Simple Summary
Enterotoxigenic Escherichia coli (ETEC) exhibits a high prevalence in Neonatal Calf Diarrhoea, leading to significant economic losses in the animal production sector. This study aimed to correlate the presence of virulence factor-producing multidrug-resistant E. coli in central France, specifically in the departments of Cantal, Haute-Loire, Loire, and Puy-de-Dôme. A large number of diarrhoeal stool samples from calves were analysed at TERANA Laboratories (France), revealing a high rate of multidrug-resistant E. coli, which harbour different virulence factors, namely fimbrial and non-fimbrial adhesins. Among E. coli strains carrying adhesins, a high prevalence of multidrug-resistance was observed, averaging 84.9%, with a predominance of CS31A-producing strains. These findings underscore the importance of monitoring the co-occurence of adhesins and multidrug-resistant profiles in E. coli from Neonatal Calf Diarrhoea, as they could serve as a reservoir for strains harbouring both determinants, thereby posing a significant public health risk. To our knowledge, this study represents the first large-scale report on the correlation between multidrug resistance profiles and virulence factors-producing E. coli isolated from cases of Neonatal Calf Diarrhoea.
Abstract
Escherichia coli is a significant cause of Neonatal Calf Diarrhoea (NCD). Its extensive antigenic diversity, coupled with the ability to acquire antimicrobial resistance determinants, hampers treatment effectiveness and compromises the control measures. This study investigated the link between the presence of multidrug-resistant (MDR) E. coli and virulence factors (VFs) in NCD from central France (Departments of Cantal, Haute-Loire, Loire, and Puy-de-Dôme), between 2016 and 2022. E. coli was identified at TERANA Laboratories, France, using API 20E (BioMérieux®) and MALDI-TOF Mass Spectrometry. Virulence factors, namely adhesins, were assessed with the slide agglutination method, and antimicrobial susceptibility testing was conducted across various antimicrobial classes. Out of 2367 E. coli strains isolated from cases of NCD, a high percentage were resistant to aminopenicillins (88.8%), aminoglycosides (89.1%), tetracyclines (79.7%), quinolones (48.4%), and sulphonamides (42.4%). More than half (58.6%) carried VFs, and 84.9% exhibited MDR profile, of which 61.34% (1233/2010) also harboured VFs. The adhesin CS31A-producing E. coli was the most prevalent, followed by the fimbrial adhesins F5 and F17 (60.8%, 20.0%, and 8.3%, respectively), all of which were associated with a high prevalence of MDR strains (79.1–93.9%). The highest occurrence of MDR profiles was observed in E. coli strains carrying CS31A and in those lacking VFs, both groups showing co-resistance to aminopenicillins, aminoglycosides, and tetracyclines or sulphonamides. The calf production sector may act as a reservoir for MDR E. coli strains, regardless of the presence of VFs, posing a major threat to public health and safety.
1. Introduction
Neonatal Calf Diarrhoea (NCD) is a leading cause of morbidity and mortality among calves under one month old, resulting in significant economic losses in dairy and beef cattle production [1]. NCD is a highly complex condition that can be caused by bacterial, viral, or parasitic pathogens, with co-infections frequently reported [1,2,3,4].
E. coli, a Gram-negative bacillus belonging to the order Enterobacterales, is highly prevalent in cases of NCD [3]. Although E. coli is part of the gastrointestinal microbiota in both humans and animals [5,6,7], it can become pathogenic when the intestinal barrier is compromised, leading to translocation into the abdominal cavity and potentially resulting in peritonitis or septicaemia [5,8]. Conversely, pathogenic strains of E. coli encode specific virulence factors (VFs) responsible for various infectious diseases collectively known as colibacillosis [7,8]. Pathogenic E. coli is classified into two groups: Extra-intestinal E. coli (ExPEC) and Intestinal E. coli (InPEC) [8,9]. Enterotoxigenic E. coli (ETEC), an InPEC pathotype, is a primary pathogen recognised as the principal bacterial agent causing NCD [1,3,10].
The initial step of the virulence mechanism of ETEC involves its adhesion to the intestinal epithelium via fimbrial and non-fimbrial adhesins [11,12]. The fimbrial adhesins implicated in this process include F5, F17, and F41 [10]. Additionally, the non-fimbrial adhesin CS31A is a surface antigen immunologically related to fimbriae F4 and F41. CS31A is an important virulence factor of E. coli, involved not only in NCD but also associated with septicaemia, particularly in young animals [10,11].
The management of NCD often involves the use of diverse antimicrobials, including beta-lactams, aminoglycosides, quinolones (including fluoroquinolones), phenicols, tetracyclines, polymyxins, and sulphonamides [13]. Some of these antibiotic classes are classified as critically important for human medicine [14,15]. A high prevalence of E. coli carrying various antimicrobial resistance (AMR) mechanisms has been documented in both humans and animals, including those from food-producing settings. This raises significant concerns regarding the implications of antimicrobial use in livestock and the potential transmission of AMR bacteria to humans through the food chain [6,7,16,17,18,19,20,21]. However, large-scale data on the association between distinct MDR profiles and VFs-producing E. coli in NCD remain limited.
The main objective of this study was to investigate potential associations between multidrug resistance (MDR) profiles and specific VFs, particularly adhesins, in E. coli isolates from cases of NCD. Unlike surveillance or risk assessment studies, our focus was on correlation analysis, aiming to identify potential biological links between AMR and adhesin patterns. To this end, we conducted a retrospective study including E. coli strains collected between 2016 and 2022 from departments in central France (Cantal, Haute-Loire, Loire, and Puy-de-Dôme), with analysis of both AMR and adhesin profiles.
2. Materials and Methods
2.1. Database Source and Management
The clinical data used in this study were provided by TERANA Laboratories (France), which operates across 10 departments in the country: Cantal, Cher, Creuse, Drôme, Indre, Loire, Haute-Loire, Nièvre, Puy-de-Dôme, and Rhône.
The data set analysed in this study included information on clinical samples (animal species, date of sample collection, and origin) and bacteriological results (bacterial identification, presence of virulence factors, and antimicrobial susceptibility testing results). Duplicate samples and repeated results (defined as isolates with the same date and reference) were excluded. Only one isolate per calf per submission was retained in the data set, ensuring that each isolate represented a unique sampling event. In addition, data containing confidential information were removed in accordance with Data Protection Law [22].
2.2. Study Design and Sample Characterisation
Between 2016 and 2022, TERANA Laboratories (https://www.labo-terana.fr/ accessed on 4 January 2023) received 2367 diarrhoeic fecal samples from young cattle diagnosed with NCD. These samples were collected during routine clinical practice in the Departments of Cantal, Haute-Loire, Loire, and Puy-de-Dôme in central France.
2.3. Identification and Virulence Profiling of Bovine Diarrheagenic Isolates
Identification of E. coli was performed using API 20E (BioMérieux®, Marcy-l’Étoile, France) for the period between 2016 and 2019 and MALDI-TOF MS (Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry) (Bruker Daltonics, Bremen, Germany) from 2020 onwards. All isolates were subsequently screened for fimbrial (F5, F6, F17, F41) and non-fimbrial (CS31A) VFs (virulence factors, namely bacterial adhesins) using the slide agglutination method [23].
2.4. Antimicrobial Susceptibility Analysis
Antimicrobial Susceptibility Testing (AST) was performed using the disk diffusion method or determination of the Minimum Inhibitory Concentration (MIC), following the guidelines of the Comité de l’Antibiogramme de la Société Française de Microbiologie (CA-SFM) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST), according to standard NF U47-107 [23]. A total of 17 antimicrobials from various classes were evaluated, including beta-lactams with or without beta-lactamase inhibitors namely amoxicillin (AML), amoxicillin-clavulanic acid (AMC); cephalexin (CL), cefoxitin (FOX), cefuroxime (CXM), ceftiofur (EFT), and cefquinome (CEQ); the aminoglycosides—gentamicin (CN), neomycin (N), and streptomycin (S); the quinolones—nalidixic acid (NA), flumequine (UB), and marbofloxacin (MBF); the phenicols—florfenicol (FFC); the tetracyclines—tetracycline (T); the polymyxins—colistin (C); and sulfamethoxazole-trimethoprim (SXT).
Susceptibility testing to colistin was performed using the Colispot method developed by Agence Nationale Sécurité Sanitaire de l’ Alimentation, de l’Environnement et du Travail (ANSES) [24,25].
The isolates exhibiting resistance to three or more classes of antimicrobials were considered MDR, by established definitions [26].
2.5. Statistical Analysis
The prevalence of AMR and VFs-producing E. coli, as well as the association between these determinants and their MDR profiles, was statistically evaluated using the Chi-square test. Statistical significance was set at p < 0.05. All analyses were performed using GraphPad Prism® (version 9.4.1).
2.6. Ethical Approval
This study was reviewed and approved by the Ethics Committee of Escola Universitária Vasco da Gama, Coimbra, Portugal, under the internal reference number 20/2024.
3. Results
3.1. Adhesins Identification
A total of 2367 E. coli isolates were identified, comprising various serotypes. Among these, 58.6% (1388/2367) carried fimbrial and non-fimbrial adhesins, while 41.4% (979/2367) carried none (p < 0.05).
The adhesin CS31A was the most prevalent VF. It was identified in 60.8% (844/1388) of isolates harbouring VFs, a significantly higher proportion compared to all other VFs detected among the E. coli isolates analysed (p < 0.05).
In addition, 6.8% (94/1388) of the isolates harboured more than one adhesin concurrently (Table 1).

Table 1.
Occurrence of fimbrial and non-fimbrial adhesins among E. coli strains isolated from NCD cases between 2016 and 2022.
3.2. Frequency of Antimicrobial Resistance
The AST of E. coli isolates obtained from NCD cases did not consistently include all antimicrobial classes, as this depended on the antimicrobial panels requested by the submitting veterinarian. Consequently, variation was observed in the number of E. coli isolates tested for each antimicrobial agent.
The results of the AST among E. coli isolates from NCD, collected during the study period revealed a high prevalence of resistance to aminopenicillins and aminoglycosides (amoxicillin: 88.8%, 1646/1854; streptomycin: 89.1%, 1776/1994), followed by tetracyclines (tetracycline: 79.7%, 1856/2330), quinolones (nalidixic acid: 48.4%, 435/899), and sulphonamides (sulfamethoxazole-trimethoprim: 42.4%, 1003/2363) (p < 0.05).
Furthermore, 30.4% (718/2360) of E. coli isolates were resistant to amoxicillin with clavulanic acid, while 39.2% (926/2360) exhibited susceptibility to increased exposure, following the EUCAST redefinition of “Intermediate Susceptibility” introduced in 2019. Among cephalosporins, resistance to cefuroxime was the most notable (26.4%, 396/1502), followed by cephalexin (10.0%, 186/1863), cefoxitin (5.7%, 95/1674), cefquinome (3.1%, 72/2352), and ceftiofur (1.7%, 40/2339) (p < 0.05). Regarding fluoroquinolones, a non-significant resistance rate of 33.6% (237/705) was detected for flumequine (p > 0.05), in contrast to a markedly lower rate recorded for marbofloxacin at 12.1% (286/2363) (p < 0.05). Resistance to colistin remained low, at 1.4% (31/2233) (Figure 1; Table 2).
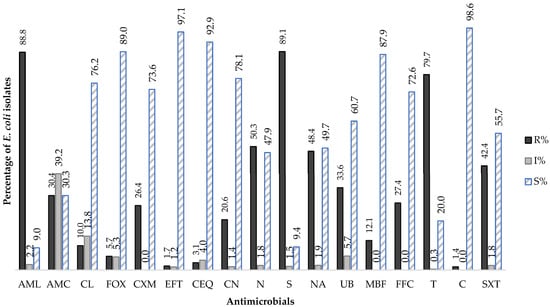
Figure 1.
Global distribution average of Antimicrobial Susceptibility profile of E. coli isolated from NDC cases (2016–2022). R—resistant; I—susceptible to increase exposure; S—susceptible; AML—amoxicillin, AMC—amoxicillin with clavulanic acid, CL—cephalexin, FOX—cefoxitin, CXM—cefuroxime, EFT—ceftiofur, CEQ—cefquinome, CN—gentamicin, N—neomycin, S—streptomycin, NA—nalidixic acid, UB—flumequine, MBF—marbofloxacin, FFC—florfenicol, T—tetracycline, C—colistin, SXT—sulphamethoxazole with trimethoprim.

Table 2.
Antimicrobial resistance profile of E. coli isolated from NCD cases annually, over a six-year (2016–2022).
Regarding the evolution of E. coli resistance profiles to the antimicrobials tested over the study period (2016–2022), the results revealed significant fluctuations in resistance to amoxicillin-clavulanic acid, cefquinome, gentamicin, cefuroxime, ceftiofur, florfenicol, cefoxitin, marbofloxacin, nalidixic acid, and tetracycline (p < 0.05). Until 2019, a progressive increase was observed in the number of E. coli isolates resistant to amoxicillin-clavulanic acid, cefquinome, gentamicin, streptomycin, florfenicol, and sulphamethoxazole-trimethoprim, followed by a significant decrease in 2020 (p < 0.05). A significant upward trend in cefuroxime resistance was observed, rising from 13% to 31% (p < 0.05). In contrast, cefquinome and ceftiofur (both fourth-generation cephalosporins), as well as marbofloxacin (a fluoroquinolone), showed significant downward trends in resistance (p < 0.05).
It is also noteworthy that the results indicate low rates of susceptible to increased exposure to antimicrobials, except for the combination of amoxicillin and clavulanic acid, for which a steady increase was recorded, rising from 25% (67/268) in 2016 to 47.4% (170/358) in 2022 (p < 0.05) (Table 3). Additionally, the resistance rate to amoxicillin was decreased when used in combination with clavulanic acid (Figure 1).

Table 3.
E. coli isolates with a ‘susceptible to increased exposure’ profile from NDC cases reported annually over the study period (2016–2022).
3.3. Adhesins Co-Carriage and Multidrug-Resistance in E. coli Isolated from Cases of Neonatal Calf Diarrhoea
The findings revealed that 84.9% (2010/2367) of the E. coli isolates collected during the study period exhibited an MDR profile (p < 0.05). The prevalence of MDR strains remained statistically stable over time, with no significant year-to-year variation detected (p > 0.05) (Table 4). Although not statistically significant, a noticeable increase in MDR prevalence was recorded in 2022 (21.9%) compared to 2016 (10.6%) (Table 4).

Table 4.
Evolution of Multidrug-Resistant E. coli strains from NCD cases (2016–2022).
It is noteworthy that more than half of the MDR E. coli isolates (61.2%; 1230/2010) harboured the adhesins tested, and 88.6% (1230/1382) of the characterised pathotypes exhibited an MDR profile. Notably, the pathotypes E. coli CS31A (37.3%; 751/2010) and E. coli F5 (12.9%; 260/2010) displayed significantly higher occurrences of MDR, whereas other pathotypes showed no significant deviation (p < 0.05; p > 0.05, respectively) (Figure 2; Table 5). Nonetheless, when assessing the association between different E. coli pathotypes and the presence or absence of MDR profiles, these findings demonstrated a statistically significant risk of being associated with MDR (p > 0.05).
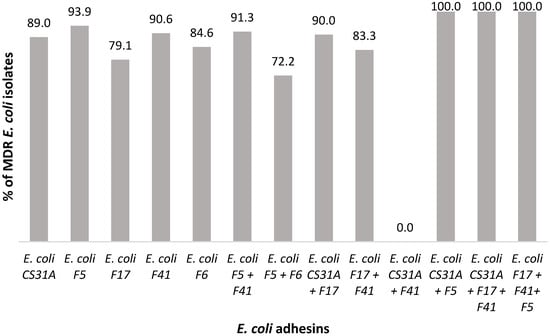
Figure 2.
Correlation between E. coli pathotypes carrying adhesins and MDR profile from NCD cases (2016–2022).

Table 5.
Distribution of MDR E. coli pathotypes from cases of NCD (2016–2022).
4. Discussion
Despite the extensive scientific literature on AMR in E. coli, there remains a notable lack of studies specifically addressing the role of defined pathovar-associated virulence factors in NCD. This study addresses that gap by focusing on E. coli strains isolated from NCD cases, demonstrating a high prevalence of AMR among calves in central France, including MDR profiles associated with strains carrying CS31A or F5.
Whenever possible, official data published by Réseau de d’épidémiosurveillance de l’antibiorésistance des bactéries pathogènes animales (RESAPATH) were used as a reference to assess whether the findings of this study align with the national data and trends.
The high prevalence of CS31A or F5-producing strains observed in this study is consistent with previous reports. Indeed, F5-producing E. coli has frequently been associated with NCD [2,12], while other studies have reported a higher prevalence of the CS31A [6]. Notably, CS31A has been linked not only to clinical cases of diarrhoea but also to septicaemia in young animals [11]. Although CS31A-producing strains displayed the highest prevalence of MDR in our data set, no adhesin, including CS31A, was statistically associated with an increased risk of MDR. The observed link should therefore be interpreted as a descriptive finding rather than a statistically proven risk factor.
Regarding the distribution of E. coli AMR patterns in animals with NCD, our results do not consistently match with official data, particularly for fluoroquinolones. For instance, the prevalence of resistance to marbofloxacin in this study (12%) is notably higher than the 5–7% reported in RESAPATH data [27]. Additionally, between 2018 and 2022, France recorded a national increase in the proportion of E. coli isolates classified as susceptible to increase exposure to amoxicillin with or without clavulanic acid. This trend is consistent with the findings of our study, which recorded an even higher prevalence of such isolates in central France [27]. It is also worth noting that the observed resistance rates to colistin, tetracycline, and quinolones in this study were broadly in agreement with the official data reported [27].
Nevertheless, RESAPATH has highlighted the potential underestimation of colistin resistance due to the limited reliability of the disc diffusion method routinely used in France, as well as the lack of routine microdilution for MIC determination [28]. It is therefore plausible that the colistin resistance rate observed in this study (1.4%) was similarly underestimated. Even so, the result remains consistent with national data, which reports resistance levels below 2% in E. coli from NCD cases [28].
According to official French data, there has been a documented decrease in the prevalence of MDR strains among E. coli isolates from animals with NCD between 2012 and 2022 [27]. However, our study identified an increase in the prevalence of MDR E. coli, rising from 10.6% in 2016 to 15.4% in 2022, with a peak of 21.9% in 2021. In this study, the most prevalent MDR profile was characterised by resistance to amoxicillin, gentamicin, tetracycline, and sulfamethoxazole-trimethoprim or nalidixic acid, closely mirroring trends observed in official data. This pattern may be attributed to the widespread use of these antimicrobials in animal production settings [29]. Furthermore, across Europe, this resistance profile has emerged as the most prevalent, with high or very high resistance rates reported in all animal categories. Notably, E. coli isolates from cattle under one year of age have shown resistance to ampicillin, sulfamethoxazole, trimethoprim, and tetracycline [20].
These findings are particularly concerning given the overlap in MDR patterns observed in E. coli from both healthy animals and those affected by NCD, suggesting a possible role for horizontal gene transfer events in disseminating resistance genetic elements, and potentially encoded plasmids adhesins like-CS31A across E. coli populations [30].
To explore a potential link between E. coli isolated from NCD cases and human infections, data from the European Antimicrobial Resistance Surveillance Network (EARS-Net) for 2021 indicated that approximately 52.3% of human E. coli isolates were resistant to at least one antimicrobial class, namely aminopenicillins, fluoroquinolones, third-generation cephalosporins, aminoglycosides, and carbapenems. Resistance was highest to aminopenicillins (53.1%), followed by fluoroquinolones (21.9%), third-generation cephalosporins (13.8%), and aminoglycosides (9.6%) [31]. As supported by other previous studies, high levels of aminopenicillin resistance have been reported in both humans and animals, including exotic pets [20,31,32,33].
Altogether, a comparative analysis of the results from this study and previously published data reveals a consistent pattern across different ecological niches, including humans and animals. This pattern warrants further research to inform strategies aimed at containing the spread of E. coli strains with MDR profiles and VFs. Ongoing surveillance of AMR and virulence determinants in other species, including humans, livestock, and companion animals, is crucial to pre-emptively address emerging public health threats.
Although this study provides valuable insights into the relationship between virulence factors and MDR profiles in E. coli strains from NCD, it is limited by the absence of molecular analyses of resistance mechanisms and genes, as only phenotypic data from the French public laboratory network TERANA were available. Even so, the findings highlight the calf production sector as a potential reservoir of MDR strains and emphasise the need for continued surveillance and future studies incorporating molecular approaches.
Additionally, as the study is based on diagnostic laboratory submissions, a potential selection bias must be acknowledged. The analysed samples originated from calves with clinical NCD for which veterinarians requested bacteriological testing, which means our data set is likely enriched in more severe or complicated cases. Consequently, the results may not fully reflect the overall epidemiology of E. coli in the general calf population. Nevertheless, given the large number of isolates analysed over a six-year period, our findings provide robust evidence of MDR–virulence associations in clinically relevant E. coli strains.
Importantly, France is currently implementing the third iteration of its National Action Plan on Antimicrobial Resistance, Le plan Écoantibio 3 (2023–2028), which aims to further reduce antimicrobial exposure across various animal species and sectors. This initiative has already contributed to a 52% reduction in antimicrobial use, addressing international concerns and complying with European regulations.
5. Conclusions
Our data reveal an alarmingly high prevalence of MDR among E. coli isolates from NCD in central France, with frequent co-resistance to antimicrobials that are critically important for human medicine.
MDR was common among CS31A-producing strains, highlighting a concerning convergence between this adhesin and resistance traits. Although no statistical association was detected between adhesin type and MDR, the consistent presence of MDR in CS31A-producing strains suggests an important epidemiological role, potentially driven by horizontal gene transfer.
Further research is essential to clarify these relationships and to support the implementation of effective control and prevention measures. Veterinarians have a pivotal role in implementing official recommendations and ensuring the responsible use of antimicrobials.
These findings point to regional hotspots for the emergence of MDR E. coli and underscore the need for targeted surveillance and robust antimicrobial stewardship.
Future studies incorporating molecular approaches will be crucial to better characterise the underlying AMR mechanisms and their epidemiological drivers.
Author Contributions
Conceptualization, C.P., A.R.P. and E.S.; methodology, C.P. and E.S.; investigation, C.P.; resources, H.Y. and G.M.; writing—original draft preparation, C.P., T.L., G.J.d.S., M.J.S. and E.S.; writing—review and editing, C.P., H.Y., G.M., A.R.P., T.L., G.J.d.S., M.J.S. and E.S.; supervision, E.S.; Co-supervision, A.R.P. All authors have read and agreed to the published version of the manuscript.
Funding
The experimental work was supported by TERANA Laboratories (https://www.labo-terana.fr/ accessed on 4 January 2023), France.
Institutional Review Board Statement
The study was approved by the Institutional Ethics Committee of University School Vasco da Gama, Coimbra, Portugal (Ref. number: 20/2024, approved on 1 July 2024).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding author under reasonable request.
Acknowledgments
We thank to TERANA Laboratories (https://www.labo-terana.fr/ accessed on 4 January 2023), which operates in 10 departments across France (Cantal, Cher, Creuse, Drôme, Indre, Loire, Haute-Loire, Nièvre, Puy-de-Dôme, Rhône) for providing the data used in this study. FCT –Portuguese Foundation for Science and Technology, under the projects: UID/NEU/04539/2020 (CNC-UC) and LA/P/0058/2020; UID UIDB/04539/2020 e UIDP/04539/2020 and LA/P/0058/2020 (CIBB); UID/04033/2025 (CITAB) and LA/P/0126/2020 (https://doi.org/10.54499/LA/P/0126/2020).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Prieto, A.; Lopez-Novo, C.; Diaz, P.; Diaz-Cao, J.M.; Lopez-Lorenzo, G.; Anton, C.; Remesar, S.; Garcia-Dios, D.; Lopez, C.; Panadero, R.; et al. Antimicrobial Susceptibility of Enterotoxigenic Escherichia coli from Diarrhoeic Neonatal Calves in Spain. Animals 2022, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.I.; Yoon, K.J. An Overview of Calf Diarrhea—Infectious Etiology, Diagnosis, and Intervention. J. Vet. Sci. 2014, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Khawaskar, D.P.; Sinha, D.K.; Lalrinzuala, M.V.; Athira, V.; Kumar, M.; Chhakchhuak, L.; Mohanapriya, K.; Sophia, I.; Abhishek; Kumar, O.R.V.; et al. Pathotyping and Antimicrobial Susceptibility Testing of Escherichia coli Isolates From Neonatal Calves. Vet. Res. Commun. 2022, 46, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Jessop, E.; Li, L.; Renaud, D.L.; Verbrugghe, A.; Macnicol, J.; Gamsjager, L.; Gomez, D.E. Neonatal Calf Diarrhea and Gastrointestinal Microbiota: Etiologic Agents and Microbiota Manipulation for Treatment and Prevention of Diarrhea. Vet. Sci. 2024, 11, 108. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- de Verdier, K.; Nyman, A.; Greko, C.; Bengtsson, B. Antimicrobial Resistance and Virulence Factors in Escherichia coli from Swedish Dairy Calves. Acta Vet. Scand. 2012, 54, 2. [Google Scholar] [CrossRef]
- Poirel, L.; Madec, J.Y.; Lupo, A.; Schink, A.K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 10-1128. [Google Scholar] [CrossRef]
- Pokharel, P.; Dhakal, S.; Dozois, C.M. The Diversity of Escherichia coli Pathotypes and Vaccination Strategies against This Versatile Bacterial Pathogen. Microorganisms 2023, 11, 344. [Google Scholar] [CrossRef]
- Moriel, D.G.; Rosini, R.; Seib, K.L.; Serino, L.; Pizza, M.; Rappuoli, R. Escherichia coli: Great diversity around a common core. mBio 2012, 3, 10-1128. [Google Scholar] [CrossRef]
- Kolenda, R.; Burdukiewicz, M.; Schierack, P. A Systematic Review and Meta-Analysis of the Epidemiology of Pathogenic Escherichia coli of Calves and the Role of Calves as Reservoirs for Human Pathogenic E. coli. Front. Cell. Infect. Microbiol. 2015, 5, 23. [Google Scholar] [CrossRef]
- Mercado, E.C.; Rodriguez, S.M.; D’Antuono, A.L.; Cipolla, A.L.; Elizondo, A.M.; Rossetti, C.A.; Malena, R.; Mendez, M.A. Occurrence and Characteristics of CS31A Antigen-Producing Escherichia coli in Calves with Diarrhoea and Septicaemia in Argentina. J. Vet. Med. Ser. B 2003, 50, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.M.; Smith, G.W. Pathophysiology of Diarrhea in Calves. Vet. Clin. N. Am. Food Anim. Pract. 2009, 25, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Constable, P.D. Antimicrobial Use in the Treatment of Calf Diarrhea. J. Vet. Intern. Med. 2004, 18, 8–17. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Categorisation of Antibiotics in the European Union; EMA/CVMP/CHMP/682198/2017; European Medicines Agency: Amsterdam, The Netherlands, 2019.
- WHO. Critically Important Antimicrobials for Human Medicine—3rd Rev. Available online: https://apps.who.int/iris/bitstream/handle/10665/77376/;jsessionid=0C947CA333F9F82CF7708F537AE6FB85?sequence=1 (accessed on 4 January 2023).
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in Europe: An Overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; Dapkevicius, M.L.E.; Canica, M.; Tejedor-Junco, M.T.; Igrejas, G.; Poeta, P. Escherichia coli as Commensal and Pathogenic Bacteria Among Food-Producing Animals: Health Implications of Extended Spectrum Beta-Lactamase (ESBL) Production. Animals 2020, 10, 2239. [Google Scholar] [CrossRef]
- Lima, T.; Fernandes, L.; Matias, M.; Mateus, A.; Silveira, E.; Domingues, S.; Pomba, C.; Da Silva, G.J. Longitudinal Study Detects the Co-Carriage of ESBL and mcr-1 and -4 Genes in Escherichia coli Strains in a Portuguese Farrow-to-Finish Swine Herd. Animals 2022, 12, 2209. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2021–2022. EFSA J. Eur. Food Saf. Auth. 2024, 22, e8583. [Google Scholar] [CrossRef]
- Lima, T.; Loureiro, D.; Henriques, A.; Ramos, F.; Pomba, C.; Domingues, S.; da Silva, G.J. Occurrence and Biological Cost of mcr-1-Carrying Plasmids Co-harbouring Beta-Lactamase Resistance Genes in Zoonotic Pathogens from Intensive Animal Production. Antibiotics 2022, 11, 1356. [Google Scholar] [CrossRef]
- European Parliament and the Council, Dated April 27, 2016, Regulation (EU) 2016/679. Available online: https://eur-lex.europa.eu/eli/reg/2016/679/oj/eng (accessed on 4 January 2023).
- Schierack, P.; Steinruck, H.; Kleta, S.; Vahjen, W. Virulence Factor Gene Profiles of Escherichia coli Isolates from Clinically Healthy Pigs. Appl. Environ. Microbiol. 2006, 72, 6680–6686. [Google Scholar] [CrossRef]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 14.0. 2024. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_14.0_Breakpoint_Tables.pdf (accessed on 4 January 2023).
- Jouy, E.; Haenni, M.; Le Devendec, L.; Le Roux, A.; Chatre, P.; Madec, J.Y.; Kempf, I. Improvement in Routine Detection of Colistin Resistance in E. coli Isolated in Veterinary Diagnostic Laboratories. J. Microbiol. Methods 2017, 132, 125–127. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Agency for Food, Environmental and Occupational Health & Safety. 2022 Anual Report. 2022. Available online: https://ansm.sante.fr/uploads/2021/12/09/20211209-ra-2020-eng.pdf (accessed on 4 January 2023).
- Agency for Food, Environmental and Occupational Health & Safety. 2020 Anual Report. 2020. Available online: https://www.anses.fr/en/system/files/ANSES-RA2022-02-EN.pdf (accessed on 4 January 2023).
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2021: Trends from 2010 to 2021: Twelfth ESVAC Report; Publications Office of the European Union: Luxembourg, 2022.
- Jallat, C.; Darfeuille-Michaud, A.; Girardeau, J.P.; Rich, C.; Joly, B. Self-transmissible R Plasmids Encoding CS31A among Human Escherichia coli Strains Isolated from Diarrheal Stools. Infect. Immun. 1994, 62, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Surveillance in Europe 2023—2021 Data; European Centre for Disease Prevention and Control and World Health Organization: Stockholm, Sweden, 2023.
- Menezes, J.; Frosini, S.M.; Belas, A.; Marques, C.; da Silva, J.M.; Amaral, A.J.; Loeffler, A.; Pomba, C. Longitudinal Study of ESBL/AmpC-Producing Enterobacterales Strains Sharing Between Cohabiting Healthy Companion Animals and Humans in Portugal and in the United Kingdom. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.; Le Loc’h, A.; Marques, I.; Almeida, A.; Sousa, S.; Saavedra, M.J.; Anastácio, S.; Silveira, E. Unveiling the Emergence of Multidrug-Resistant Pathogens in Exotic Pets from France: A Comprehensive Study (2017–2019). One Health Implement. Res. 2023, 3, 161–176. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).