Sericin Enhances Cryopreserved Sperm Quality in Chengde Hornless Black Goats by Increasing Glutamine Metabolism
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Semen Extender Supplemented with Sericin
2.3. Semen Collection, Quality Assessment, and Isothermal Dilution
2.4. Freezing and Thawing of Semen
2.5. Sperm Motility Assessment
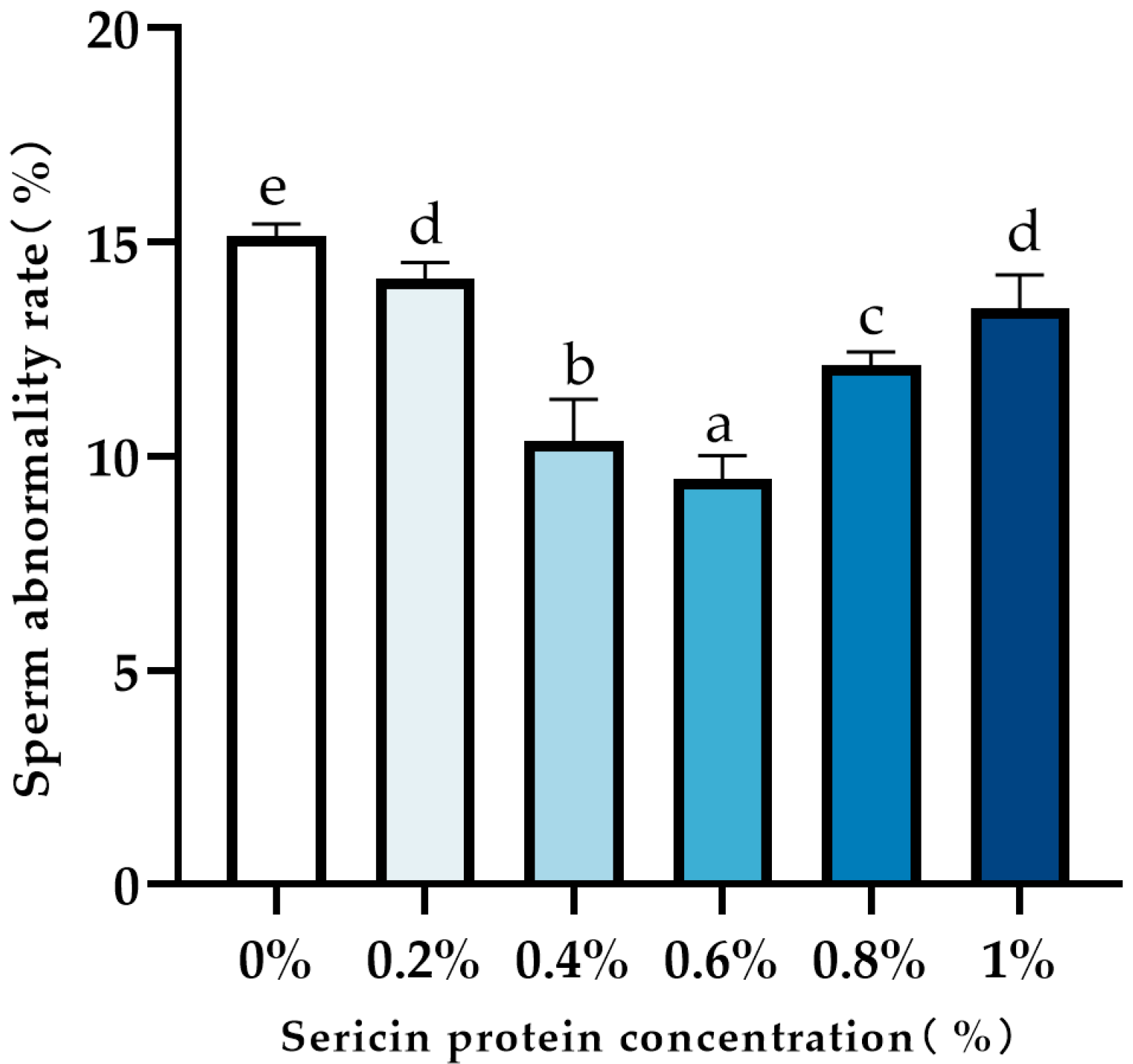
2.6. Sperm Abnormality Analysis
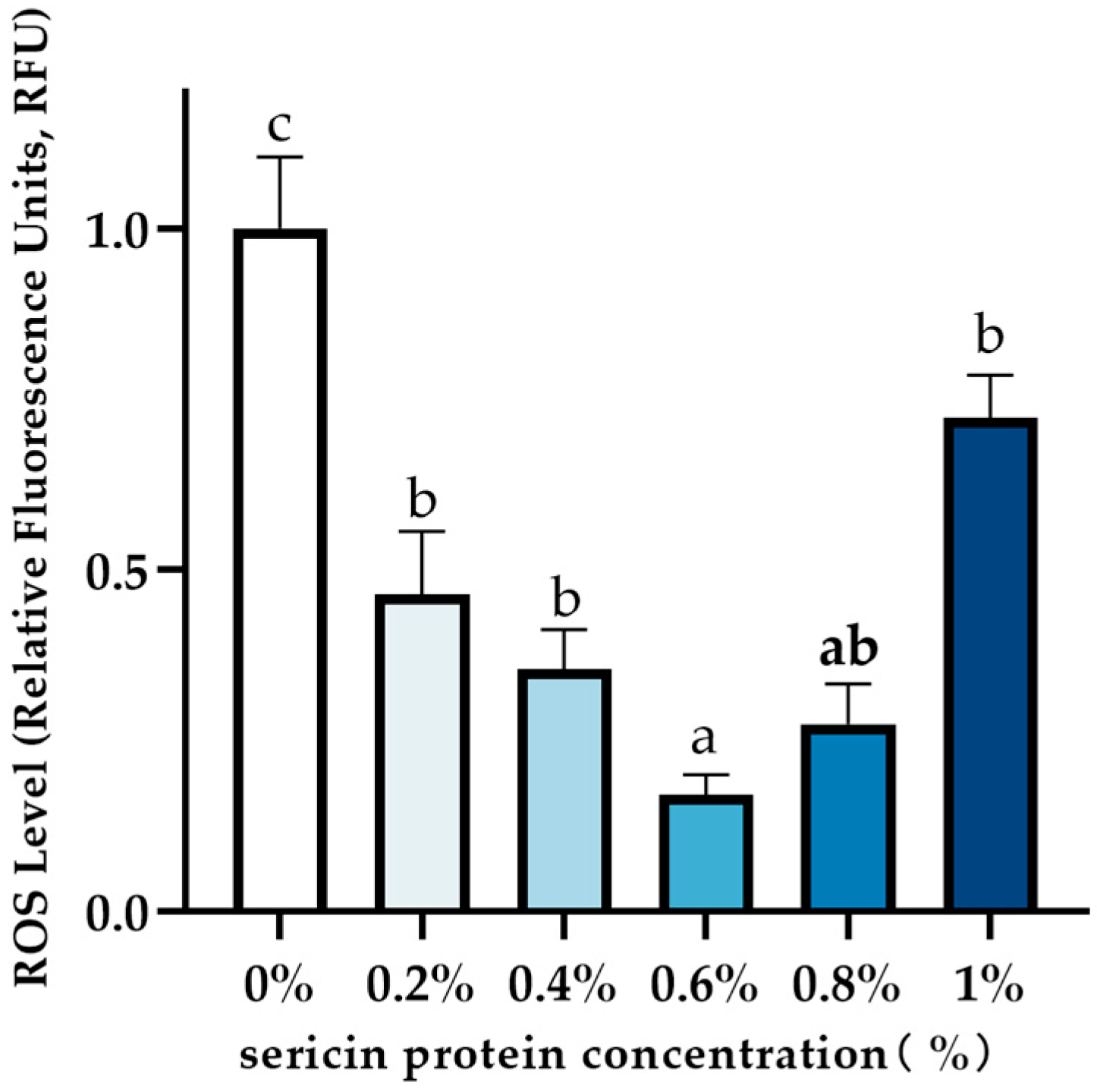
2.7. Measurement of Sperm ROS Levels
2.8. Metabolite Extraction
2.9. Metabolome Sequencing and Data Analysis
2.10. Protein Extraction
2.11. Proteome Sequencing and Data Analysis
2.12. qRT-PCR
2.13. Statistical Analysis
3. Results
3.1. Effect of Sericin on Post-Thaw Sperm Viability
3.2. Formatting of Mathematical Components
3.3. Effect of Sericin on Post-Thaw Sperm ROS Levels
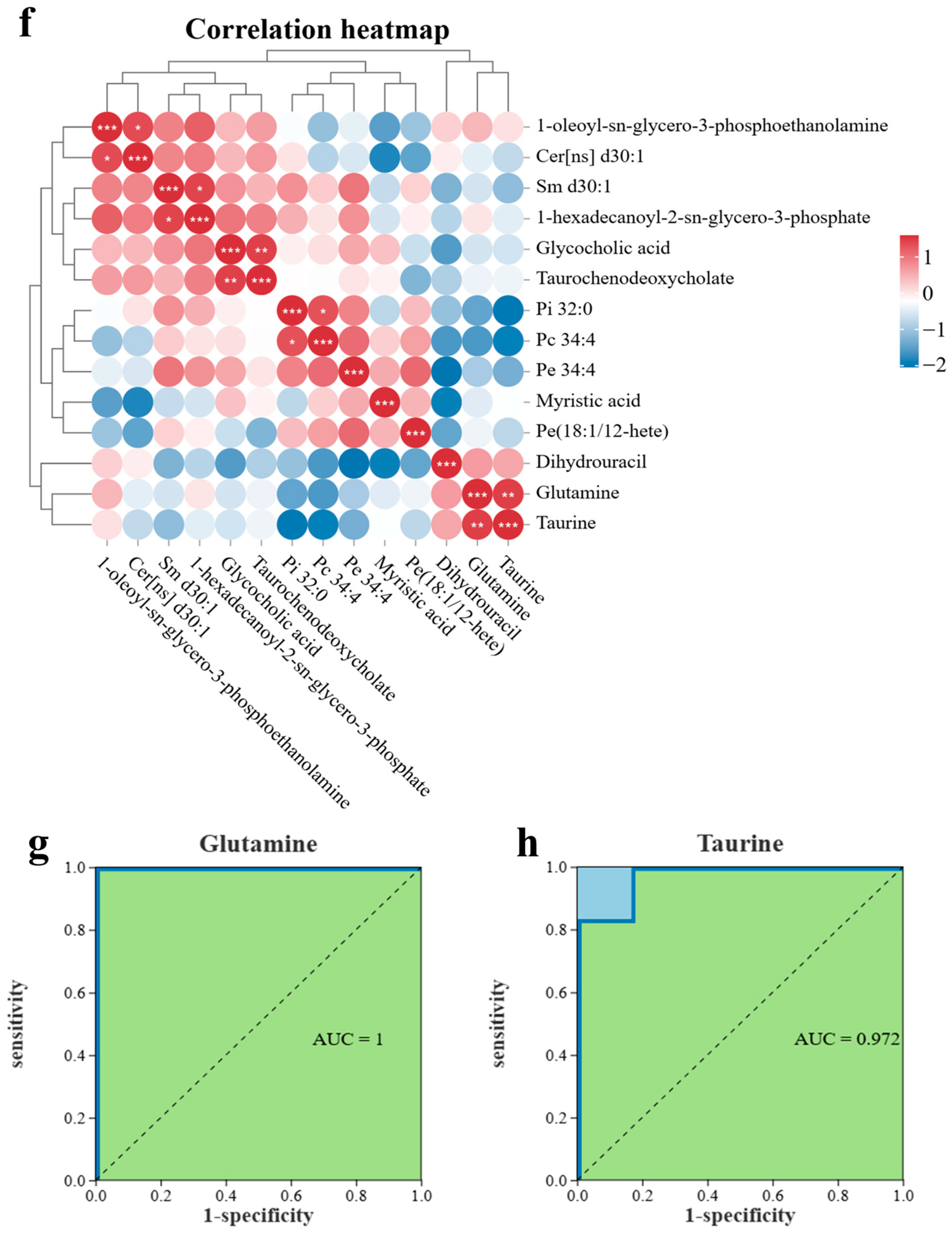
3.4. Untargeted Metabolomics-Based Analysis of the Effect of Sericin Proteins on the Cryopreservation of Semen from Chengde Hornless Goats
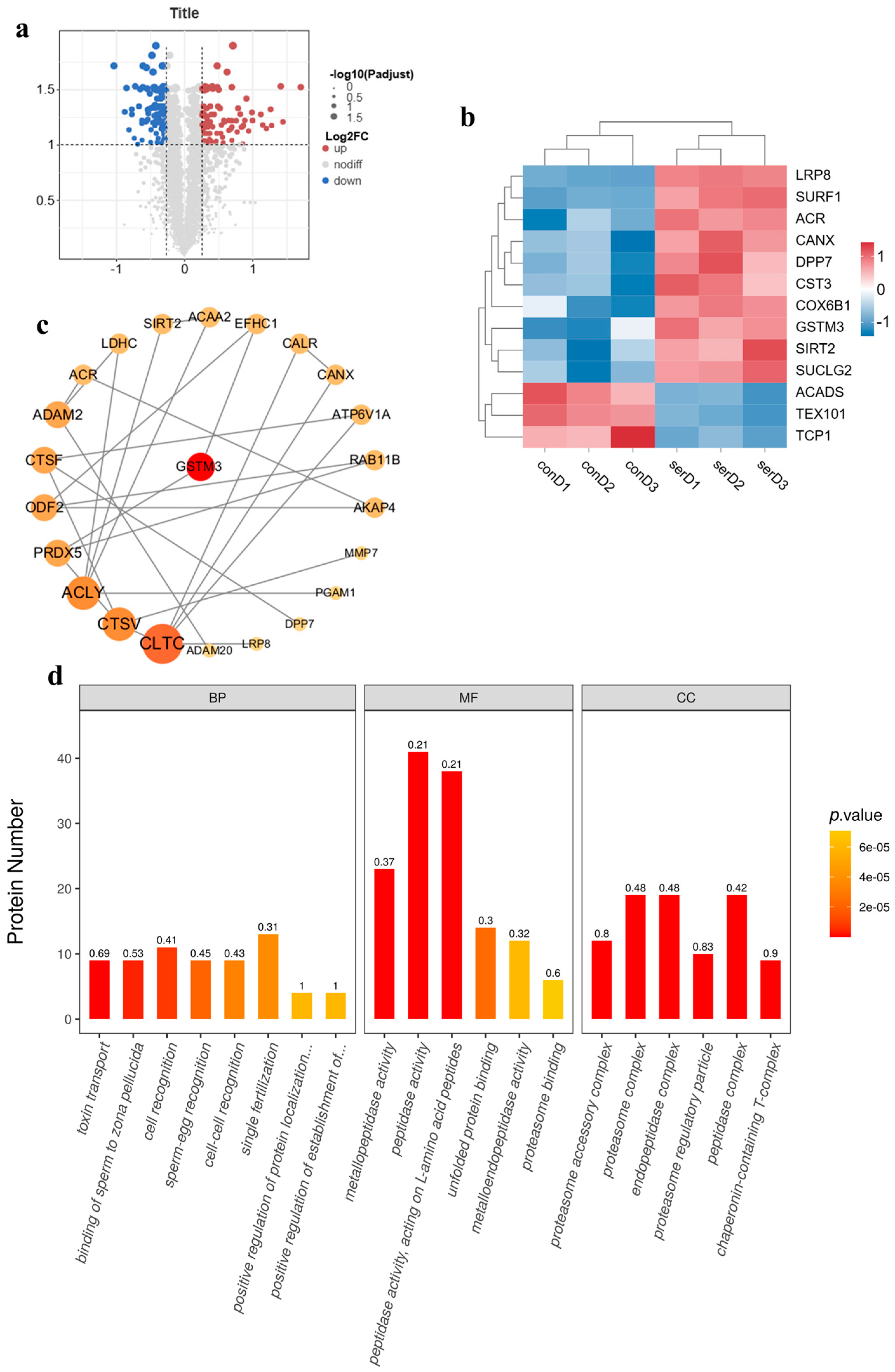
3.5. TMT-Tagged Quantitative Proteomics-Based Analysis of the Effect of Sericin Proteins on the Cryopreservation of Semen from Chengde Hornless Goats
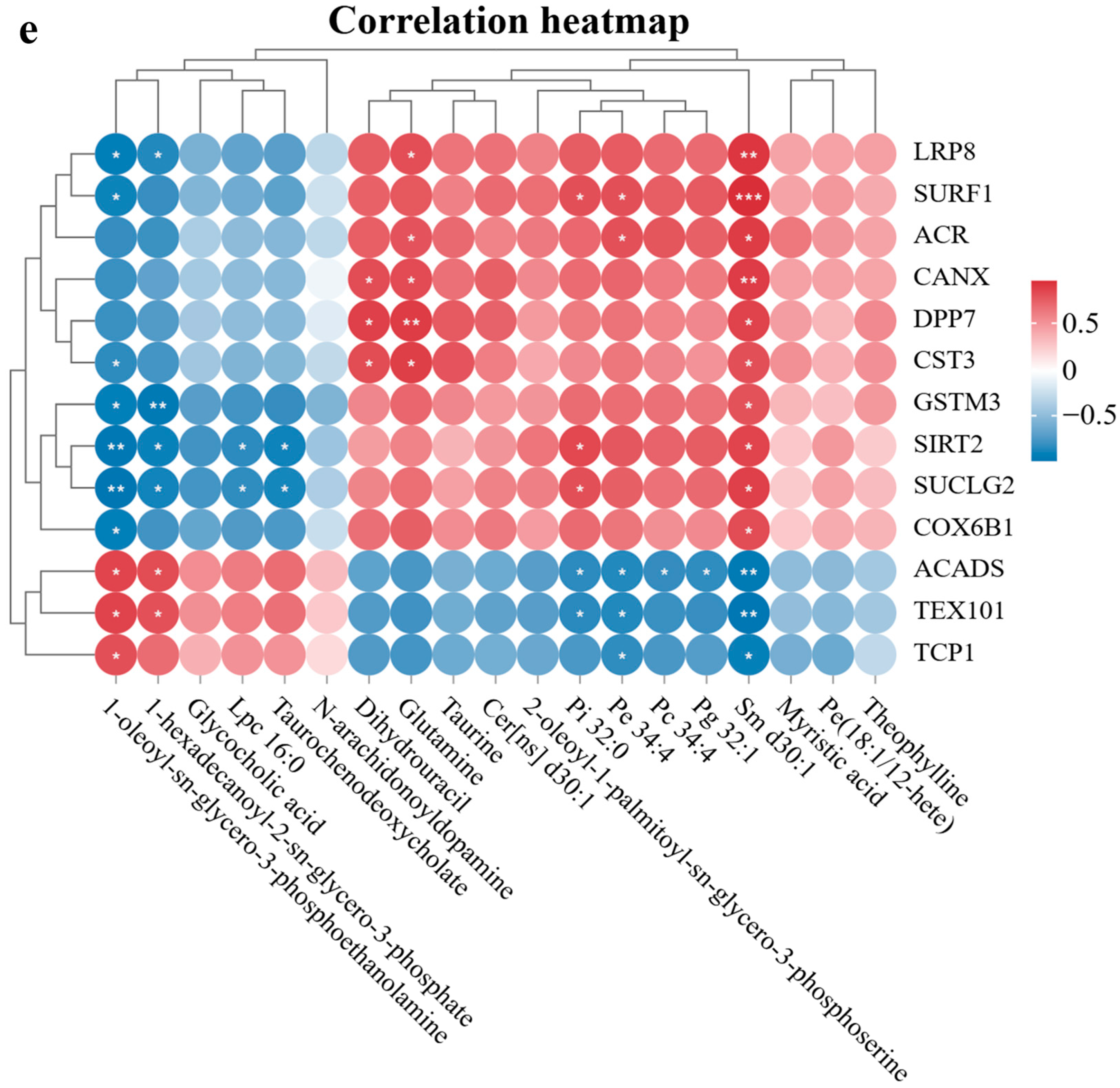
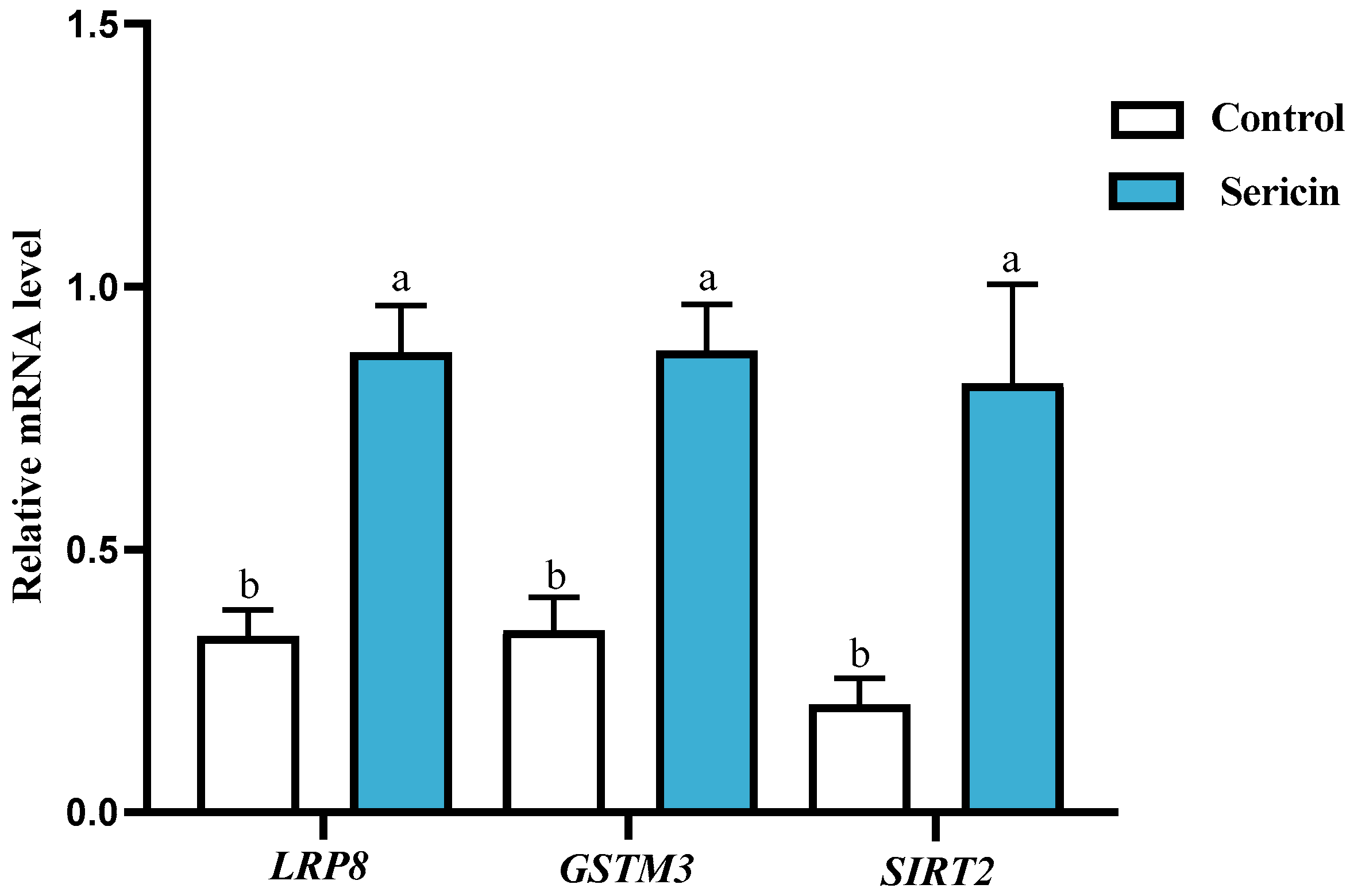
3.6. Verification of Sericin’s Antioxidant Effects via Key Proteins in Chengde Hornless Black Goat Sperm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Liu, Y.; Duan, C.; Zhang, Y.; Wang, Y.; Guo, Y. Analysis of Genetic Diversity and Genetic Structure in 7 Local Goat Breeds. aBIOTECH 2020, 36, 183–190. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, S.; Han, C.; Hong, Z.; Xie, Y.; Qiao, X.; Liu, Z.; Gong, Y. Identification of the Core Promoter Region of KCNJ15 Gene in Chengde Polled Goat (Capra hircus). J. Agric. Biotechnol. 2024, 32, 2081–2087. [Google Scholar]
- Saha, A.; Asaduzzaman, M.; Bari, F.Y. Cryopreservation Techniques for Ram Sperm. Vet. Med. Int. 2022, 2022, 7378379. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, R.; Wang, Z.; Liu, H.; Wang, Z.; Zhang, W.; Zhang, Y.; Su, R.; Liu, Z.; Liu, Y.; et al. Evaluation of lipidomic change in goat sperm after cryopreservation. Front. Vet. Sci. 2022, 9, 1004683. [Google Scholar] [CrossRef] [PubMed]
- Darin-Bennett, A.; White, I.G. Influence of the cholesterol content of mammalian spermatozoa on susceptibility to cold-shock. Cryobiology 1977, 14, 466–470. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhao, Z. Freezing-induced changes in goat cheese: Effect of freezing rate, storage and freeze–thaw cycles. Int. J. Dairy Technol. 2023, 76, 436–441. [Google Scholar] [CrossRef]
- Aghaz, F.; Asadi, Z.; Sajadimajd, S.; Kashfi, K.; Arkan, E.; Rahimi, Z. Codelivery of resveratrol melatonin utilizing pHresponsive sericin based nanocarriers inhibits the proliferation of breast cancer cell line at the different pH. Sci. Rep. 2023, 13, 11090. [Google Scholar] [CrossRef]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef]
- Saad, M.; El-Samad, L.M.; Gomaa, R.A.; Augustyniak, M.; Hassan, M.A. A comprehensive review of recent advances in silk sericin: Extraction approaches, structure, biochemical characterization, and biomedical applications. Int. J. Biol. Macromol. 2023, 250, 126067. [Google Scholar] [CrossRef]
- Liu, J.; Shi, L.; Deng, Y.; Zou, M.; Cai, B.; Song, Y.; Wang, Z.; Wang, L. Silk sericin-based materials for biomedical applications. Biomaterials 2022, 287, 121638. [Google Scholar] [CrossRef]
- Raza, S.; Uçan, U.; Aksoy, M.; Erdoğan, G.; Ceylan, A.; Serin, I. Silk protein sericin pretreatment enhances osmotic tolerance and post-thaw sperm quality but reduces the ability of sperm cells to undergo in vitro induced acrosome reaction in rabbit. Cryobiology 2019, 90, 1–7. [Google Scholar] [CrossRef]
- Kunz, R.I.; Brancalhão, R.M.; Ribeiro, L.F.; Natali, M.R. Silkworm Sericin: Properties and Biomedical Applications. Biomed. Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef]
- Cao, T.T.; Zhang, Y.Q. Processing and characterization of silk sericin from Bombyx mori and its application in biomaterials and biomedicines. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Aghaz, F.; Hajarian, H.; Shabankareh, H.K.; Abdolmohammadi, A. Effect of sericin supplementation in maturation medium on cumulus cell expansion, oocyte nuclear maturation, and subsequent embryo development in Sanjabi ewes during the breeding season. Theriogenology 2015, 84, 1631–1635. [Google Scholar] [CrossRef] [PubMed]
- Yamatoya, K.; Nagai, Y.; Teramoto, N.; Kang, W.; Miyado, K.; Nakata, K.; Yagi, T.; Miyamoto, Y. Cryopreservation of undifferentiated and differentiated human neuronal cells. Regen. Ther. 2022, 19, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Yangngam, Y.; Chapanya, S.; Vongpralub, T.; Boonkum, W.; Chankitisakul, V. Effect of semen extender supplementation with sericin on post-thaw dairy bull sperm quality lipid peroxidation Czech. J. Anim. Sci. 2021, 66, 13–20. [Google Scholar] [CrossRef]
- Yadava, C.L.; Saxena, A.; Yadav, B.; Singh, V.; Reddy, A.V.S.; Patel, A.; Kumar, A.; Yadav, S. Effect of sericin supplementation on the semen quality of cryopreserved hariana bull semen. Rumin. Sci. 2018, 7, 93–96. [Google Scholar]
- Ratchamak, R.; Ratsiri, T.; Kheawkanha, T.; Vongpralub, T.; Boonkum, W.; Chankitisakul, V. Evaluation of cryopreserved boar semen after supplementation sericin form silkworm (Bombyx mori) in semen extender. Anim. Sci. J. 2020, 91, e13428. [Google Scholar] [CrossRef]
- Chaturvedi, D.; Dhami, A.; Chaudhari, D.; Patel, A. Effect of Mifepristone, Sericin and Taurine in Tris Extender on Oxidative Markers and Quality of Fresh and Frozen-Thawed Bovine Spermatozoa. Int. J. Livest. Res. 2020, 10, 61–67. [Google Scholar] [CrossRef]
- Meyers, S.A. Cryostorage Oxidative Stress in Mammalian Spermatozoa. In Studies on Men’s Health and Fertility; Agarwal, A., Aitken, R., Alvarez, J., Eds.; Oxidative Stress in Applied Basic Research and Clinical Practice; Humana Press: Totowa, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Reddy, V.S.; Yadav, B.; Yadav, C.L.; Anand, M.; Swain, D.K.; Kumar, D.; Kritania, D.; Madan, A.K.; Kumar, J.; Yadav, S. Effect of sericin supplementation on heat shock protein 70 (HSP70) expression, redox status and post thaw semen quality in goat. Cryobiology 2018, 84, 33–39. [Google Scholar] [CrossRef]
- Nuntapaitoon, M.; Tummaruk, P.; Suwimonteerabutr, J. Supplementation of glutamine in a short-term boar semen extender during 17 °C holding time enhances post-thaw sperm quality for cryopreservation. Porc. Health Manag. 2024, 10, 50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yurekten, O.; Payne, T.; Tejera, N.; Amaladoss, F.X.; Martin, C.; Williams, M.; O’Donovan, C. MetaboLights: Open data repository for metabolomics. Nucleic Acids Res. 2024, 52, D640–D646. [Google Scholar] [CrossRef]
- Ma, J.; Chen, T.; Wu, S.; Yang, C.; Bai, M.; Shu, K.; Li, K.; Zhang, G.; Jin, Z.; He, F.; et al. iProX: An integrated proteome resource. Nucleic Acids Res. 2019, 47, D1211–D1217. [Google Scholar] [CrossRef]
- Chen, T.; Ma, J.; Liu, Y.; Chen, Z.; Xiao, N.; Lu, Y.; Fu, Y.; Yang, C.; Li, M.; Wu, S.; et al. iProX in 2021: Connecting proteomics data sharing with big data. Nucleic Acids Res. 2021, 50, D1522–D1527. [Google Scholar] [CrossRef]
- Zini, A.; San Gabriel, M.; Baazeem, A. Antioxidants and sperm DNA damage: A clinical perspective. J. Assist. Reprod. Genet. 2009, 26, 427–432. [Google Scholar] [CrossRef]
- Blount, J.; Møller, A.; Houston, D. Antioxidants, showy males and sperm quality. Ecol. Lett. 2001, 4, 393–396. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, D.; Sikka, P.; Singh, P. Sericin supplementation improves semen freezability of buffalo bulls by minimizing oxidative stress during cryopreservation. Anim. Reprod. Sci. 2015, 152, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Violante, S.; Kyaw, A.; Kouatli, L.; Paladugu, K.; Apostolakis, L.; Jenks, M.; Johnson, A.; Sheldon, R.D.; Schilmiller, A.L.; Visconti, P.E.; et al. Sperm meet the elevated energy demands to attain fertilization competence by increasing flux through aldolase. bioRxiv, 2025; preprint. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rotimi, D.E.; Iyobhebhe, M.; Oluwayemi, E.T.; Olajide, O.P.; Akinsanola, B.A.; Evbuomwan, I.O.; Asaleye, R.M.; Ojo, O.A. Energy metabolism and spermatogenesis. Heliyon 2024, 10, e38591. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.A.; Akhigbe, T.M.; Akhigbe, R.E.; Aremu, A.O.; Oyedokun, P.A.; Gbadamosi, J.A.; Anifowose, P.E.; Adewole, M.A.; Aboyeji, O.O.; Yisau, H.O.; et al. Glutamine restores testicular glutathione-dependent antioxidant defense and upregulates NO/cGMP signaling in sleep deprivation-induced reproductive dysfunction in rats. Biomed. Pharmacother. 2022, 148, 112765. [Google Scholar] [CrossRef]
- De la Puerta, C.; Arrieta, F.J.; Balsa, J.A.; Botella-Carretero, J.I.; Zamarrón, I.; Vázquez, C. Taurine and glucose metabolism: A review. Nutr. Hosp. 2010, 25, 910–919. [Google Scholar] [PubMed]
- Zhang, L.; Wang, Y.; Sohail, T.; Kang, Y.; Niu, H.; Sun, X.; Ji, D.; Li, Y. Effects of Taurine on Sperm Quality during Room Temperature Storage in Hu Sheep. Animals 2021, 11, 2725. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alirezaei, M.; Jelodar, G.; Ghayemi, Z. Antioxidant Defense of Betaine Against Oxidative Stress Induced by Ethanol in the Rat Testes. Int. J. Pept. Res. Ther. 2012, 18, 239–247. [Google Scholar] [CrossRef]
- Mori, N.; Ishihara, M.; Tasaki, H.; Sankai, T.; Otsuki, J. The effect of betaine for mouse sperm cryopreservation. Cryobiology 2022, 106, 157–159. [Google Scholar] [CrossRef]
- Attia, Y.A.; El-Naggar, A.S.; Abou-Shehema, B.M.; Abdella, A.A. Effect of Supplementation with Trimethylglycine (Betaine) and/or Vitamins on Semen Quality, Fertility, Antioxidant Status, DNA Repair and Welfare of Roosters Exposed to Chronic Heat Stress. Animals 2019, 9, 547. [Google Scholar] [CrossRef]
- Batra, V.; Dagar, K.; Diwakar, M.P.; Kumaresan, A.; Kumar, R.; Datta, T.K. The proteomic landscape of sperm surface deciphers its maturational and functional aspects in buffalo. Front. Physiol. 2024, 15, 1413817. [Google Scholar] [CrossRef]
- Iniesta-Cuerda, M.; Havránková, J.; Řimnáčová, H.; García-Álvarez, O.; Nevoral, J. Male SIRT1 insufficiency leads to sperm with decreased ability to hyperactivate and fertilize. Reprod. Domest. Anim. 2022, 57 (Suppl. 5), 72–77. [Google Scholar] [CrossRef]
- Llavanera, M.; Delgado-Bermúdez, A.; Mateo-Otero, Y.; Padilla, L.; Romeu, X.; Roca, J.; Barranco, I.; Yeste, M. Exploring Seminal Plasma GSTM3 as a Quality and In Vivo Fertility Biomarker in Pigs-Relationship with Sperm Morphology. Antioxidants 2020, 9, 741. [Google Scholar] [CrossRef]
- Suzuki-Toyota, F.; Ito, C.; Toyama, Y.; Maekawa, M.; Yao, R.; Noda, T.; Iida, H.; Toshimori, K. Factors maintaining normal sperm tail structure during epididymal maturation studied in Gopc−/− mice. Biol. Reprod. 2007, 77, 71–82. [Google Scholar] [CrossRef][Green Version]
- Lima, A.C.; Okhovat, M.; Stendahl, A.M.; Yang, R.; VanCampen, J.; Nevonen, K.A.; Herrera, J.; Li, W.; Harshman, L.; Fedorov, L.M.; et al. Deletion of an evolutionarily conserved TAD boundary impacts spermatogenesis in mice. Biol. Reprod. 2025, 112, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Llavanera, M.; Delgado-Bermúdez, A.; Fernandez-Fuertes, B.; Recuero, S.; Mateo, Y.; Bonet, S.; Barranco, I.; Yeste, M. GSTM3, but not IZUMO1, is a cryotolerance marker of boar sperm. J. Anim. Sci. Biotechnol. 2019, 10, 61. [Google Scholar] [CrossRef]
- Hemachand, T.; Gopalakrishnan, B.; Salunke, D.M.; Totey, S.M.; Shaha, C. Sperm plasma-membrane-associated glutathione S-transferases as gamete recognition molecules. J. Cell Sci. 2002, 115 Pt 10, 2053–2065. [Google Scholar] [CrossRef]
- Llavanera, M.; Delgado-Bermúdez, A.; Olives, S.; Mateo-Otero, Y.; Recuero, S.; Bonet, S.; Fernández-Fuertes, B.; Yeste, M.; Barranco, I. Glutathione S-Transferases Play a Crucial Role in Mitochondrial Function, Plasma Membrane Stability and Oxidative Regulation of Mammalian Sperm. Antioxidants 2020, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, V.S.; Shahid, M.; Deo, P.; Fenech, M. Reduced SIRT1 and SIRT3 and Lower Antioxidant Capacity of Seminal Plasma Is Associated with Shorter Sperm Telomere Length in Oligospermic Men. Int. J. Mol. Sci. 2024, 25, 718. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Di Emidio, G.; Barbonetti, A.; Carta, G.; Luciano, A.M.; Falone, S.; Amicarelli, F. Sirtuins in gamete biology and reproductive physiology: Emerging roles and therapeutic potential in female and male infertility. Hum. Reprod. Update 2018, 24, 267–289. [Google Scholar] [CrossRef] [PubMed]

| Group | pH | Osmolality (mOsmol/kg) |
|---|---|---|
| Control | 6.4 | 1645.67 ± 6.11 |
| SER 0.2% | 6.4 | 1652.67 ± 8.02 |
| SER 0.4% | 6.4 | 1645.33 ± 6.66 |
| SER 0.6% | 6.4 | 1652.33 ± 9.72 |
| SER 0.8% | 6.4 | 1658.33 ± 9.19 |
| SER 1% | 6.4 | 1659.67 ± 8.02 |
| Primers Name | Primer Sequences (5′-3′) |
|---|---|
| LRP8 | F: CAAACGCCGATGTACCCT R: TGAGCCCGGACTTCTCAA |
| GSTM3 | F: CCCAGAGCAATGCCATCTTG R: TGTTCCAAGTACCGAGGCTT |
| SIRT2 | F: AAGGAGAAGACTGGCCAGAC R: GGAAGCTGAAGTAGTGGGGT |
| β-actin | F: CTCTTCCAGCCTTCCTTCCT R: GGGCAGTGATCTCTTTCTGC |
| GAPDH | F: ATGGCAAGTTCCACGGCACAGTC |
| R: CAGCCTTCTCCATGGTAGTGAAG |
| Movement Parameters | Control | SER 0.2% | SER 0.4% | SER 0.6% | SER 0.8% | SER 1% |
|---|---|---|---|---|---|---|
| TM/(%) | 50.10 ± 1.69 d | 57.10 ± 1.79 c | 63.89 ± 2.43 ab | 65.25 ± 1.76 a | 60.24 ± 2.84 bc | 58.14 ± 1.25 bc |
| VSL/(μm/s) | 18.20 ± 0.50 d | 19.88 ± 0.51 c | 21.61 ± 0.71 bc | 22.90 ± 0.76 a | 20.81 ± 0.88 bc | 20.02 ± 0.23 c |
| VCL/(μm/s) | 49.54 ± 2.04 c | 53.55 ± 3.87 bc | 56.60 ± 2.66 ab | 59.73 ± 2.91 a | 54.27 ± 3.50 abc | 51.49 ± 2.43 bc |
| VAP/(μm/s) | 23.60 ± 1.64 c | 25.01 ± 1.76 c | 28.53 ± 3.13 bc | 33.17 ± 2.01 a | 29.33 ± 1.88 abc | 25.46 ± 1.43 c |
| ALH/(μm) | 11.98 ± 1.09 b | 13.22 ± 0.94 b | 13.75 ± 1.33 b | 16.30 ± 1.73 a | 14.12 ± 1.21 ab | 12.94 ± 0.75 b |
| Protein Name | Control Group | Sericin Group | FC |
|---|---|---|---|
| LRP8 | 0.691 | 1133 | 1.641 |
| GSTM3 | 0.869 | 1.094 | 1.259 |
| SIRT2 | 0.903 | 1.112 | 1.231 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Xia, W.; Zhang, W.; Tao, C.; Tian, X.; Li, M.; Zhang, X.; Zhang, J.; Zhao, S.; Qi, Y.; et al. Sericin Enhances Cryopreserved Sperm Quality in Chengde Hornless Black Goats by Increasing Glutamine Metabolism. Animals 2025, 15, 2830. https://doi.org/10.3390/ani15192830
Yu Y, Xia W, Zhang W, Tao C, Tian X, Li M, Zhang X, Zhang J, Zhao S, Qi Y, et al. Sericin Enhances Cryopreserved Sperm Quality in Chengde Hornless Black Goats by Increasing Glutamine Metabolism. Animals. 2025; 15(19):2830. https://doi.org/10.3390/ani15192830
Chicago/Turabian StyleYu, Yang, Wei Xia, Wentao Zhang, Chenyu Tao, Xiaofeng Tian, Mengqi Li, Xiaosheng Zhang, Jinlong Zhang, Shunran Zhao, Yatian Qi, and et al. 2025. "Sericin Enhances Cryopreserved Sperm Quality in Chengde Hornless Black Goats by Increasing Glutamine Metabolism" Animals 15, no. 19: 2830. https://doi.org/10.3390/ani15192830
APA StyleYu, Y., Xia, W., Zhang, W., Tao, C., Tian, X., Li, M., Zhang, X., Zhang, J., Zhao, S., Qi, Y., Qin, T., & Li, J. (2025). Sericin Enhances Cryopreserved Sperm Quality in Chengde Hornless Black Goats by Increasing Glutamine Metabolism. Animals, 15(19), 2830. https://doi.org/10.3390/ani15192830





