Diurnal Habitat Selection and Use of Wintering Bar-Headed Geese (Anser indicus) Across Heterogeneous Landscapes on the Yunnan–Guizhou Plateau, Southwest China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
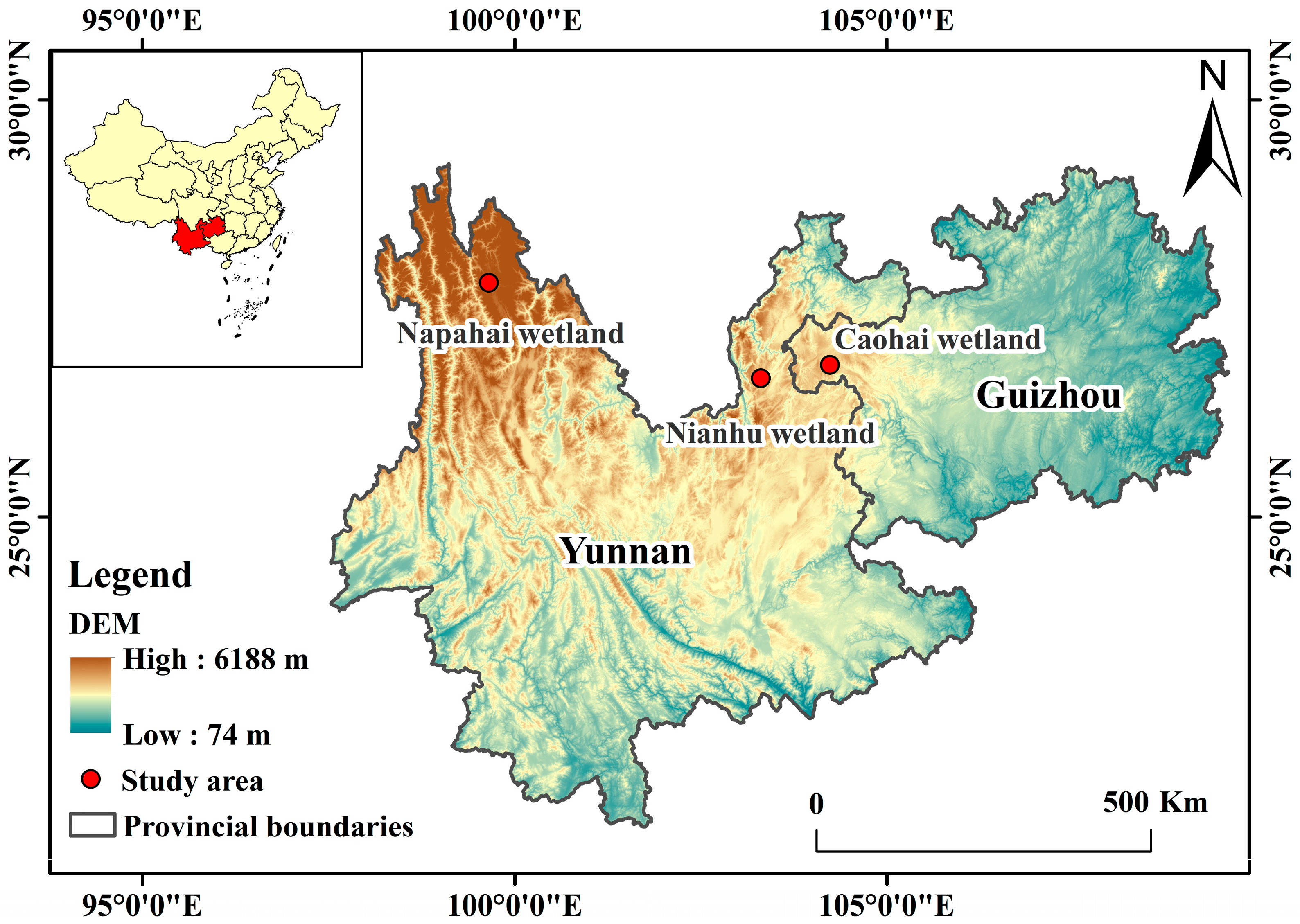
2.1. Study Area
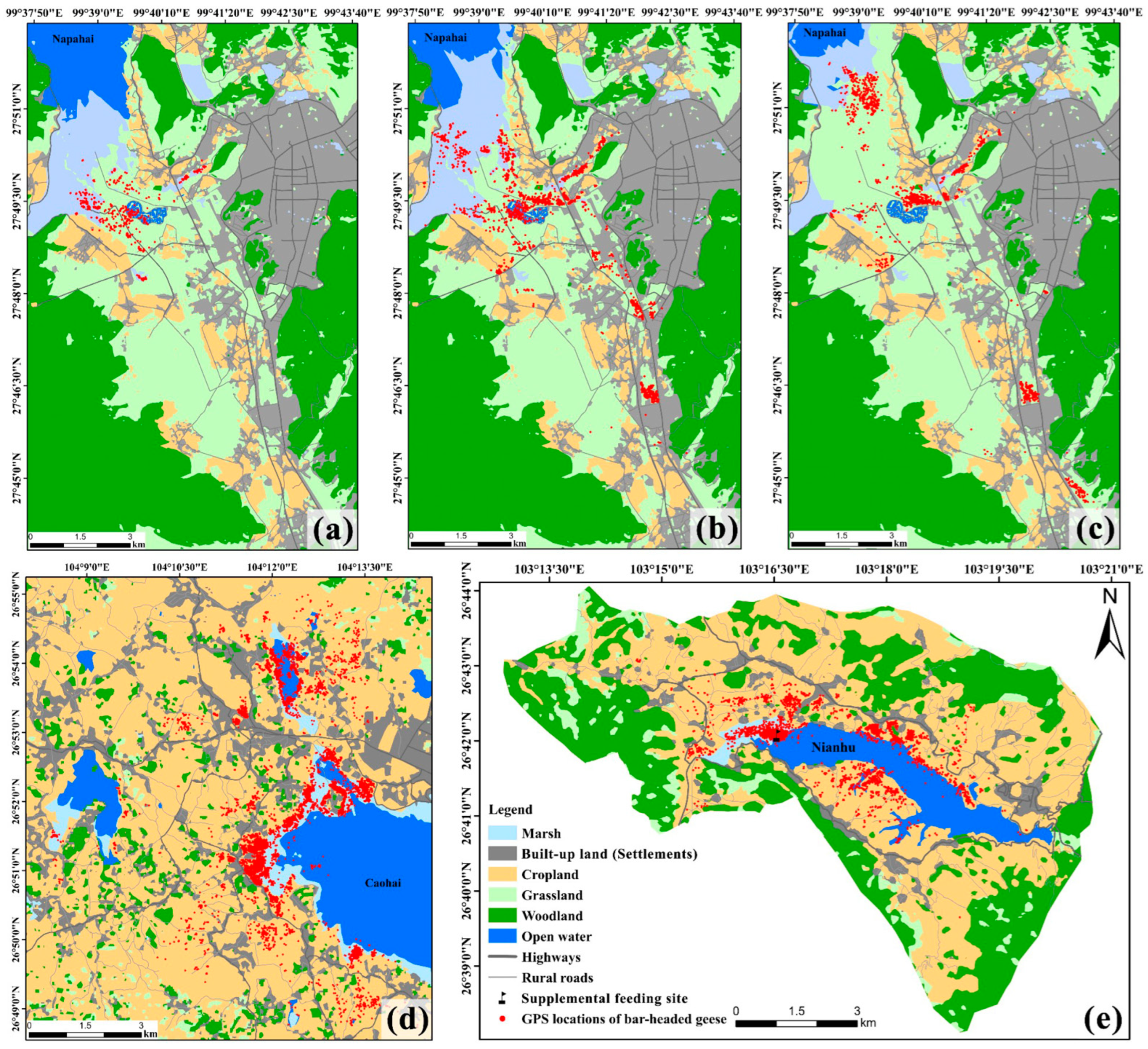
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Habitat Characteristics
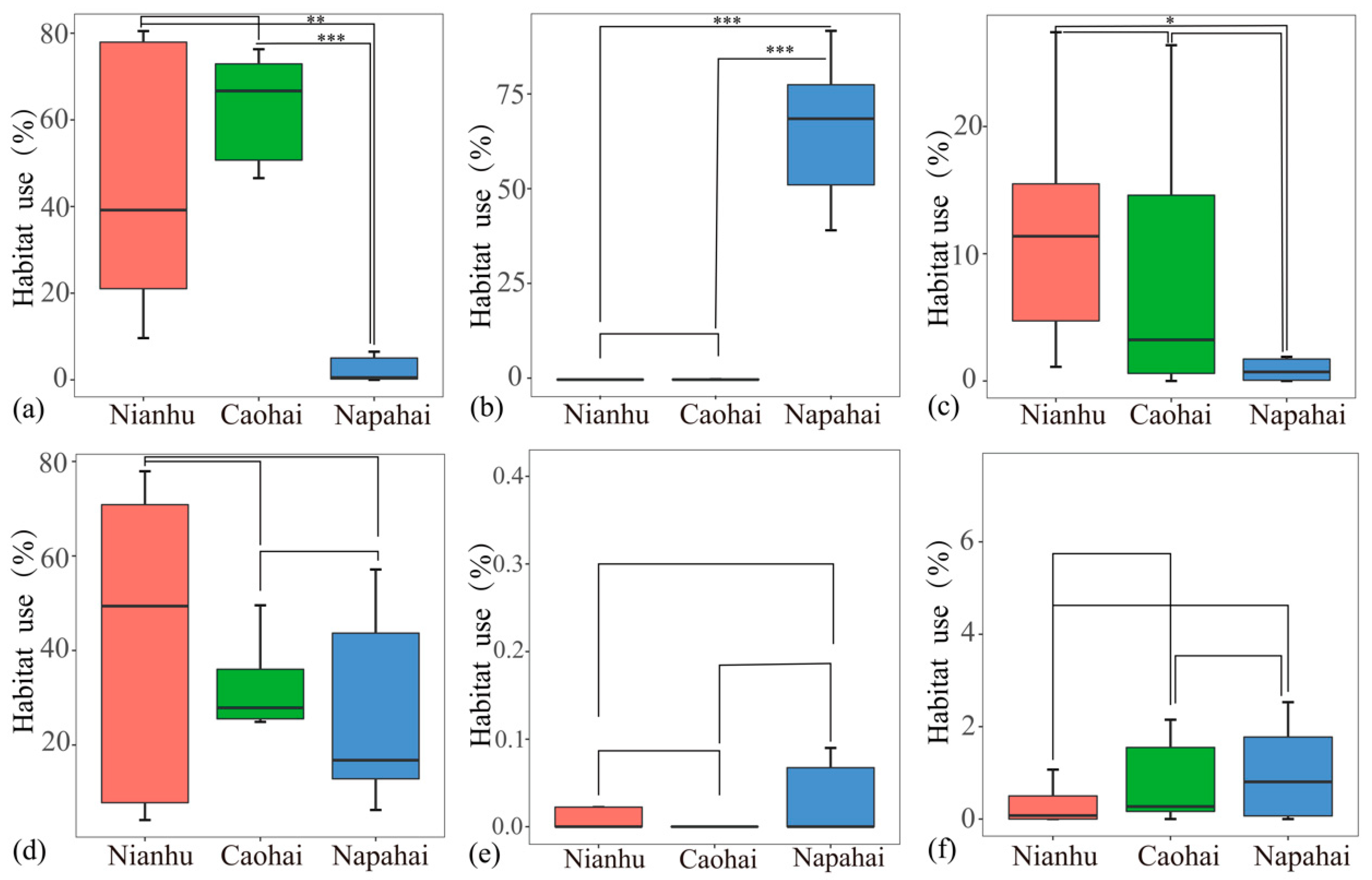
3.2. Differences in Diurnal Habitat Use Across Wetlands
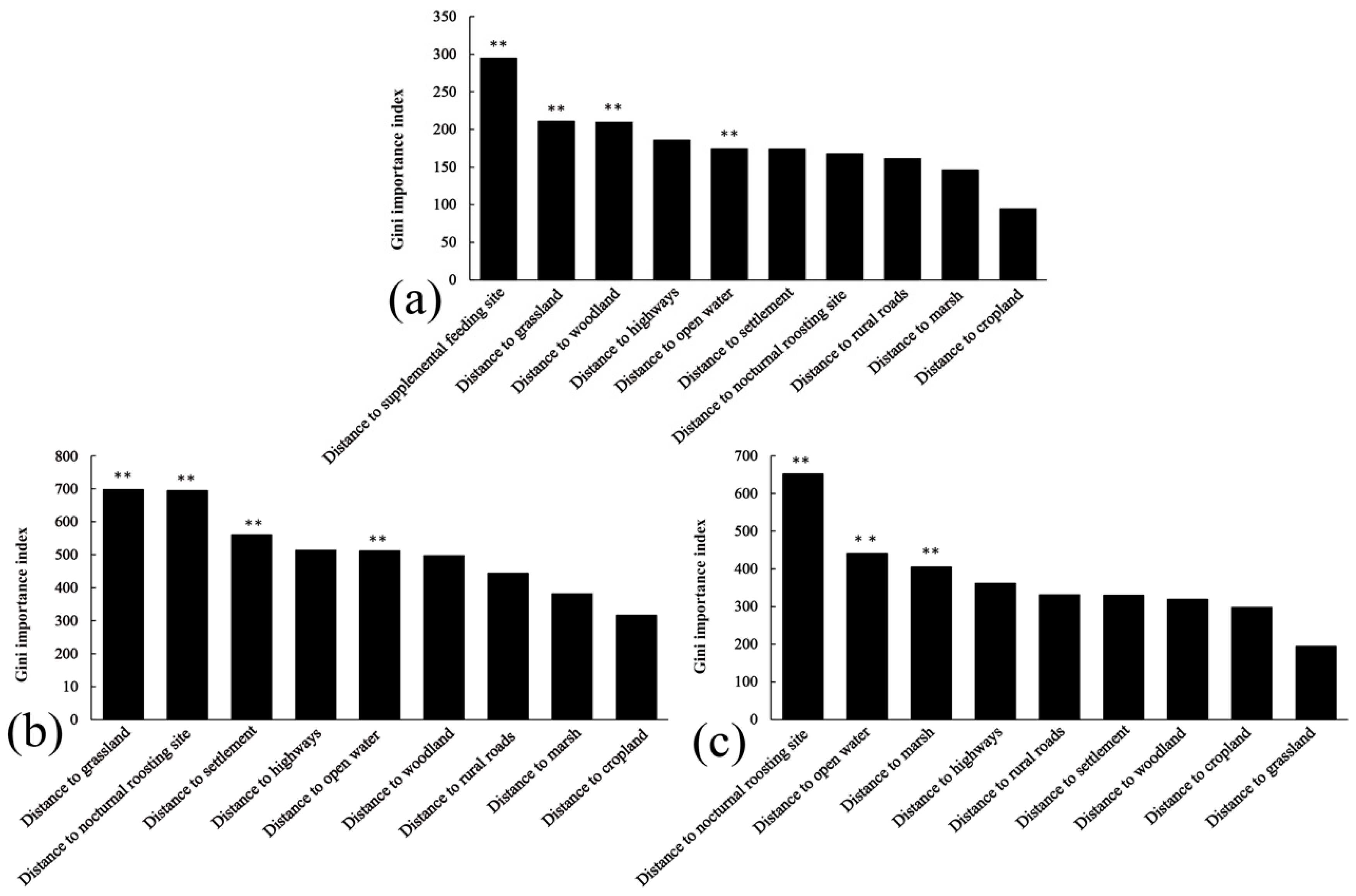
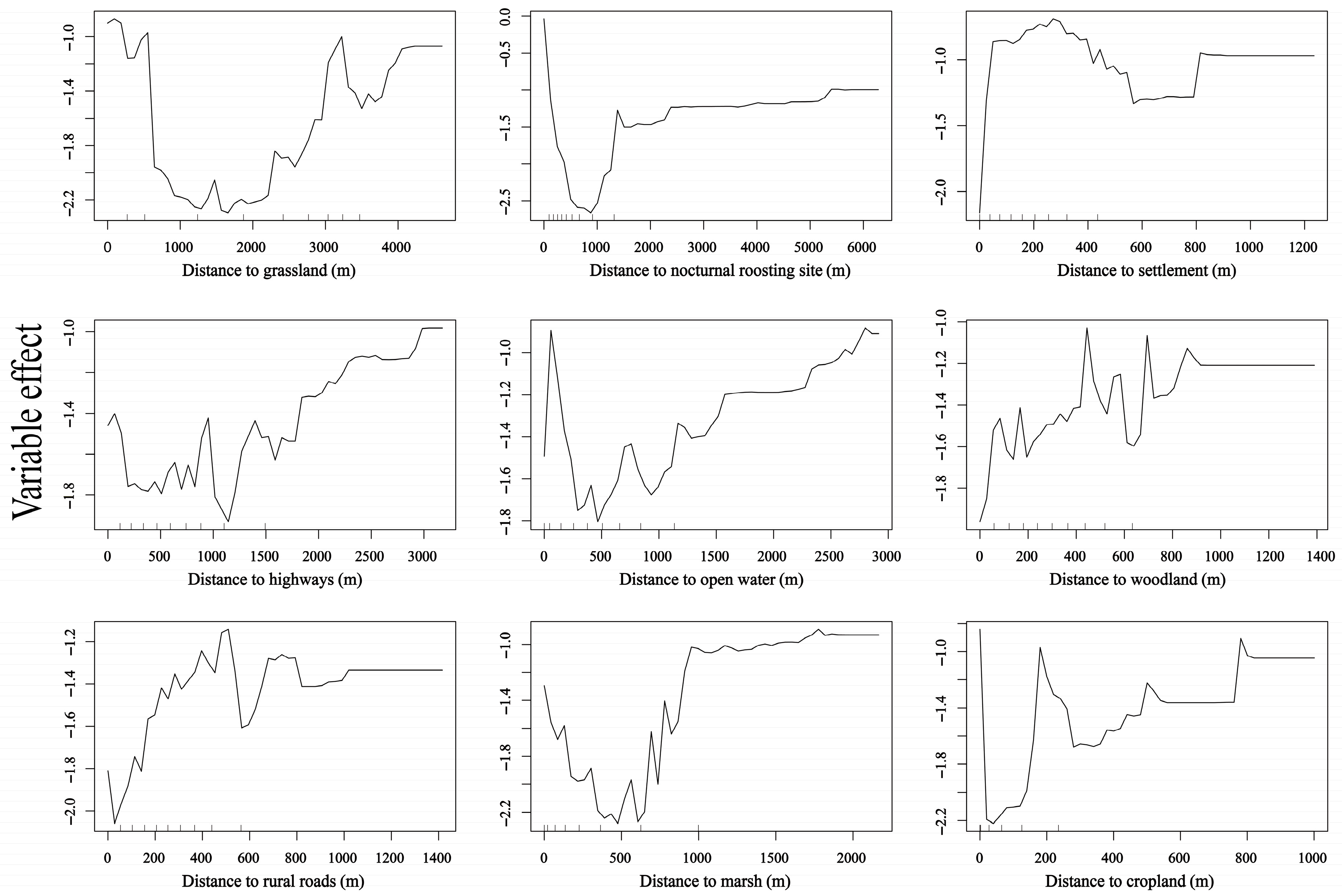
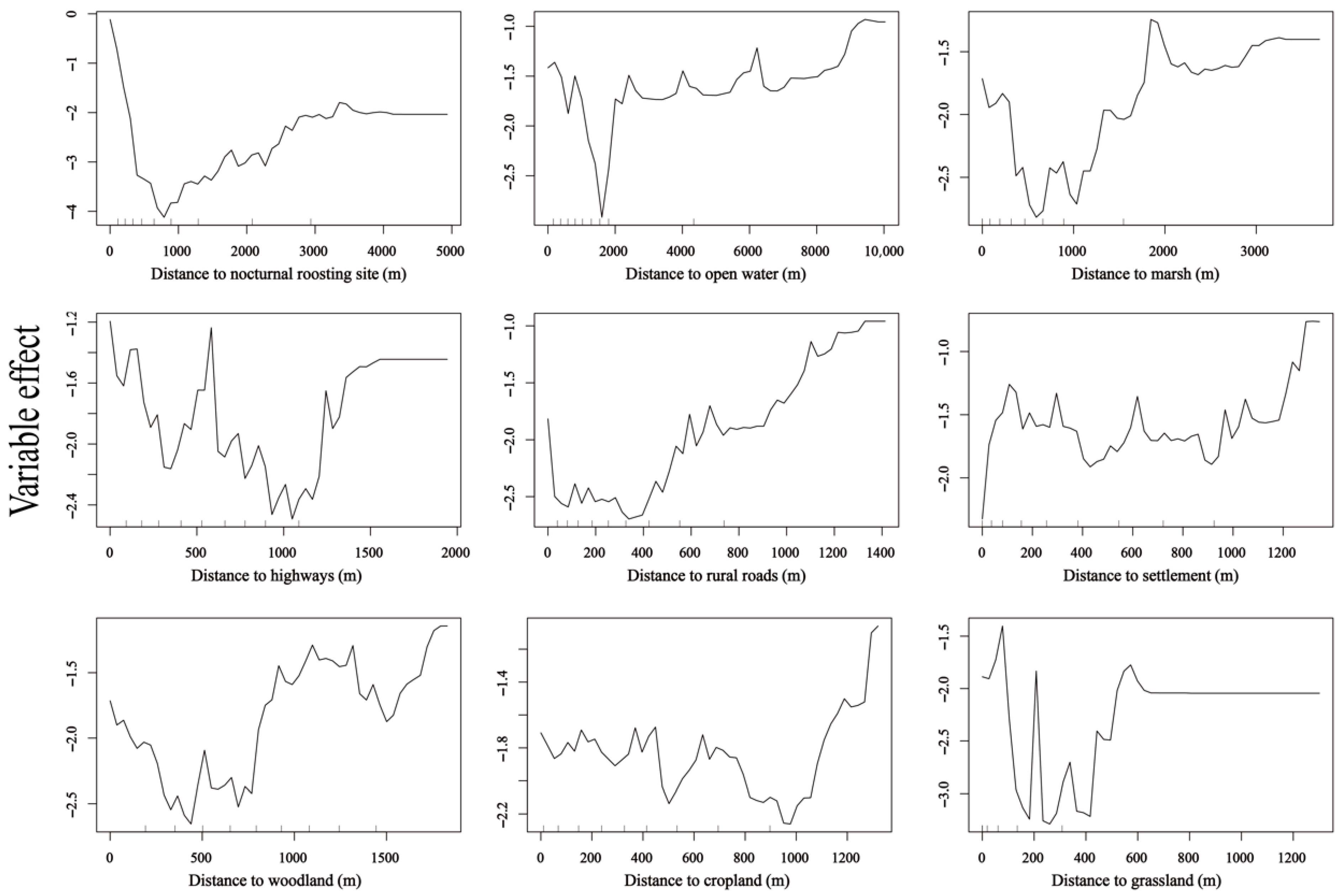
3.3. Differences in Diurnal Habitat Selection Across Wetlands
4. Discussion
4.1. Habitat Use
4.2. Habitat Selection
4.3. Behavioral Plasticity
4.4. Regionalized Conservation Management
4.5. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UD | utilization distribution |
| dBBMM | dynamic Brownian bridge movement model |
References
- Wilson, M.C.; Chen, X.Y.; Corlett, R.T.; Didham, R.K.; Ding, P.; Holt, R.D.; Holyoak, M.; Hu, G.; Hughes, A.C.; Jiang, L.; et al. Habitat Fragmentation and Biodiversity Conservation: Key Findings and Future Challenges. Landsc. Ecol. 2016, 31, 219–227. [Google Scholar] [CrossRef]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; Chan, K.M.A.; et al. Pervasive Human-Driven Decline of Life on Earth Points to the Need for Transformative Change. Science 2019, 366, eaax3100. [Google Scholar] [CrossRef]
- Yuan, R.; Zhang, N.; Zhang, Q. The Impact of Habitat Loss and Fragmentation on Biodiversity in Global Protected Areas. Sci. Total Environ. 2024, 931, 173004. [Google Scholar] [CrossRef]
- Kirby, J.S.; Stattersfield, A.J.; Butchart, S.H.; Evans, M.I.; Grimmett, R.F.; Jones, V.R.; O’Sullivan, J.; Tucker, G.M.; Newton, I. Key Conservation Issues for Migratory Land- and Waterbird Species on the World’s Major Flyways. Bird Conserv. Int. 2008, 18, S49–S73. [Google Scholar] [CrossRef]
- Pereira, H.M.; Navarro, L.M.; Martins, I.S. Global Biodiversity Change: The Bad, the Good, and the Unknown. Annu. Rev. Environ. Resour. 2012, 37, 25–50. [Google Scholar] [CrossRef]
- Rosenberg, K.V.; Dokter, A.M.; Blancher, P.J.; Sauer, J.R.; Smith, A.C.; Smith, P.A.; Stanton, J.C.; Panjabi, A.; Helft, L.; Parr, M.; et al. Decline of the North American Avifauna. Science 2019, 366, 120–124. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Moore, J.N.; Casazza, M.L.; Coons, S.P. Functional Wetland Loss Drives Emerging Risks to Waterbird Migration Networks. Front. Ecol. Evol. 2022, 10, 844278. [Google Scholar] [CrossRef]
- Zhao, Q.; Arnold, T.W.; Devries, J.H.; Howerter, D.W.; Clark, R.G.; Weegman, M.D. Land-Use Change Increases Climatic Vulnerability of Migratory Birds: Insights from Integrated Population Modelling. J. Anim. Ecol. 2019, 88, 1625–1637. [Google Scholar] [CrossRef]
- Li, Y.; Mao, D.; Wang, Z.; Wang, X.; Tan, X.; Jia, M.; Ren, C. Identifying Variable Changes in Wetlands and Their Anthropogenic Threats Bordering the Yellow Sea for Waterbird Conservation. Glob. Ecol. Conserv. 2021, 27, e01613. [Google Scholar]
- Duan, H.; Yu, X.; Shan, K.; Zhang, C.; Liu, H. Effects of Habitat Loss on Migratory Shorebird Community Structure at Stopover Sites: A Case Study in the Yellow River Delta, China. Front. Mar. Sci. 2022, 9, 1049765. [Google Scholar] [CrossRef]
- Liu, J.; Lei, W.; Mo, X.; Hassell, C.J.; Zhang, Z.; Coulson, T. Unravelling the Processes between Phenotypic Plasticity and Population Dynamics in Migratory Birds. J. Anim. Ecol. 2022, 91, 983–995. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Zhou, L. The Influence of the Abundance and Availability of Alternative Food on the Foraging Behavior of Wintering Siberian Cranes (Leucogeranus leucogeranus). Avian Res. 2025, 16, 100254. [Google Scholar] [CrossRef]
- Wang, C.; Xia, S.; Yu, X.; Wen, L. Responses to Extreme Drought in Wintering Waterbirds: A Multi-Species Approach. Front. Zool. 2025, 22, 3. [Google Scholar] [CrossRef]
- McDuie, F.; Lorenz, A.A.; Klinger, R.C.; Overton, C.T.; Feldheim, C.L.; Ackerman, J.T.; Casazza, M.L. Informing Wetland Management with Waterfowl Movement and Sanctuary Use Responses to Human-Induced Disturbance. J. Environ. Manag. 2021, 297, 113170. [Google Scholar] [CrossRef]
- Elmore, J.A.; Londe, D.W.; Davis, C.A.; Fuhlendorf, S.D.; Loss, S.R. Associations with Landscape- and Local-Scale Wetland Habitat Conditions Vary Among Migratory Shorebird Species during Stopovers. Wildl. Biol. 2024, 2024, e01132. [Google Scholar] [CrossRef]
- Xing, X.; Qian, F.; Ma, K. From Sky to Ground: Decoding Migratory Bird’s Habitat Selection along the Multi-Scale Sequence. Landsc. Ecol. 2025, 40, 33. [Google Scholar] [CrossRef]
- Hawkes, L.A.; Balachandran, S.; Batbayar, N.; Butler, P.J.; Frappell, P.B.; Milsom, W.K.; Tseveenmyadag, N.; Newman, S.H.; Scott, G.R.; Sathiyaselvam, P.; et al. The Trans-Himalayan Flights of Bar-Headed Geese (Anser indicus). Proc. Natl. Acad. Sci. USA 2011, 108, 9516–9519. [Google Scholar] [CrossRef]
- Scott, G.R.; Hawkes, L.A.; Frappell, P.B.; Butler, P.J.; Bishop, C.M.; Milsom, W.K. How Bar-Headed Geese Fly Over the Himalayas. Physiology 2015, 30, 107–115. [Google Scholar] [CrossRef]
- Meir, J.U.; York, J.M.; Chua, B.A.; Jardine, W.; Hawkes, L.A.; Milsom, W.K. Reduced Metabolism Supports Hypoxic Flight in the High-Flying Bar-Headed Goose (Anser indicus). eLife 2019, 8, e44986. [Google Scholar] [CrossRef]
- Lu, P. Distribution of Bar-Headed Goose in China. J. Ecol. Soc. 1997, 10, 8–9. [Google Scholar]
- Uttangi, D.J. Wintering Status & Site Loyalty of Bar-Headed Goose (Anser indicus) in Areas of Dharwad District, Karnataka, India. J. Ecol. Soc. 1997, 10, 22–24. [Google Scholar]
- Liu, D.; Zhang, G.; Li, F.; Ma, T.; Lu, J.; Qian, F. A Revised Species Population Estimate for the Bar-Headed Goose (Anser indicus). Avian Res. 2017, 8, 7. [Google Scholar] [CrossRef]
- Bishop, M.A.; Liu, D.; Zhang, G.; Tsamchu, D.; Yang, L.; Qian, F.; Li, F. Rapid Growth of the Bar-Headed Goose (Anser indicus) Wintering Population in Tibet, China: 1991–2017. Bird Conserv. Int. 2022, 32, 398–413. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Z.; Chen, P.; Xu, C.; Gao, B.; Ruan, L. Shallow Sub-Lakes Are Essential for Sustaining the Successful Wintering of Waterbirds in Poyang Lake, China. Avian Res. 2024, 15, 100178. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Y.; Li, L.; Batbayar, N.; Deng, X.; Damba, I.; Meng, F.; Cao, L.; Fox, A.D. Assessing Site-Safeguard Effectiveness and Habitat Preferences of Bar-Headed Geese (Anser indicus) at Their Stopover Sites within the Qinghai–Tibet Plateau Using GPS/GSM Telemetry. Avian Res. 2020, 11, 49. [Google Scholar] [CrossRef]
- Wu, Z.; Ye, X.; Kuang, Z.; Ye, H.; Zhao, X. Positive Effects of Land Use Change on Wintering Bar-Headed Geese between 2010 and 2021. Animals 2022, 12, 3142. [Google Scholar] [CrossRef]
- Li, T.; Bao, R.; Li, L.; Tang, M.; Deng, H. Temporal and Spatial Changes of Habitat Quality and Their Potential Driving Factors in Southwest China. Land 2023, 12, 346. [Google Scholar] [CrossRef]
- Liu, H.; Lei, Y.; Meng, Z.W.; Zhang, J.J.; Chen, N.Y.; Liu, Q. Effects of Maca Lepidium meyenii Planting on Habitat Utilization and Selection of Wintering Bar-Headed Geese Anser indicus in Huize, Yunnan. Acta Ecol. Sin. 2024, 44, 9221–9232. (In Chinese) [Google Scholar]
- Qiu, G.; Yang, X. Yunnan Huize Black-Necked Crane National Nature Reserve; Yunnan Science and Technology Press: Kunming, China, 2012. (In Chinese) [Google Scholar]
- Kong, D.; Luo, W.; Liu, Q.; Li, Z.; Huan, G.; Zhang, J.; Yang, X. Habitat Use, Preference, and Utilization Distribution of Two Crane Species (Grus spp.) in Huize National Nature Reserve, Yunnan-Guizhou Plateau, China. PeerJ 2018, 6, e5105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ying, H. Discussion on the Current Status of the Huize Black-Necked Crane Nature Reserve, Yunnan. J. Green Sci. Technol. 2018, 24, 151–152. (In Chinese) [Google Scholar]
- Chen, Y.; Chen, Q.; Feng, T. Comprehensive Scientific Investigation Report of Guizhou Caohai National Nature Reserve; China Forestry Publishing House: Beijing, China, 2021. (In Chinese) [Google Scholar]
- Zhang, H.; Su, H.; Liu, W.; Zhang, M.; Li, Z. Relationship of Community Structure of Main Waterfowl with Habitat in Caohai National Nature Reserve in Winter. J. Ecol. Rural Environ. 2014, 30, 601–607. (In Chinese) [Google Scholar]
- Tu, S.; Ran, J.; Yang, J.; Ma, S.; Liu, Y.; Zhang, Y.; Ni, N. Study on the Dynamics of Overwintering Birds in Caohai Wetland. J. Anhui Agric. Sci. 2023, 51, 76–79. (In Chinese) [Google Scholar]
- Tian, K.; Guo, H.; Yang, Y. Research and Practice on Ecological Structure Characteristics and Functional Zoning in Plateau Wetland Reserves; Science Press: Beijing, China, 2009. (In Chinese) [Google Scholar]
- Liu, Q.; Yang, J.; Yang, X.; Zhao, J.; Yu, H. Foraging Habitats and Utilization Distributions of Black-Necked Cranes Wintering at the Napahai Wetland, China. J. Field Ornithol. 2010, 81, 21–30. [Google Scholar] [CrossRef]
- Fu, W. Composition and Spatial Distribution Patterns of Anatidae in Yunnan Wetlands during Winter. Master’s Thesis, Yunnan University, Kunming, China, 2018. (In Chinese). [Google Scholar]
- Jones, J. Habitat Selection Studies in Avian Ecology: A Critical Review. Auk 2001, 118, 557–562. [Google Scholar] [CrossRef]
- Yan, L.; Hu, M.; Meng, F.; Liu, N. Foraging Habitat Selection of Anser indicus During Winter at Lashihai Lake in Yunnan Province. Mod. Agric. Sci. Technol. 2014, 10, 262, 263–273. (In Chinese) [Google Scholar]
- Deng, S. ENVI Remote Sensing Image Processing Methods; Science Press: Beijing, China, 2010. (In Chinese) [Google Scholar]
- Flint, P.L.; Meixell, B.W. Movements and Habitat Use of White-Fronted Geese (Anser albifrons frontalis) During the Remigial Molt in Arctic Alaska, USA. Waterbirds 2017, 40, 272–281. [Google Scholar] [CrossRef]
- Byrne, M.E.; McCoy, J.C.; Hinton, J.W.; Chamberlain, M.J.; Collier, B.A. Using Dynamic Brownian Bridge Movement Modelling to Measure Temporal Patterns of Habitat Selection. J. Anim. Ecol. 2014, 83, 1234–1243. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y. Applying Various Algorithms for Species Distribution Modelling. Integr. Zool. 2013, 8, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Pichler, M.; Hartig, F. Machine Learning and Deep Learning—A Review for Ecologists. Methods Ecol. Evol. 2023, 14, 994–1016. [Google Scholar] [CrossRef]
- Gao, R.; Su, X.; Xie, Y.; Xie, Y.; Xu, Y. Suitability Prediction of Adaptability of Cunninghamia lanceolata Based on Random Forest. J. Beijing For. Univ. 2017, 39, 36–43. (In Chinese) [Google Scholar]
- Jiao, S.; Chen, W.; Wang, J.; Du, N.; Li, Q.; Wei, G. Soil Microbiomes with Distinct Assemblies Through Vertical Soil Profiles Drive the Cycling of Multiple Nutrients in Reforested Ecosystems. Microbiome 2018, 6, 146. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, G.; Qian, F.; Hou, Y.; Dai, M.; Jiang, H.; Lu, J.; Xiao, W. Population, Distribution and Home Range of Wintering Bar-Headed Geese along Yaluzangbu River, Tibet. Acta Ecol. Sin. 2010, 30, 4173–4179. (In Chinese) [Google Scholar]
- Fox, A.D.; Abraham, K.F. Why Geese Benefit from the Transition from Natural Vegetation to Agriculture. Ambio 2017, 46, 188–197. [Google Scholar] [PubMed]
- Liu, Q.; Jiang, W. Winter Landscape Dynamics of Napahai Wetland in Yunnan. J. Southwest For. Univ. 2018, 38, 139–145. (In Chinese) [Google Scholar]
- Zou, Y.A.; Zhang, P.Y.; Zhang, S.Q.; Chen, X.S.; Li, F.; Deng, Z.M.; Yang, S.; Zhang, H.; Li, F.Y.; Xie, Y.H.; et al. Crucial Sites and Environmental Variables for Wintering Migratory Waterbird Population Distributions in the Natural Wetlands of East Dongting Lake, China. Sci. Total Environ. 2019, 655, 147–157. [Google Scholar] [PubMed]
- Ma, Q. Monitoring Report on Black-Necked Cranes and Other Birds in Xundian Black-Necked Crane Provincial Nature Reserve. Green Sci. Technol. 2021, 23, 156–158. (In Chinese) [Google Scholar]
- Feng, C.; Liang, W. Behavioral Responses of Black-Headed Gulls (Chroicocephalus ridibundus) to Artificial Provisioning in China. Glob. Ecol. Conserv. 2020, 21, e00873. [Google Scholar] [CrossRef]
- Hale, R.; Swearer, S.E. Ecological Traps: Current Evidence and Future Directions. Proc. R. Soc. B 2016, 283, 20152647. [Google Scholar]
- Stephens, D.W.; Krebs, J.R. Foraging Theory; Princeton University Press: Princeton, NJ, USA, 1986. [Google Scholar]
- Duan, Y.; Liu, Q.; Lei, Y.; Li, J.; Liu, W.; Li, Z.; Wang, R. Habitat Selection of Overwintering Common Cranes (Grus grus) Based on Individuals Tracking by Satellite Technology. Chin. J. Ecol. 2020, 39, 2392–2399. (In Chinese) [Google Scholar]
- Huang, Y. Spatio-Temporal Habitat Selection of Wild Crested Ibis (Nipponia nippon) and Modeling of Reintroduction Sites. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2021. (In Chinese). [Google Scholar]
- Zhong, L.; Li, Y.; Li, Y.; Dai, C. Hungry Wintering Birds and Angry Farmers: Crop Damage and Management Implications in a Protected Wetland in China. Glob. Ecol. Conserv. 2025, 57, e03402. [Google Scholar] [CrossRef]
- Weller, M.W. Wetland Birds: Habitat Resources and Conservation Implications; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Meng, Z.; Xiao, L.; Lei, Y.; Wang, L.; Liu, Q. Home Range of Wintering Bar-headed Goose in Napahai Wetland, Yunnan Province, China. Chin. J. Zool. 2022, 57, 554–563. (In Chinese) [Google Scholar]
- Snell-Rood, E.C. An Overview of the Evolutionary Causes and Consequences of Behavioural Plasticity. Anim. Behav. 2013, 85, 1004–1011. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Batbayar, N.; Zhu, B.; Dong, S.; Barma, A.; Sasin, A.; Cao, L. Discussion of Existing Protection for Three Waterbirds’ Habitats in the Yellow River Basin Nature Reserves, Based on Satellite Tracking. Biodivers. Sci. 2020, 28, 1483–1495. (In Chinese) [Google Scholar] [CrossRef]
- Pearse, A.T.; Caven, A.J.; Baasch, D.M.; Bidwell, M.T.; Conkin, J.A.; Brandt, D.A. Flexible Migration and Habitat Use Strategies of an Endangered Waterbird during Hydrological Drought. Conserv. Sci. Pract. 2024, 6, e13120. [Google Scholar] [CrossRef]
- Lei, J.; Jia, Y.; Wang, Y.; Lei, G.; Lu, C.; Saintilan, N.; Wen, L. Behavioural Plasticity and Trophic Niche Shift: How Wintering Geese Respond to Habitat Alteration. Freshw. Biol. 2019, 64, 1183–1195. [Google Scholar] [CrossRef]
- Yang, L.; Wang, R.; Liu, Q.; Shah, T.; Zhou, J.; Zhao, W.; Wang, Y.; Deng, L.; Wang, B. Genomic Characterization and Phylogenetic Analysis of Five Avian Influenza H5N1 Subtypes from Wild Anser indicus in Yunnan, China. Vet. Sci. 2025, 12, 280. [Google Scholar] [CrossRef]


| Habitat Type | Nianhu | Caohai | ||
|---|---|---|---|---|
| Area (ha) | Proportion (%) | Area (ha) | Proportion (%) | |
| Cropland | 3553.15 | 49 | 8181.07 | 60 |
| Woodland | 2304.06 | 32 | 846.64 | 6 |
| Grassland | 379.83 | 5 | 277.85 | 2 |
| Marsh | 88.29 | 1 | 533.07 | 4 |
| Open water | 573.85 | 8 | 1618.84 | 12 |
| Built-up land | 395.98 | 5 | 2117.01 | 16 |
| Habitat Type | Early Wintering Period | Mid Wintering Period | Late Wintering Period | |||
|---|---|---|---|---|---|---|
| Area (ha) | Proportion (%) | Area (ha) | Proportion (%) | Area (ha) | Proportion (%) | |
| Cropland | 1352.36 | 8 | 1348.11 | 8 | 1343.38 | 8 |
| Woodland | 5913.54 | 36 | 5910.66 | 36 | 5899.51 | 36 |
| Grassland | 4264.05 | 26 | 4273.01 | 26 | 4998.63 | 30 |
| Marsh | 801.68 | 5 | 1038.69 | 6 | 616.88 | 4 |
| Open water | 857.31 | 5 | 612.46 | 4 | 321.01 | 2 |
| Built-up land | 3371.67 | 20 | 3377.87 | 20 | 3381.39 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Liu, H.; Meng, Z.; Yan, W.; Xiao, L.; Lei, Y.; Zhao, X.; Chen, Z.; Liu, Q. Diurnal Habitat Selection and Use of Wintering Bar-Headed Geese (Anser indicus) Across Heterogeneous Landscapes on the Yunnan–Guizhou Plateau, Southwest China. Animals 2025, 15, 2826. https://doi.org/10.3390/ani15192826
Li C, Liu H, Meng Z, Yan W, Xiao L, Lei Y, Zhao X, Chen Z, Liu Q. Diurnal Habitat Selection and Use of Wintering Bar-Headed Geese (Anser indicus) Across Heterogeneous Landscapes on the Yunnan–Guizhou Plateau, Southwest China. Animals. 2025; 15(19):2826. https://doi.org/10.3390/ani15192826
Chicago/Turabian StyleLi, Chao, Hong Liu, Ziwen Meng, Weike Yan, Linna Xiao, Yu Lei, Xuyan Zhao, Zhiming Chen, and Qiang Liu. 2025. "Diurnal Habitat Selection and Use of Wintering Bar-Headed Geese (Anser indicus) Across Heterogeneous Landscapes on the Yunnan–Guizhou Plateau, Southwest China" Animals 15, no. 19: 2826. https://doi.org/10.3390/ani15192826
APA StyleLi, C., Liu, H., Meng, Z., Yan, W., Xiao, L., Lei, Y., Zhao, X., Chen, Z., & Liu, Q. (2025). Diurnal Habitat Selection and Use of Wintering Bar-Headed Geese (Anser indicus) Across Heterogeneous Landscapes on the Yunnan–Guizhou Plateau, Southwest China. Animals, 15(19), 2826. https://doi.org/10.3390/ani15192826





