Effectual Environmental Enrichments for Commercial Broiler Chickens
Abstract
Simple Summary
Abstract
1. Introduction
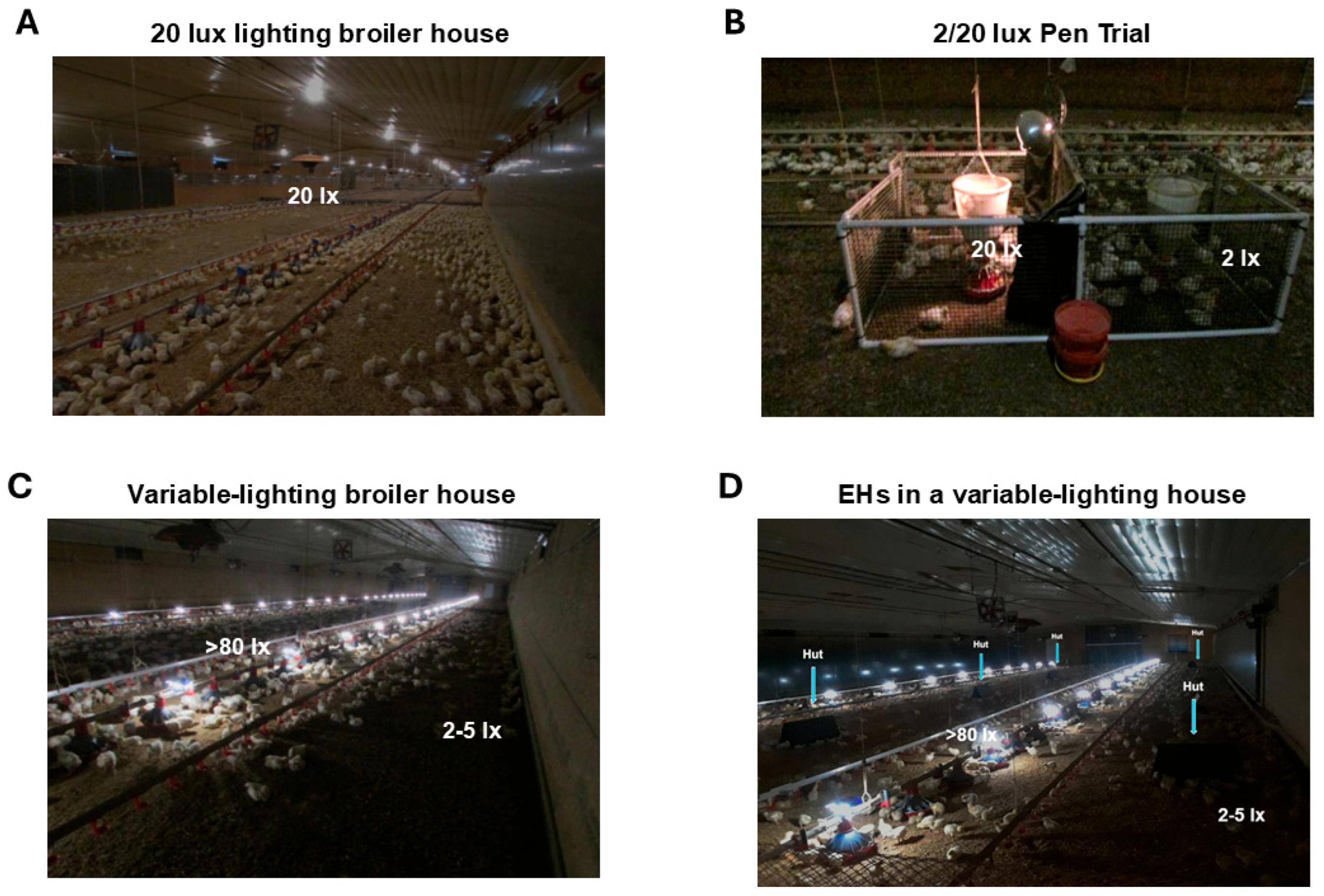
2. Behavioral Preference of Broiler Chickens for Variable Light Intensity
3. Effect of Daytime Resting in Darkness on the Welfare of Broiler Chickens
4. Improved Welfare and Performance by Environmental Enrichment Lighting and Huts in Commercial Broiler Houses
5. Future Perspectives
6. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davinelli, S.; Medoro, A.; Savino, R.; Scapagnini, G. Sleep and Oxidative Stress: Current Perspectives on the Role of NRF2. Cell Mol. Neurobiol. 2024, 44, 52. [Google Scholar] [CrossRef]
- Villafuerte, G.; Miguel-Puga, A.; Rodriguez, E.M.; Machado, S.; Manjarrez, E.; Arias-Carrion, O. Sleep deprivation and oxidative stress in animal models: A systematic review. Oxidative Med. Cell. Longev. 2015, 2015, 234952. [Google Scholar] [CrossRef]
- Eugene, A.R.; Masiak, J. The Neuroprotective Aspects of Sleep. MEDtube Sci. 2015, 3, 35–40. [Google Scholar]
- Chen, S.; Xie, Y.; Li, Y.; Fan, X.; Xing, F.; Mao, Y.; Xing, N.; Wang, J.; Yang, J.; Wang, Z.; et al. Sleep deprivation and recovery sleep affect healthy male resident’s pain sensitivity and oxidative stress markers: The medial prefrontal cortex may play a role in sleep deprivation model. Front. Mol. Neurosci. 2022, 15, 937468. [Google Scholar] [CrossRef]
- Wiesner, C.D.; Davoli, V.; Schurger, D.; Prehn-Kristensen, A.; Baving, L. Melatonin Secretion during a Short Nap Fosters Subsequent Feedback Learning. Front. Hum. Neurosci. 2018, 11, 648. [Google Scholar] [CrossRef]
- Mantua, J.; Spencer, R.M.C. Exploring the nap paradox: Are mid-day sleep bouts a friend or foe? Sleep Med. 2017, 37, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tao, Q.; Ren, C. A Comprehensive Overview of the Neural Mechanisms of Light Therapy. Neurosci. Bull. 2024, 40, 350–362. [Google Scholar] [CrossRef]
- Alvino, G.M.; Blatchford, R.A.; Archer, G.S.; Mench, J.A. Light intensity during rearing affects the behavioural synchrony and resting patterns of broiler chickens. Br. Poult. Sci. 2009, 50, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Deep, A.; Schwean-Lardner, K.; Crowe, T.G.; Fancher, B.I.; Classen, H.L. Effect of light intensity on broiler production, processing characteristics, and welfare. Poult. Sci. 2010, 89, 2326–2333. [Google Scholar] [CrossRef]
- Olanrewaju, H.A.; Miller, W.W.; Maslin, W.R.; Collier, S.D.; Purswell, J.L.; Branton, S.L. Effects of light sources and intensity on broilers grown to heavy weights. Part 1: Growth performance, carcass characteristics, and welfare indices. Poult. Sci. 2016, 95, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, J.; Quan, S.; Yang, Y. Light regimen on health and growth of broilers: An update review. Poult. Sci. 2022, 101, 101545. [Google Scholar] [CrossRef]
- Blatchford, R.A.; Archer, G.S.; Mench, J.A. Contrast in light intensity, rather than day length, influences the behavior and health of broiler chickens. Poult. Sci. 2012, 91, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, H.A.; Miller, W.W.; Maslin, W.R.; Collier, S.D.; Purswell, J.L.; Branton, S.L. Influence of light sources and photoperiod on growth performance, carcass characteristics, and health indices of broilers grown to heavy weights. Poult. Sci. 2018, 97, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Prayitno, D.S.; Phillips, C.J.; Stokes, D.K. The effects of color and intensity of light on behavior and leg disorders in broiler chickens. Poult. Sci. 1997, 76, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Raccoursier, M.; Thaxton, Y.V.; Christensen, K.; Aldridge, D.J.; Scanes, C.G. Light intensity preferences of broiler chickens: Implications for welfare. Animal 2019, 13, 2857–2863. [Google Scholar] [CrossRef]
- van der Eijk, J.A.J.; Izquierdo Garcia-Faria, T.; Melis, S.; van Riel, J.W.; Te Beest, D.E.; de Jong, I.C. Light intensity preferences of broiler chickens is affected by breed, age, time of day and behaviour. Sci. Rep. 2025, 15, 6302. [Google Scholar] [CrossRef]
- Sirigu, A.; Daprati, E.; Ciancia, S.; Giraux, P.; Nighoghossian, N.; Posada, A.; Haggard, P. Altered awareness of voluntary action after damage to the parietal cortex. Nat. Neurosci. 2004, 7, 80–84. [Google Scholar] [CrossRef]
- Desmurget, M.; Sirigu, A. A parietal-premotor network for movement intention and motor awareness. Trends Cogn. Sci. 2009, 13, 411–419. [Google Scholar] [CrossRef]
- van Kempen, J.; Brandt, C.; Distler, C.; Bellgrove, M.A.; Thiele, A. Dopamine influences attentional rate modulation in Macaque posterior parietal cortex. Sci. Rep. 2022, 12, 6914. [Google Scholar] [CrossRef]
- Foster, R.G.; Helfrich-Forster, C. The regulation of circadian clocks by light in fruitflies and mice. Philos. Trans. R. Soc. B Biol. Sci. 2001, 356, 1779–1789. [Google Scholar] [CrossRef]
- Lucas, R.J.; Allen, A.E.; Brainard, G.C.; Brown, T.M.; Dauchy, R.T.; Didikoglu, A.; Do, M.T.H.; Gaskill, B.N.; Hattar, S.; Hawkins, P.; et al. Recommendations for measuring and standardizing light for laboratory mammals to improve welfare and reproducibility in animal research. PLoS Biol. 2024, 22, e3002535. [Google Scholar] [CrossRef]
- Vandewalle, G.; Maquet, P.; Dijk, D.J. Light as a modulator of cognitive brain function. Trends Cogn. Sci. 2009, 13, 429–438. [Google Scholar] [CrossRef]
- Sabbah, S.; Worden, M.S.; Laniado, D.D.; Berson, D.M.; Sanes, J.N. Luxotonic signals in human prefrontal cortex as a possible substrate for effects of light on mood and cognition. Proc. Natl. Acad. Sci. USA 2022, 119, e2118192119. [Google Scholar] [CrossRef]
- Coria-Avila, G.A.; Pfaus, J.G.; Orihuela, A.; Dominguez-Oliva, A.; Jose-Perez, N.; Hernandez, L.A.; Mota-Rojas, D. The Neurobiology of Behavior and Its Applicability for Animal Welfare: A Review. Animals 2022, 12, 928. [Google Scholar] [CrossRef] [PubMed]
- Crozier, W.J.; Pincus, G. Stereotropism in Rats and Mice. J. Gen. Physiol. 1926, 10, 195–203. [Google Scholar] [CrossRef]
- Gong, Z.; Liu, J.; Guo, C.; Zhou, Y.; Teng, Y.; Liu, L. Two pairs of neurons in the central brain control Drosophila innate light preference. Science 2010, 330, 499–502. [Google Scholar] [CrossRef]
- Ward, A.; Liu, J.; Feng, Z.; Xu, X.Z. Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nat. Neurosci. 2008, 11, 916–922. [Google Scholar] [CrossRef]
- Yamanaka, N.; Romero, N.M.; Martin, F.A.; Rewitz, K.F.; Sun, M.; O’Connor, M.B.; Leopold, P. Neuroendocrine control of Drosophila larval light preference. Science 2013, 341, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Navara, K.J.; Nelson, R.J. The dark side of light at night: Physiological, epidemiological, and ecological consequences. J. Pineal Res. 2007, 43, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, Q.; Gong, N. A modified light-dark box test for the common marmoset. Neurosci. Bull 2014, 30, 394–400. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Fero, K.; Arrenberg, A.B.; Bergeron, S.A.; Driever, W.; Burgess, H.A. Deep brain photoreceptors control light-seeking behavior in zebrafish larvae. Curr. Biol. 2012, 22, 2042–2047. [Google Scholar] [CrossRef] [PubMed]
- Hones, V.I.; Mizumori, S.J.Y. Response Flexibility: The Role of the Lateral Habenula. Front Behav. Neurosci. 2022, 16, 852235. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qu, N.; Gonzalez, N.V.; Palma, M.A.; Chen, H.; Xiong, J.; Choubey, A.; Li, Y.; Li, X.; Yu, M.; et al. A Light-Responsive Neural Circuit Suppresses Feeding. J. Neurosci. 2024, 44, e2192232024. [Google Scholar] [CrossRef]
- Derdeyn, P.; Hui, M.; Macchia, D.; Beier, K.T. Uncovering the Connectivity Logic of the Ventral Tegmental Area. Front. Neural Circuits 2021, 15, 799688. [Google Scholar] [CrossRef]
- Kang, S.W. Central Nervous System Associated with Light Perception and Physiological Responses of Birds. Front. Physiol. 2021, 12, 723454. [Google Scholar] [CrossRef]
- Kristensen, H.H.; Aerts, J.M.; LeRoy, T.; Berckmans, D.; Wathes, C.M. Using light to control activity in broiler chickens. Br. Poult. Sci. 2004, 45 (Suppl. 1), S30–S31. [Google Scholar] [CrossRef]
- Bizeray, D.; Estevez, I.; Leterrier, C.; Faure, J.M. Influence of increased environmental complexity on leg condition, performance, and level of fearfulness in broilers. Poult. Sci. 2002, 81, 767–773. [Google Scholar] [CrossRef]
- Dawson, L.C.; Widowski, T.M.; Liu, Z.; Edwards, A.M.; Torrey, S. In pursuit of a better broiler: A comparison of the inactivity, behavior, and enrichment use of fast- and slower growing broiler chickens. Poult. Sci. 2021, 100, 101451. [Google Scholar] [CrossRef]
- Dawkins, M.S. Active walking in broiler chickens: A flagship for good welfare, a goal for smart farming and a practical starting point for automated welfare recognition. Front. Veter Sci. 2024, 10, 1345216. [Google Scholar] [CrossRef]
- Blatchford, R.A.; Klasing, K.C.; Shivaprasad, H.L.; Wakenell, P.S.; Archer, G.S.; Mench, J.A. The effect of light intensity on the behavior, eye and leg health, and immune function of broiler chickens. Poult. Sci. 2009, 88, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Newberry, R.C.; Hunt, J.R.; Gardiner, E.E. Influence of light intensity on behavior and performance of broiler chickens. Poult. Sci. 1988, 67, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Christensen, K.D.; Aldridge, D.; Kuenzel, W.J. Effects of light intensity and dual light intensity choice on plasma corticosterone, central serotonergic and dopaminergic activities in birds, Gallus gallus. Gen. Comp. Endocrinol. 2020, 285, 113289. [Google Scholar] [CrossRef]
- Kang, S.W.; Christensen, K.D. Effects of enrichment hut on natural behavior and leg-health of broilers in light-enriched commercial houses. In Proceedings of the International Poultry Scientific Forum, Atlanta, GA, USA, 29–30 January 2024. [Google Scholar]
- Kang, S.W.; Christensen, K.D. Effects of environmental enrichment hut on eye dimensions of broilers. In Proceedings of the Poultry Science Association Annual Meeting, Louisville, KY, USA, 15–18 July 2024. [Google Scholar]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Korkmaz, A.; Reiter, R.J.; Topal, T.; Manchester, L.C.; Oter, S.; Tan, D.X. Melatonin: An established antioxidant worthy of use in clinical trials. Mol. Med. 2009, 15, 43–50. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Hardeland, R.; Poeggeler, B. Melatonin and synthetic melatonergic agonists: Actions and metabolism in the central nervous system. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 189–216. [Google Scholar] [CrossRef]
- Skene, D.J.; Vivien-Roels, B.; Sparks, D.L.; Hunsaker, J.C.; Pevet, P.; Ravid, D.; Swaab, D.F. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the human pineal gland: Effect of age and Alzheimer’s disease. Brain Res. 1990, 528, 170–174. [Google Scholar] [CrossRef]
- Uchida, K.; Okamoto, N.; Ohara, K.; Morita, Y. Daily rhythm of serum melatonin in patients with dementia of the degenerate type. Brain Res. 1996, 717, 154–159. [Google Scholar] [CrossRef]
- Qian, J.; Morris, C.J.; Phillips, A.J.K.; Li, P.; Rahman, S.A.; Wang, W.; Hu, K.; Arendt, J.; Czeisler, C.A.; Scheer, F. Unanticipated daytime melatonin secretion on a simulated night shift schedule generates a distinctive 24-h melatonin rhythm with antiphasic daytime and nighttime peaks. J. Pineal Res. 2022, 72, e12791. [Google Scholar] [CrossRef] [PubMed]
- Mesas, A.E.; Nunez de Arenas-Arroyo, S.; Martinez-Vizcaino, V.; Garrido-Miguel, M.; Fernandez-Rodriguez, R.; Bizzozero-Peroni, B.; Torres-Costoso, A.I. Is daytime napping an effective strategy to improve sport-related cognitive and physical performance and reduce fatigue? A systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 2023, 57, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Romdhani, M.; Dergaa, I.; Moussa-Chamari, I.; Souissi, N.; Chaabouni, Y.; Mahdouani, K.; Abene, O.; Driss, T.; Chamari, K.; Hammouda, O. The effect of post-lunch napping on mood, reaction time, and antioxidant defense during repeated sprint exercice. Biol. Sport 2021, 38, 629–638. [Google Scholar] [CrossRef]
- Van Cauter, E.; Moreno-Reyes, R.; Akseki, E.; L’Hermite-Baleriaux, M.; Hirschfeld, U.; Leproult, R.; Copinschi, G. Rapid phase advance of the 24-h melatonin profile in response to afternoon dark exposure. Am. J. Physiol. 1998, 275, E48–E54. [Google Scholar] [CrossRef]
- Iacovitti, L.; Stull, N.D.; Johnston, K. Melatonin rescues dopamine neurons from cell death in tissue culture models of oxidative stress. Brain Res. 1997, 768, 317–326. [Google Scholar] [CrossRef]
- Jimenez-Delgado, A.; Ortiz, G.G.; Delgado-Lara, D.L.; Gonzalez-Usigli, H.A.; Gonzalez-Ortiz, L.J.; Cid-Hernandez, M.; Cruz-Serrano, J.A.; Pacheco-Moises, F.P. Effect of Melatonin Administration on Mitochondrial Activity and Oxidative Stress Markers in Patients with Parkinson’s Disease. Oxidative Med. Cell. Longev. 2021, 2021, 5577541. [Google Scholar] [CrossRef]
- Georgakopoulou, V.E.; Sklapani, P.; Trakas, N.; Reiter, R.J.; Spandidos, D.A. Exploring the association between melatonin and nicotine dependence (Review). Int. J. Mol. Med. 2024, 54, 82. [Google Scholar] [CrossRef]
- Uz, T.; Arslan, A.D.; Kurtuncu, M.; Imbesi, M.; Akhisaroglu, M.; Dwivedi, Y.; Pandey, G.N.; Manev, H. The regional and cellular expression profile of the melatonin receptor MT1 in the central dopaminergic system. Brain Res. Mol. Brain Res. 2005, 136, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Venero, J.L.; Absi, E.H.; Cano, J.; Machado, A. Melatonin induces tyrosine hydroxylase mRNA expression in the ventral mesencephalon but not in the hypothalamus. J. Pineal Res. 2002, 32, 6–14. [Google Scholar] [CrossRef]
- Kang, S.W.; Christensen, K.D.; Kidd, M.T., Jr.; Orlowski, S.K.; Clark, J. Effects of a variable light intensity lighting program on the welfare and performance of commercial broiler chickens. Front. Physiol. 2023, 14, 1059055. [Google Scholar] [CrossRef]
- Kang, S.W.; Christensen, K.D.; Kidd, M.T., Jr.; Orlowski, S.K. Effects of Environmental Enrichments on Welfare and Hepatic Metabolic Regulation of Broiler Chickens. Animals 2024, 14, 557. [Google Scholar] [CrossRef] [PubMed]
- Bankoski, A.; Harris, T.B.; McClain, J.J.; Brychta, R.J.; Caserotti, P.; Chen, K.Y.; Berrigan, D.; Troiano, R.P.; Koster, A. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care 2011, 34, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.M.; Molina-Hidalgo, C.; Aghjayan, S.L.; Fanning, J.; Erlenbach, E.D.; Gothe, N.P.; Velazquez-Diaz, D.; Erickson, K.I. Differentiating the influence of sedentary behavior and physical activity on brain health in late adulthood. Exp. Gerontol. 2023, 180, 112246. [Google Scholar] [CrossRef]
- Sherlock, L.; Demmers, T.G.; Goodship, A.E.; McCarthy, I.D.; Wathes, C.M. The relationship between physical activity and leg health in the broiler chicken. Br. Poult. Sci. 2010, 51, 22–30. [Google Scholar] [CrossRef]
- van der Berg, J.D.; Stehouwer, C.D.; Bosma, H.; van der Velde, J.H.; Willems, P.J.; Savelberg, H.H.; Schram, M.T.; Sep, S.J.; van der Kallen, C.J.; Henry, R.M.; et al. Associations of total amount and patterns of sedentary behaviour with type 2 diabetes and the metabolic syndrome: The Maastricht Study. Diabetologia 2016, 59, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Reiter, K.; Bessei, W. [Effect of locomotor activity on leg disorder in fattening chicken]. Berl. Munch. Tierarztl. Wochenschr. 2009, 122, 264–270. [Google Scholar]
- Englund, M.D.; Cronin, K.A. Choice, control, and animal welfare: Definitions and essential inquiries to advance animal welfare science. Front. Veter Sci. 2023, 10, 1250251. [Google Scholar] [CrossRef]
- Bastioli, G.; Arnold, J.C.; Mancini, M.; Mar, A.C.; Gamallo-Lana, B.; Saadipour, K.; Chao, M.V.; Rice, M.E. Voluntary Exercise Boosts Striatal Dopamine Release: Evidence for the Necessary and Sufficient Role of BDNF. J. Neurosci. 2022, 42, 4725–4736. [Google Scholar] [CrossRef]
- Ruiz-Tejada, A.; Neisewander, J.; Katsanos, C.S. Regulation of Voluntary Physical Activity Behavior: A Review of Evidence Involving Dopaminergic Pathways in the Brain. Brain Sci. 2022, 12, 333. [Google Scholar] [CrossRef]
- Knab, A.M.; Bowen, R.S.; Hamilton, A.T.; Gulledge, A.A.; Lightfoot, J.T. Altered dopaminergic profiles: Implications for the regulation of voluntary physical activity. Behav. Brain Res. 2009, 204, 147–152. [Google Scholar] [CrossRef]
- Sherwin, C.M. Voluntary wheel running: A review and novel interpretation. Anim. Behav. 1998, 56, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Xie, Y.; Fan, R.; Wang, Q.; Luo, Y.; Dong, P. Exercise orchestrates systemic metabolic and neuroimmune homeostasis via the brain-muscle-liver axis to slow down aging and neurodegeneration: A narrative review. Eur. J. Med. Res. 2025, 30, 475. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Lee, H.; Lim, Y. Potential Effects of Resistant Exercise on Cognitive and Muscle Functions Mediated by Myokines in Sarcopenic Obese Mice. Biomedicines 2022, 10, 2529. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.H.; Wang, Q.Y.; Lv, Q.A.; Zhang, Y.Q.; Gao, G.X.; Lu, S. Advances in the research on myokine-driven regulation of bone metabolism. Heliyon 2024, 10, e22547. [Google Scholar] [CrossRef]
- Lawler, J.M.; Powers, S.K. Oxidative stress, antioxidant status, and the contracting diaphragm. Can. J. Appl. Physiol. 1998, 23, 23–55. [Google Scholar] [CrossRef]
- Thirupathi, A.; Pinho, R.A. Effects of reactive oxygen species and interplay of antioxidants during physical exercise in skeletal muscles. J. Physiol. Biochem. 2018, 74, 359–367. [Google Scholar] [CrossRef]
- Kwon, C.H.; Sun, J.L.; Kim, M.J.; Abd El-Aty, A.M.; Jeong, J.H.; Jung, T.W. Clinically confirmed DEL-1 as a myokine attenuates lipid-induced inflammation and insulin resistance in 3T3-L1 adipocytes via AMPK/HO-1- pathway. Adipocyte 2020, 9, 576–586. [Google Scholar] [CrossRef]
- Leal, L.G.; Lopes, M.A.; Batista, M.L. Physical Exercise-Induced Myokines and Muscle-Adipose Tissue Crosstalk: A Review of Current Knowledge and the Implications for Health and Metabolic Diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef]
- Machado, M.V. Aerobic Exercise in the Management of Metabolic Dysfunction Associated Fatty Liver Disease. Diabetes Metab. Syndr. Obes. 2021, 14, 3627–3645. [Google Scholar] [CrossRef] [PubMed]
- van der Windt, D.J.; Sud, V.; Zhang, H.; Tsung, A.; Huang, H. The Effects of Physical Exercise on Fatty Liver Disease. Gene Expr. 2018, 18, 89–101. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Y.; Liu, H.; Guo, Y. Protective effect of exercise on metabolic dysfunction-associated fatty liver disease: Potential epigenetic mechanisms (Review). Int. J. Mol. Med. 2025, 56, 146. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, L.P.; Hannivoort, R.A.; Gerken, G.; Hotamisligil, G.S.; Trauner, M.; Canbay, A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012, 56, 952–964. [Google Scholar] [CrossRef]
- Parhofer, K.G. Interaction between Glucose and Lipid Metabolism: More than Diabetic Dyslipidemia. Diabetes Metab. J. 2015, 39, 353–362. [Google Scholar] [CrossRef]
- Han, H.S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef]
- Shah, A.M.; Wondisford, F.E. Tracking the carbons supplying gluconeogenesis. J. Biol. Chem. 2020, 295, 14419–14429. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, J.; Tang, K.; Huang, B. Beyond energy storage: Roles of glycogen metabolism in health and disease. FEBS J. 2021, 288, 3772–3783. [Google Scholar] [CrossRef]
- Casas-Grajales, S.; Muriel, P. Antioxidants in liver health. World J. Gastrointest. Pharmacol. Ther. 2015, 6, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L.; Gunn, P.J. The regulation of hepatic fatty acid synthesis and partitioning: The effect of nutritional state. Nat. Rev. Endocrinol. 2019, 15, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Dennis, K.M.J.H.; Hodson, L. The ins and outs of liver fat metabolism: The effect of phenotype and diet on risk of intrahepatic triglyceride accumulation. Exp. Physiol. 2025, 110, 936–948. [Google Scholar] [CrossRef]
- Franssen, W.M.A.; Nieste, I.; Verboven, K.; Eijnde, B.O. Sedentary behaviour and cardiometabolic health: Integrating the potential underlying molecular health aspects. Metabolism 2025, 170, 156320. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, Z.X.; Qian, M.Z.; Li, Z.; Feng, Z.H.; Luo, S.Y.; Gao, Q.F.; Hou, Z.S. Low light intensity dysregulated growth and behavior of juvenile rainbow trout via microbiota-gut-brain axis. Aquaculture 2025, 603, 742388. [Google Scholar] [CrossRef]
- Kim, H.J.; Son, J.; Kim, H.S.; Hong, E.C.; Kim, J.H. Effects of light intensity on growth performance, blood components, carcass characteristics, and welfare of broilers. J. Anim. Sci. Technol. 2022, 64, 985–996. [Google Scholar] [CrossRef]
- Li, T.; Troilo, D.; Glasser, A.; Howland, H.C. Constant light produces severe corneal flattening and hyperopia in chickens. Vision Res. 1995, 35, 1203–1209. [Google Scholar] [CrossRef]
- Li, T.; Howland, H.C. The effects of constant and diurnal illumination of the pineal gland and the eyes on ocular growth in chicks. Investig. Opthalmology Vis. Sci. 2003, 44, 3692–3697. [Google Scholar] [CrossRef]
- Iuvone, P.M.; Tosini, G.; Pozdeyev, N.; Haque, R.; Klein, D.C.; Chaurasia, S.S. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog. Retin. Eye Res. 2005, 24, 433–456. [Google Scholar] [CrossRef]
- Ostrin, L.A. Ocular and systemic melatonin and the influence of light exposure. Clin. Exp. Optom. 2019, 102, 99–108. [Google Scholar] [CrossRef]
- Tosini, G.; Baba, K.; Hwang, C.K.; Iuvone, P.M. Melatonin: An underappreciated player in retinal physiology and pathophysiology. Exp. Eye Res. 2012, 103, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Pintor, J.; Martin, L.; Pelaez, T.; Hoyle, C.H.; Peral, A. Involvement of melatonin MT(3) receptors in the regulation of intraocular pressure in rabbits. Eur. J. Pharmacol. 2001, 416, 251–254. [Google Scholar] [CrossRef]
- Cahill, G.M.; Besharse, J.C. Circadian Clock Functions Localized in Xenopus Retinal Photoreceptors. Neuron 1993, 10, 573–577. [Google Scholar] [CrossRef]
- Liu, C.M.; Fukuhara, C.; Wessel, J.H.; Iuvone, P.M.; Tosini, G. Localization of Aa-nat mRNA in the rat retina by fluorescence in situ hybridization and laser capture microdissection. Cell Tissue Res. 2004, 315, 197–201. [Google Scholar] [CrossRef]
- Tosini, G.; Menaker, M. Circadian rhythms in cultured mammalian retina. Science 1996, 272, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Brennan, R.; Jan, J.E.; Lyons, C.J. Light, dark, and melatonin: Emerging evidence for the importance of melatonin in ocular physiology. Eye 2007, 21, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Moreno, M.C.; Campanelli, J.; Sande, P.; Sáenz, D.A.; Sarmiento, M.I.S.K.; Rosenstein, R.E. Retinal oxidative stress induced by high intraocular pressure. Free. Radic. Biol. Med. 2004, 37, 803–812. [Google Scholar] [CrossRef]
- Lauber, J.K. Light-Induced Avain Glaucoma as an Animal-Model for Human Primary Glaucoma. J. Ocul. Pharmacol. 1987, 3, 77–100. [Google Scholar] [CrossRef]
- Oishi, T.; Lauber, J.K.; Vriend, J. Experimental Myopia and Glaucoma in Chicks. Zool. Sci. 1987, 4, 455–464. [Google Scholar]
- Martínez-Aguila, A.; Fonseca, B.; de Lara, M.J.P.; Pintor, J. Effect of Melatonin and 5-Methoxycarbonylamino-N-Acetyltryptamine on the Intraocular Pressure of Normal and Glaucomatous Mice. J. Pharmacol. Exp. Ther. 2016, 357, 293–299. [Google Scholar] [CrossRef]
- Fowler, W.C.; Chang, D.H.; Roberts, B.C.; Zarovnaya, E.L.; Proia, A.D. A new paradigm for corneal wound healing research: The white leghorn chicken (Gallus gallus domesticus). Curr. Eye Res. 2004, 28, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Shynkaruk, T.; Parsons, M.; Adler, C.A.B.; Goeree, C.; Long, K.; Schwean-Lardner, K. Does the distribution of light intensity within the barn impact broiler production and welfare? Br. Poult. Sci. 2025, 66, 281–289. [Google Scholar] [CrossRef]
- Piccirillo, R. Exercise-Induced Myokines with Therapeutic Potential for Muscle Wasting. Front. Physiol. 2019, 10, 287. [Google Scholar] [CrossRef]
- Zhang, X.; Smith, S.W.; Zaldivar, L.R.; Lesak, D.J.; Schilling, M.W. Study of emerging chicken meat quality defects using OMICs: What do we know? J. Proteom. 2023, 276, 104837. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, P.; Beitia, A.; Weil, J.; Suesuttajit, N.; Hilton, K.; Caldas, J.; Umberson, C.; Martinez, D.; Kong, B.; Owens, C.M.; et al. Woody breast myopathy broiler show age-dependent adaptive differential gene expression in Pectoralis major and altered in-vivo triglyceride kinetics in adipogenic tissues. Poult. Sci. 2021, 100, 101092. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic-Racic, M.; Perdomo, G.; Mantell, B.S.; Sipula, I.J.; Brown, N.F.; O’Doherty, R.M. A moderate increase in carnitine palmitoyltransferase 1a activity is sufficient to substantially reduce hepatic triglyceride levels. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E969–E977. [Google Scholar] [CrossRef] [PubMed]

| Trial | Treatment | DWG (g) | FCR |
|---|---|---|---|
| Trial 1 (56 days) | 5 lx | 67.1 | 1.96 |
| 20 lx | 66.3 | 1.98 | |
| NL | 65.7 | 2.00 | |
| VL | 67.3 | 1.93 | |
| Trial 2 (51 days) | 5 lx | 66.4 | 1.86 |
| 20 lx | 64.2 | 1.84 | |
| NL | 64.0 | 1.87 | |
| VL | 66.5 | 1.82 | |
| Trial 3 (49 days) | 5 lx | 63.9 | 1.92 |
| 20 lx | 66.2 | 1.91 | |
| NL | 61.6 | 1.99 | |
| VL | 66.3 | 1.89 | |
| Trial 4 (55 days) | 20 lx | 69.0 | 1.97 |
| 20 lx | 67.6 | 2.07 | |
| VL | 68.7 | 1.94 | |
| VL | 70.6 | 1.95 |
| Treatment | BF (mm) | SS (mm) | CD (mm) | CD/BW (mm/0.1 kg) | EW (g) | EW/BW (g/kg) |
|---|---|---|---|---|---|---|
| 20 lx_Con | 16.107 a | 19.246 a,b | 9.343 a | 1.056 a | 2.824 a | 0.319 b |
| 20 lx_Hut | 15.784 b | 18.934 b | 9.039 b | 1.088 a | 2.641 b | 0.318 b |
| VL_Con | 15.852 a,b | 19.352 a | 9.194 a | 1.055 a | 2.916 a | 0.335 a |
| VL_Hut | 15.797 a,b | 19.357 a | 8.818 b | 0.996 b | 2.774 a,b | 0.313 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.W. Effectual Environmental Enrichments for Commercial Broiler Chickens. Animals 2025, 15, 2829. https://doi.org/10.3390/ani15192829
Kang SW. Effectual Environmental Enrichments for Commercial Broiler Chickens. Animals. 2025; 15(19):2829. https://doi.org/10.3390/ani15192829
Chicago/Turabian StyleKang, Seong W. 2025. "Effectual Environmental Enrichments for Commercial Broiler Chickens" Animals 15, no. 19: 2829. https://doi.org/10.3390/ani15192829
APA StyleKang, S. W. (2025). Effectual Environmental Enrichments for Commercial Broiler Chickens. Animals, 15(19), 2829. https://doi.org/10.3390/ani15192829






