects on wildlife: A cross-national analysis of current policies and planning regulations. Renew. Sustain. Energy Rev. 2022, 168, 112801. [Google Scholar] [CrossRef]
- Moilanen, A. Landscape Zonation, benefit functions and target-based planning: Unifying reserve selection strategies. Biol. Conserv. 2007, 134, 571–579. [Google Scholar] [CrossRef]
- Lehtomäki, J.; Moilanen, A. Methods and workflow for spatial conservation prioritization using Zonation. Environ. Model. Softw. 2013, 47, 128–137. [Google Scholar] [CrossRef]
- Belote, R.T.; Barnett, K.; Dietz, M.S.; Burkle, L.; Jenkins, C.N.; Dreiss, L.; Aycrigg, J.L.; Aplet, G.H. Options for prioritizing sites for biodiversity conservation with implications for “30 by 30”. Biol. Conserv. 2021, 264, 109378. [Google Scholar] [CrossRef]
- Moilanen, A.; Lehtinen, P.; Kohonen, I.; Jalkanen, J.; Virtanen, E.A.; Kujala, H. Novel methods for spatial prioritization with applications in conservation, land use planning and ecological impact avoidance. Methods Ecol. Evol. 2022, 13, 1062–1072. [Google Scholar] [CrossRef]
- Shin, Y.C.; Min, D.K. Estimating the Economic Value of the First-Grade Area in Ecological Nature Status. Environ. Resour. Econ. Review. 2005, 14, 25–52. [Google Scholar]
- NIE (National Institute of Ecology). Guideline of the Ecological and Nature Map; National Institute of Ecology: Seocheon, Republic of Korea, 2022; Available online: https://www.nie.re.kr (accessed on 1 March 2025).
- NIER (National Institute of Environmental Research). Report on Constructing Comprehensive GIS Database for Natural Environment; National Institute of Environmental Research: Incheon, Republic of Korea, 2007. Available online: https://ecolibrary.me.go.kr/nier (accessed on 1 March 2025).
- Baldwin, R.A. Use of Maximum Entropy Modeling in Wildlife Research. Entropy 2009, 11, 854–866. [Google Scholar] [CrossRef]
- Park, Y.H.; Lee, H.W.; Kim, K.G.; Lee, G.G.; Choi, J.Y.; Heo, S.J.; Seo, G.W. Development of Designation Criteria for Ecological Protected Areas and its Application Methodology. J. Environ. Impact Assess. 2008, 17, 177–188. [Google Scholar]
- Lee, G.G. Distributional Characteristics and Improvements for Wildlife Protection Areas in South Korea. J. Environ. Impact Assess. 2011, 20, 685–695. [Google Scholar]

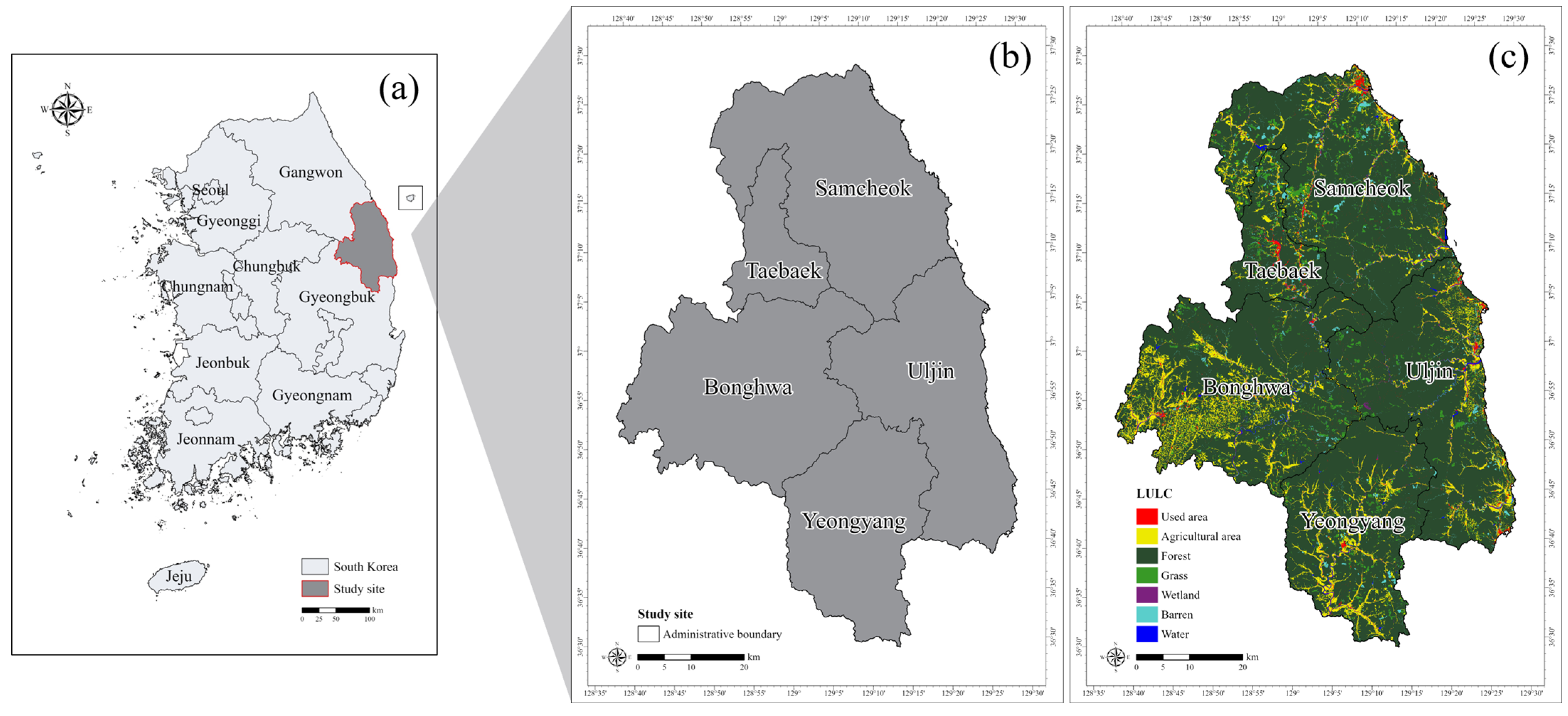


| Classification | Variable Code | Description | Data Type | Data Source |
|---|---|---|---|---|
| Topography | DEM | Elevation | Continuous | National Geographic Information Institute (2023) (https://map.ngii.go.kr, accessed on 19 March 2025) |
| SLOPE | Gradient | |||
| ASPECT | Aspect | |||
| Distance | DFW | Distance from water bodies | Environmental Geographic Information Service (2022) (https://egis.me.go.kr, accessed on 10 March 2025) | |
| DFA | Distance from agricultural areas | |||
| DFT | Distance from traffic areas | |||
| DFM | Distance from mountain trails | Korea Forest Service (2023) (https://map.forest.go.kr, accessed on 31 March 2025) | ||
| Vegetation | NDVI | Normalized difference vegetation index | Korea Institute of Geoscience and Mineral Resources (2022) (https://www.bigdata-environment.kr, accessed on 7 October 2024) | |
| FRTP | Forest type class | Categorical | Korea Forest Service (2023) (https://map.forest.go.kr, accessed on 4 October 2024) | |
| AGCLS | Forest age class | |||
| Land cover | LULC | Land use and land cover | Environmental Geographic Information Service (2022) (https://egis.me.go.kr, accessed on 10 March 2025) |
| Habitat Resilience Attributes | Criteria Type | Rating | Data Quality | Weight |
|---|---|---|---|---|
| Recruitment rate | Consequence | 3 | 1 | 2 |
| Natural mortality rate | 2 | 1 | 2 | |
| Connectivity rate | 3 | 1 | 2 | |
| Recovery time | 2 | 1 | 2 |
| Stressor Code | Description | Data Source |
|---|---|---|
| ROAD | Roads | Environmental Geographic Information Service (2022) (https://egis.me.go.kr, accessed on 21 April 2025) |
| DEFOR | Deforestation | |
| QUAR | Quarries | |
| MTT | Mountain trails | Korea Forest Service (2023) (https://map.forest.go.kr, accessed on 31 March 2025) |
| WPP | Wind power plants | Korea Energy Agency (2022) (https://nr.energy.or.kr, accessed on 28 March 2025) |
| Stressor | Criteria Type | Rating | Data Quality | Weight | References | |
|---|---|---|---|---|---|---|
| ROAD | Exposure | TOR | 3 | 2 | 1 | [27,35,36,38,39,40,41,42,43] |
| MER | 2 | 2 | 3 | |||
| IR | 2 | 2 | 1 | |||
| Consequence | FDR | 3 | 2 | 3 | ||
| CAR | 2 | 2 | 1 | |||
| CSR | 3 | 2 | 1 | |||
| MTT | Exposure | TOR | 2 | 2 | 2 | [35,36,44,45,46,47] |
| MER | 3 | 2 | 2 | |||
| IR | 3 | 2 | 1 | |||
| Consequence | FDR | 3 | 2 | 3 | ||
| CAR | 2 | 2 | 2 | |||
| CSR | 3 | 2 | 1 | |||
| DEFOR | Exposure | TOR | 3 | 2 | 3 | [36,38,48,49,50,51,52,53,54,55,56] |
| MER | 3 | 2 | 3 | |||
| IR | 2 | 2 | 2 | |||
| Consequence | FDR | 2 | 2 | 2 | ||
| CAR | 3 | 2 | 1 | |||
| CSR | 3 | 2 | 1 | |||
| QUAR | Exposure | TOR | 3 | 2 | 2 | [36,57,58,59,60] |
| MER | 2 | 2 | 3 | |||
| IR | 3 | 2 | 1 | |||
| Consequence | FDR | 3 | 2 | 3 | ||
| CAR | 3 | 2 | 1 | |||
| CSR | 3 | 2 | 1 | |||
| WPP | Exposure | TOR | 3 | 2 | 3 | [37,61,62,63,64,65,66,67,68] |
| MER | 3 | 2 | 3 | |||
| IR | 2 | 2 | 2 | |||
| Consequence | FDR | 1 | 2 | 3 | ||
| CAR | 3 | 2 | 3 | |||
| CSR | 3 | 2 | 1 | |||
| Stressor Code | Description | Exposure | Consequence | Risk |
|---|---|---|---|---|
| ROAD | Roads | 1.685 | 1.752 | 1.122 |
| MTT | Mountain trails | 0.391 | 0.365 | 0.266 |
| DEFOR | Deforestation | 0.131 | 1.134 | 0.072 |
| QUAR | Quarries | 0.090 | 0.086 | 0.047 |
| WPP | Wind power plants | 0.027 | 0.027 | 0.015 |
| Average value | 0.465 | 0.473 | 0.304 | |
| Province | Priority Rank | ||||
|---|---|---|---|---|---|
| Classification | Low | Moderately low | Moderate | Moderately High | High |
| Uljin | 185.9 | 138.28 | 132.98 | 183.69 | 351.25 |
| Samcheok | 269.38 | 215.86 | 251.96 | 249.37 | 198.18 |
| Yeongyang | 134.14 | 154.64 | 174.52 | 181.69 | 172.54 |
| Bonghwa | 232.68 | 301.29 | 257.05 | 236.13 | 172.23 |
| Taebaek | 59.53 | 89.21 | 82.76 | 48.39 | 22.71 |
| Total | 881.63 | 899.28 | 899.27 | 899.27 | 916.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kim, M.; Lee, S. Integrating Habitat Prediction and Risk Assessment to Prioritize Conservation Areas for the Long-Tailed Goral (Naemorhedus caudatus). Animals 2025, 15, 2848. https://doi.org/10.3390/ani15192848
Park S, Kim M, Lee S. Integrating Habitat Prediction and Risk Assessment to Prioritize Conservation Areas for the Long-Tailed Goral (Naemorhedus caudatus). Animals. 2025; 15(19):2848. https://doi.org/10.3390/ani15192848
Chicago/Turabian StylePark, Soyeon, Minkyung Kim, and Sangdon Lee. 2025. "Integrating Habitat Prediction and Risk Assessment to Prioritize Conservation Areas for the Long-Tailed Goral (Naemorhedus caudatus)" Animals 15, no. 19: 2848. https://doi.org/10.3390/ani15192848
APA StylePark, S., Kim, M., & Lee, S. (2025). Integrating Habitat Prediction and Risk Assessment to Prioritize Conservation Areas for the Long-Tailed Goral (Naemorhedus caudatus). Animals, 15(19), 2848. https://doi.org/10.3390/ani15192848







