Selected Pathologies of the Male Genital Organs in Bulls, Including Frequency, Significance, and Risk Factors: A Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Testicular and Epididymal Pathologies
2.1. Orchitis
2.2. Hypoplasia Testis
2.3. Degeneratio Testis
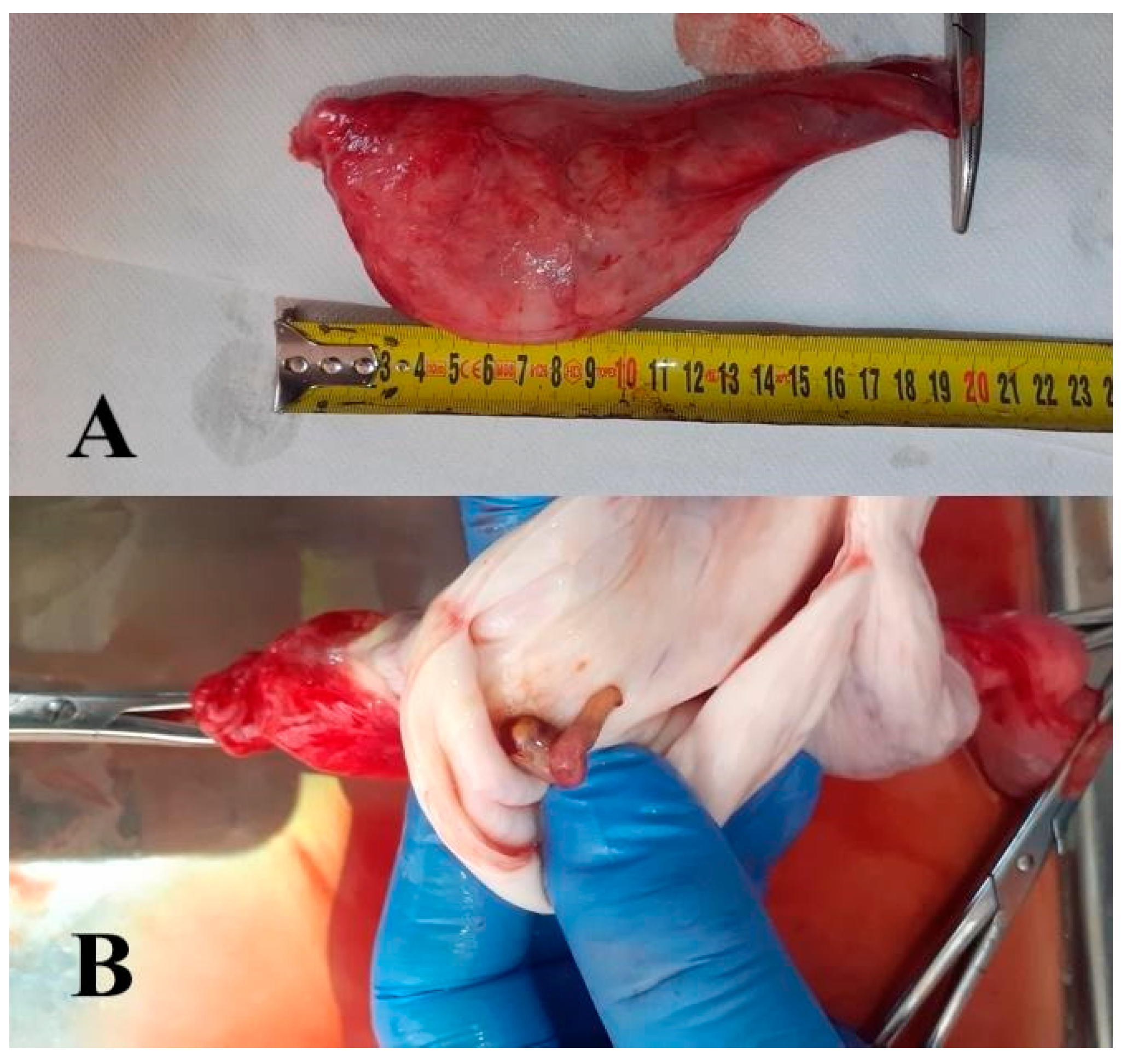
2.4. Hydrocele Testis and Cysts Testes
2.5. Tumores Neoplasmatices
2.6. Cryptorchidism
2.7. Epididymitis
2.8. Spermatocele
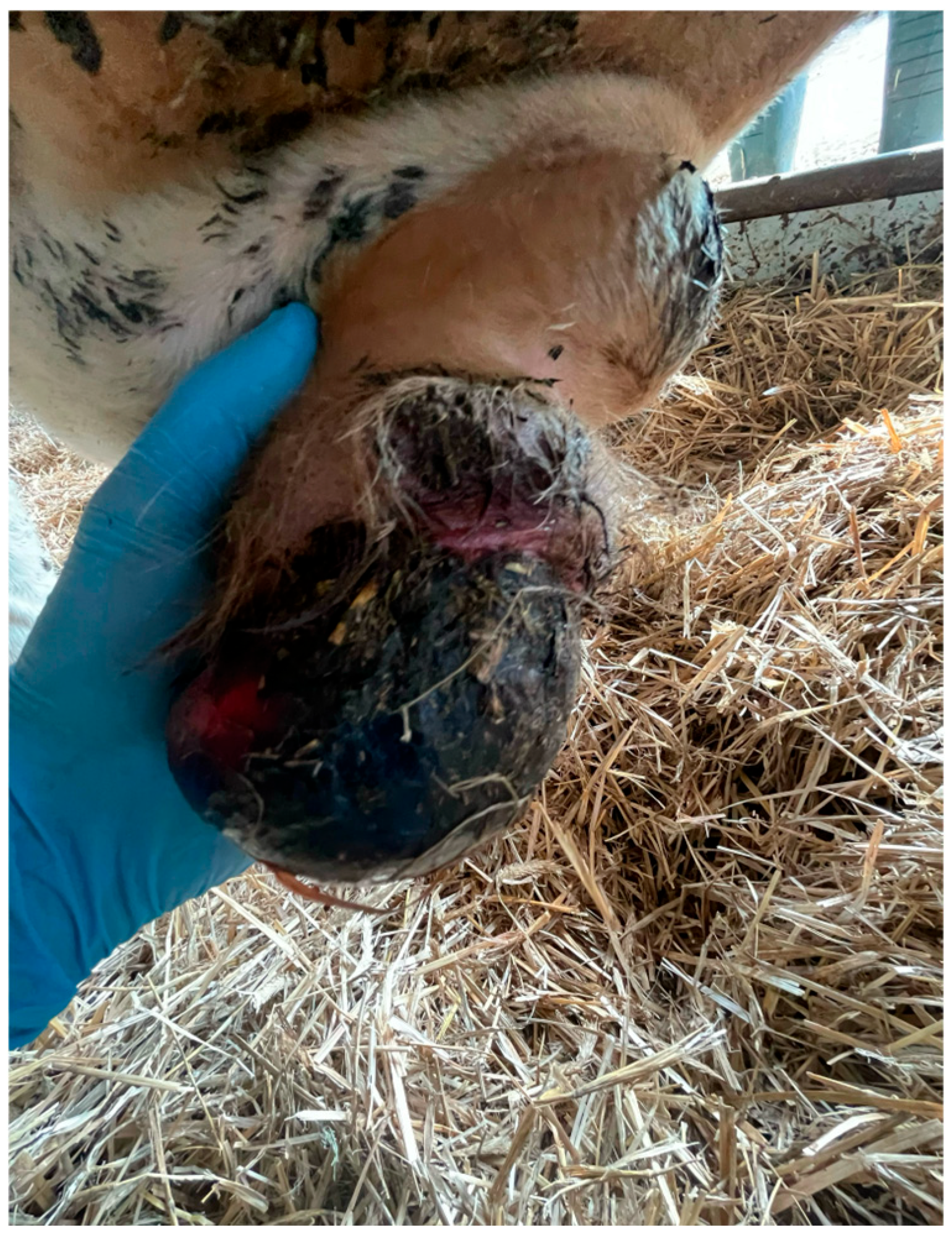
2.9. Hernia Scrotalis
3. Penile and Foreskin Pathologies
3.1. Fibropapillomatosis
3.2. Penile Hematoma
3.3. Penile Hair Ring
3.4. Penile Tuberculosis
3.5. Tumors of the Copulatory Organ
3.6. Frenulum Preputii Persistens
3.7. Phimosis and Paraphimosis
3.8. Other Penile Defects
4. Disorders of the Accessory Sex Glands
5. Sterilitas Secundaria
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Latos-Bieleńska, A.; Materna-Kiryluk, A.; PRCM Working Group. Polish registry of congenital malformations—Aims and organization of the registry monitoring 300 000 births a year. J. Appl. Genet. 2005, 46, 341–348. [Google Scholar] [PubMed]
- Wolfe, D.F. Review: Abnormalities of the bull—Occurrence, diagnosis and treatment of abnormalities of the bull, including structural soundness. Animal 2018, 12, 148–157. [Google Scholar] [CrossRef]
- Hopper, R.M. Bovine Reproduction, 2nd ed.; John Wiley and Sons Ltd.: Starkville, MS, USA, 2021; 1232p. [Google Scholar]
- Engelken, T. The development of beef breeding bulls. Theriogenology 2008, 70, 573–575. [Google Scholar] [CrossRef]
- Society for Theriogenology. Veterinary Professionals Dedicated to Animal Reproduction. Available online: https://www.therio.org/ (accessed on 15 July 2023).
- Kastelic, J.P.; Thundathil, J.C. Breeding soundness evaluation and semen analysis for predicting bull fertility. Reprod. Domest. Anim. 2008, 43, 368–373. [Google Scholar] [CrossRef]
- Kouamo, J.; Nyonga, G.V. Gross reproductive organs abnormalities in bulls of northern regions of Cameroon. J. Infertil. Reprod. Biol. 2022, 10, 41–47. [Google Scholar]
- Bousmaha, F.; Benchaib Khoudja, F. Comparative and pathological study of the testis and epididymis in rams, bucks and bulls of Algeria. Asian J. Anim. Vet. Adv. 2012, 7, 950–959. [Google Scholar] [CrossRef]
- Silva, C.; Delgado, R.; Magaña, J.; Reyes, A. Abnormalities of testicular and scrotal development in bulls of three breeds in southeast México. Av. En Investig. Agropecu. 2008, 12, 21–31. [Google Scholar]
- McGowan, M.R.; Bertram, J.D.; Fordyce, G.; Fitzpatrick, L.A.; Miller, R.G.; Jayawardhana, G.A.; Doogang, V.J.; De Faveri, J.; Holroyd, R.G. Bull selection and use in northern Australia. 1. Physical traits. Anim. Reprod. Sci. 2002, 71, 25–37. [Google Scholar] [CrossRef]
- Migbaru, K.; Sisay, G.; Kasa, T. Study on Gross Testicular Disorders of Bulls Slaughtered at Addis Ababa Abattoirs Enterprise. J. Reprod. Fertil. 2014, 5, 45–49. [Google Scholar]
- Gemeda, A.E. Gross testicular abnormalities in indigenous breeds of bulls in Eastern Ethiopia. JAVAR 2017, 4, 200–206. [Google Scholar] [CrossRef]
- Kouamo, J.; Eta, L.E. Prevalence and associated risk factors of genital abnormalities in bulls slaughtered at the SODEPA Industrial Abattoir, Douala, Cameroon. J. Infertil. Reprod. Biol. 2021, 9, 136–141. [Google Scholar]
- Uyar, A.; Uslu, B.A.; Yurdakul, İ. Evaluation on the histopathology of testes anomalies in the bulls slaughtered at the city of Van. DUVETFD 2019, 12, 30–36. [Google Scholar]
- Kandiwa, E.; Nyirakunzimana, L.; Habarugira, G.; Mushonga, B.; Samkange, A. A 4-year study of the proportional distribution of male reproductive organ abnormalities in cattle slaughtered at Nyagatare abattoir. Vet. Med. Sci. 2017, 3, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.P.L.; Lobo Júnior, A.R.; Castilho, E.F.; Guimarães, J.D.; Melo, T.V.; Mota, D.A.; Siqueira, J.B. Reproductive disorders affecting 21-month-old bulls assessed by andrological examination. Rev. Fac. Cienc. Agrar. 2020, 41, 3199–3209. [Google Scholar] [CrossRef]
- Amaer, E.; Habtemariam, K.; Samulel, A.; Shimellis, D.; Belina, D. Incidence of gross pathological conditions of the reproductive organs in bulls. Indian J. Vet. Pathol. 2016, 40, 305–311. [Google Scholar]
- Boryczko, Z. Zaburzenia płodności. In Fizjologia i Patologia Rozrodu Bydła, 1st ed.; Boryczko, Z., Bostedt, H., Jaśkowski, J.M., Eds.; University of Nicolaus Copernicus: Toruń, Poland, 2021; p. 86. [Google Scholar]
- Parsonson, I.M.; Winter, J.; McEntee, K. Allergic epididymo-orchitis in guinea pigs and bulls. Vet. Pat. 1971, 8, 333–351. [Google Scholar] [CrossRef]
- Sivahoth, S.; Sudnakara Reddy, B. Rare report of orchitis in a bull due to Trypanosoma evansi infection. J. Parasit. Dis. 2019, 43, 28–30. [Google Scholar]
- González-Huecas, M.; Ferre, I.; Jiménez-Meléndez, A.; Criado, F.; Gutiérrez-Expósito, D.; Ortega-Mora, L.M.; Álvarez-Garcia, G. Vascular wall injury and inflammation are key pathogenic mechanisms responsible for early testicular degeneration during acute besnoitiosis in bulls. Parasit Vectors 2020, 13, 113. [Google Scholar] [CrossRef]
- Jaśkowski, L.; Sadowski, J.M.; Hoffmann-Woźniak, K.; Wieczór, H.; Truszczyński, M. Experimental chlamydiosis in bulls. Effects of unilateral intratesticular inoculation. Bull. Vet. Inst. Puławy 1979, 23, 14–20. [Google Scholar]
- Machmoud, M.A.M.; Megahed, G.; Yousef, M.S.; Zakib Ali, F.A.; Zako, R.S.; Abdelhafeza, H.H. Salmonella Typhimurium triggered unilaateral epididimo-orchitis and splenomegaly in a holstein bulls Assiut, Egypt: A case report. Pathogens 2020, 9, 314. [Google Scholar] [CrossRef]
- Mahesh, V.; Rashmitha, H.; Preetham Kumar, S.V.; Shivaraju, E.; Nagaraja, B.N. Surgical management of orchitis in Gir bullock by scrotal ablation technique. J. Livest. Sci. 2022, 13, 38–40. [Google Scholar] [CrossRef]
- Youngquist, R.S.; Threlfall, W.R. Current Therapy in Large Animal Theriogenology, 2nd ed.; Elsevier Health Sciences: London, UK, 2006; 1088p. [Google Scholar]
- Neves, H.H.; Vargas, G.; Brito, L.F.; Schenkel, F.S.; Albuquerque, L.G.; Carvalheiro, R. Genetic and genomic analyses of testicular hypoplasia in Nellore cattle. PLoS ONE 2019, 14, e0211159. [Google Scholar] [CrossRef]
- Salvador, D.F.; Salvador, S.C. Testicular hypoplasia in cattle: Causes and consequences. Ciência Anim. 2021, 31, 67–79. [Google Scholar]
- Barth, A.D. Congenital and acquired abnormalities of the scrotum, testes and epididymides of bulls. Large Anim. Vet. 2006, 6, 1–6. [Google Scholar]
- Saunders, P.J.; Ladds, P.W. Congenital and developmental anomalies of the genital of slaughtered bulls. Aust. Vet. J. 1978, 54, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Chenoweth, P.J.; Osborne, H.G. Breed differences in abnormalities of the reproductive organs of young beef bulls. Aust. Vet. J. 1978, 54, 463–468. [Google Scholar] [CrossRef]
- Hoflack, G.; Van der Broeck, W.; Maes, D.; Van Damme, K.; Opsomer, G.; Duchateau, L.; de Kruif, A.; Rodriguez-Martinez, H.; Van Soom, A. Testicular dysfunction is responsible for low sperm quality in Belgian Blue bulls. Theriogenology 2008, 69, 232–233. [Google Scholar] [CrossRef]
- Borel, N.; Janett, F.; Teankum, K.; Zlinszky, K.; Iten, C.; Hilbe, M. Testicular hypoplasia in a bull persistently infected with bovine diarrhoea virus. J. Comp. Pathol. 2007, 137, 169–173. [Google Scholar] [CrossRef]
- Dunn, H.O.; Lein, D.H.; McKentee, K. Testicular hypoplasia in a Hereford bull with 61,XXY karyotype: The bovine counterpart of human Klinefelter’s syndrome. Cornell Vet. 1980, 70, 137–146. [Google Scholar]
- Bongso, T.A.; Jainudeen, M.R.; Lee, Y.J. Testicular hypoplasia in a bull with XX/XY chimerism. Cornell Vet. 1981, 71, 376–382. [Google Scholar]
- Krumrych, W.; Jaśkowski, J.M.; Gehrke, M.; Zbylut, E.N. Legitimacy of systematic karyotype evaluation of cattle qualified for reproduction. J. Bull. Vet. Inst. Puławy 2002, 46, 307–315. [Google Scholar]
- Adamu, S.; Fatihu, M.Y.; Useh, N.M.; Mamman, M.; Sekoni, V.O.; Esievo, K.A.N. Sequential testicular and epididymal damage in Zebu bulls experimentally infected with Trypanosoma vivax. Vet. Parasitol. 2007, 143, 29–34. [Google Scholar]
- Koziol, J.; Palmer, C. Pathophysiology, diagnosis, and management of testicular degeneration in the bull. Clin. Theriogenology 2023, 15, 1–7. [Google Scholar] [CrossRef]
- Contri, A.; Gloria, A.; Wegher, L.; Carluccio, A. Successful use of gonadotropin-releasing hormone (GnRH) analog for the treatment of tertiary hypogonadism (GnRH deficiency) in a 5-year-old Belgian Blue bull. Vet. Q. 2012, 32, 51–54. [Google Scholar] [PubMed]
- Moran, K.; Picco, R.; Morrell, E.; Verna, A.; Zapata, L.; Moiraghi, L.; Bilbao, G.; Bartolomé, J. Testicular degeneration, fibrosis, and mineralization in Limangus bulls. Clin. Theriogenology 2025, 17, 1–5. [Google Scholar] [CrossRef]
- Hassan, M.F. Biometrical and pathological studies on testes of cow bulls. In Proceedings of the First International Congress of Veterinary Phatmacology & Pharmaceutical Sciences (1st ICVPS), Teheran, Iran, 4–5 October 2008. [Google Scholar]
- Abu-Seida, A.M. Ultrasonographic diagnosis of some scrotal swellings in the bulls. Pak. Vet. J. 2012, 32, 378–381. [Google Scholar]
- McClure, M.; King, H. Scrotal hydrocele in an Angus bull. In Proceedings of the SFT Theriogenology Annual Conference, Online, 22–25 July 2020; TherioConference; Society for Theriogenology: Sacramento, CA, USA, 2020. [Google Scholar]
- Singh, K.B. Hydrocele in an Ongole bull. Indian Vet. J. 1985, 62, 529–530. [Google Scholar]
- Dalal, J.; Kumar, A.; Gupta, G. Management of unilateral chronic orchitis and hydrocele in a Sahiwal Bull. Indian Vet. J. 2017, 94, 68–69. [Google Scholar]
- Sindh, R.S. Hydrocele in a yearling bull. The Vet. J. 1916, 72, 310. [Google Scholar]
- Ali, K.M.; Ahmad, N.; Akhtar, N.; Ali, S.; Ahmad, M.; Younis, M. Ultrasound imaging testes and epididymitis of normal and infertile breeding bulls. Pak. Vet. J. 2011, 31, 345–350. [Google Scholar]
- Jaśkowski, J.M.; Kaptur, M.D.; Jaśkowski, B.M.; Kulynych, S.; Gehrke, M. A premier case report of a congenital pseudocyst of the testis (cystis spuria testiculi congenita) in a three-week-old calf. BMC Vet. Res. 2025, 21, 306. [Google Scholar] [CrossRef]
- Prado, T.M.; Dawson, L.J.; Schumacher, J. Surgical procedures of the genital organs of bulls. Vet. Clin. N. Am. Food. Anim. Pract. 2016, 32, 701–725. [Google Scholar] [CrossRef]
- Luby, C.D.; Middleton, J.R.; Youngquist, R.S.; Kim, D.Y.; Evans, A.T. Theriogenology question of the month. Sertoli cell tumor. J. Am. Vet. Med. Assoc. 2007, 231, 1503–1505. [Google Scholar] [CrossRef]
- Schiendewolffs, L.; Dierks, C.; Heppelmann, M.; Gähle, M.; Piechotta, M.; Bieneke, A.; Brehem, L.; Distl, O. Testicular yolk sac tumor and impaired spermatogenesis in a Holstein Friesian calf. Syst. Biol. Reprod. Med. 2015, 61, 314–319. [Google Scholar]
- Jean, G.; Gaughan, E.M.; Constable, P.D. Cryptorchidism in North American cattle: Breed predisposition and clinical findings. Theriogenology 1992, 38, 951–958. [Google Scholar] [CrossRef]
- Ayodeji, A.A.; Suwaiba, W. Cryptorchidism among breeds of bulls in a semi-arid region of Nigeria. Maced. Vet. Rev. 2013, 36, 123–128. [Google Scholar]
- Miyake, Y.; Kaneda, Y. A new type of Robertsonian translocation (1/26) in a bull with unilateral cryptorchidism, probably occurring de novo. Jpn. J. Vet. Sci. 1987, 49, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Torisu, S.; Kitahara, G.; Hidaka, Y.; Satoh, H.; Asanuma, T.; Mizutani, S.; Osawa, T.; Naganobu, K. Laparoscopic cryptorchidectomy in standing bulls. J. Vet. Med. Sci. 2015, 77, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Van Camp, S.D. Common causes of infertility in the bull. Vet. Clin. N. Am. Food Anim. Pract. 1997, 13, 203–231. [Google Scholar] [CrossRef]
- Pagliarani, S.; Johnston, S.A.; Beagly, K.W.; Dief, H.; Palmieri, C. The occurrence and pathology of chlamydiosis in the male reproductive tract of non-human mammals: A review. Theriogenology 2020, 154, 152–160. [Google Scholar] [CrossRef]
- McEntee, K. Reproductive Pathology of Domestic Mammals, 1st ed.; Academic Press: Cambridge, MA, USA, 2012; 400p. [Google Scholar]
- Dorne, M. Spermatocele. J. Urol. 1926, 15, 389–396. [Google Scholar] [CrossRef]
- Stachura, J.; Domagała, W. Patologia Znaczy Słowo o Chorobie, 3rd ed.; Polska Akademia Umiejętności: Kraków, Poland, 2016; 676p. [Google Scholar]
- Threlfall, W.R.; Robertson, J.T.; Munsterman, A.S.; Oglesbee, M.J.; Hubbell, J.A. Theriogenology question of the month. Seminoma, spermatocele, sustentacular cell tumor. J. Am. Vet. Med. Assoc. 2005, 226, 1649–1650. [Google Scholar] [CrossRef] [PubMed]
- Statham, J. Differential diagnosis of scrotal enlargement in bulls. Practice 2010, 32, 200–206. [Google Scholar] [CrossRef]
- Grindflek, E.; Moe, M.; Taubert, H.; Similander, H.; Lien, S.; Moen, T. Genome-wide linkage analysis of inguinal hernia in pigs using affected sib pairs. BMC Genet. 2006, 7, 25. [Google Scholar] [CrossRef]
- Ebert, E.F. Surgical repair of scrotal hernia in the bull. Can. Vet. J. 1960, 1, 441–443. [Google Scholar]
- Baba, A.I. Oncologie Comparată; Editura Academiei Române: Bucureşti, Romania, 2002; 684p. [Google Scholar]
- Knipe, D.M.; Howley, P.M. Fields Virology, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1996; 2664p. [Google Scholar]
- Campo, M.S. Papillomavirus Research: From Natural History to Vaccine and Beyond, 1st ed.; Caister Academic Press: Glasgow, UK, 2006; 424p. [Google Scholar]
- McEntee, K. Fibropapilloma of the external genitalia of cattle. Cornell Vet. 1950, 40, 304–312. [Google Scholar]
- Batamuzi, E.K.; Kassuku, A.A.; Agger, J.F. Risk factors associated with canine transmissible venereal tumour in Tanzania. Prev. Vet. Med. 1992, 13, 13–17. [Google Scholar] [CrossRef]
- Borzacchiello, G.; Roperto, F. Bovine papillomaviruses, papillomas and cancer in cattle. Vet. Res. 2008, 39, 1. [Google Scholar]
- Patnaik, A.K.; Matthiesen, D.T.; Zarvie, D.A. Two cases of canine penile neoplasm: Squamous cell carcinoma and mesenchymal chondrosarcoma. J. Am. Anim. Hosp. Assoc. 1987, 24, 403–406. [Google Scholar]
- Kennedy, P.C.; Cullen, J.M.; Edwards, J.F.; Goldschmidt, M.H.; Larsen, S.; Munson, L.; Nielsen, S. Histological Classification of Tumors of the Genital System of Domestic Animals, 2nd ed.; Armed Forces Institute of Pathology: Washington, DC, USA, 1998; 79p. [Google Scholar]
- Lindsey, C.L.; Almeida, M.E.; Vicari, C.F.; Carvalho, C.; Yaguiu, A.; Freitas, A.C.; Beçak, W. Stocco dos Santos RC. Bovine papillomavirus DNA in milk, blood, urine, semen, and spermatozoa of bovine papillomavirus-infected animals. Genet. Mol. Res. 2009, 8, 310–318. [Google Scholar] [CrossRef]
- Stocco dos Santos, R.C.; Lindsey, C.J.; Ferraz, O.P.; Pinto, J.R.; Mirandola, R.S.; Benesi, F.J.; Birgel, E.H.; Pereira, C.A.; Beçak, W. Bovine papillomavirus transmission and chromosomal aberrations: An experimental model. J. Gen. Virol. 1998, 79, 2127–2135. [Google Scholar] [CrossRef]
- Beckett, S.D.; Reynolds, T.M.; Walker, D.F.; Hudson, R.S.; Purohit, R.C. Experimentally induced rupture of corpus cavernosum penis of the bull. Am. J. Vet. Res. 1974, 35, 765–767. [Google Scholar] [CrossRef]
- Wolfe, D.F.; Beckett, S.D.; Carson, R.L.; Powe, T.A.; Pugh, D.G. Acquired conditions of the penis and prepuce. In Large Animal Urogenital Surgery, 1st ed.; Wolfe, D.F., Moll, H.D., Eds.; Williams and Wilkins: Baltimore, MD, USA, 1998; pp. 237–272. [Google Scholar]
- Akin, I. A Case of penile hair ring in a young bull. Anim. Health Prod. Hyg. 2013, 2, 212. [Google Scholar]
- Armstrong, C.L.; Koziol, J.H. Understanding a breeding soundness evaluation and factors that impact bull fertility. In Proceedings of the Applied Reproductive Strategies in Beef Cattle, San Antonio, TX, USA, 30–31 August 2022. Beef Reproduction Task Force. [Google Scholar]
- Wolfe, D.F.; Carson, R.L. Juvenile anomalies of the penis and prepuce: Bulls. In Large Animal Urogenital Surgery, 1st ed.; Wolfe, D.F., Moll, H.D., Eds.; Williams and Wilkins: Baltimore, MD, USA, 1998; pp. 233–235. [Google Scholar]
- Trotter, A.M. Tuberculosis of the penis of a bull. J. Com. Pathol. 1903, 16, 252. [Google Scholar] [CrossRef]
- Thoen, C.O.; Himes, E.M.; Stumpff, C.D.; Parks, T.W.; Sturkie, H.N. Isolation of Mycobacterium bovis from the prepuce of a herd bull. Am. J. Vet. Res. 1977, 30, 877–878. [Google Scholar] [CrossRef]
- Foster, R.A. Male genital system. In Jubb, Kennedy and Palmer’s Pathology of Domestic Animals, 6th ed.; Maxie, M.G., Ed.; Elsevier: St. Louis, MO, USA, 2016; Volume 3, pp. 465–510. [Google Scholar]
- Vielmo, A.; Lopes, B.C.; Panziera, W.; Bianchi, R.M.; Mayer, F.Q.; Vielmo, L.A.; Barros, C.S.L.; Driemeier, D. Penile Tuberculosis in a bull. J. Comp. Pathol. 2020, 180, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Riojas, M.A.; McGough, K.J.; Rider-Riojas, C.J.; Rastogi, N.; Hazbón, M.H. Phylogenomic analysis of the species of the Mycobacterium tuberculosis complex demonstrates that Mycobacterium africanum, Mycobacterium bovis, Mycobacterium caprae, Mycobacterium microti and Mycobacterium pinnipedii are later heterotypic synonyms of Mycobacterium tuberculosis. Int. J. Syst. Evol. Microbiol. 2018, 68, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Terefe, D. Gross pathological lesions of bovine tuberculosis and efficiency of meat inspection procedure to detect-infected cattle in Adama municipal abattoir. J. Vet. Med. Anim. Health 2014, 6, 48–53. [Google Scholar]
- Domingo, M.; Vidal, E.; Marco, A. Pathology of bovine tuberculosis. Res. Vet. Sci. 2014, 97, 20–29. [Google Scholar] [CrossRef]
- Schlafer, D.H.; Foster, R.A. Female Genital System. In Jubb, Kennedy and Palmer’s Pathology of Domestic Animals, 6th ed.; Maxie, M.G., Ed.; Elsevier: St. Louis, MO, USA, 2016; Volume 3, pp. 358–464. [Google Scholar]
- Hesaraki, S.; Abedi, G.; Rismanchi, S. Penile fibrosarcoma tumor in a bull. Iran J. Vet. Res. 2010, 11, 283–286. [Google Scholar]
- Pandit, G.A.; Kudrimoti, J.K.; Kokandakar, H.R.; Bhople, K.S. Fibrosarcoma of penis—A case report. Indian J. Pathol. Microbiol. 2004, 47, 389–390. [Google Scholar] [PubMed]
- Smirnov, A.V. Fibrosarcoma: Immunohistochemical study of the extracellular matrix. Arkh. Pathol. 1988, 50, 17–24. [Google Scholar]
- Fletcher, C.D.M.; Unni, K.K.; Mertens, F. Pathology and Genetics of Tumors of Soft Tissue and Bone, 1st ed.; IARC Press: Lyon, France, 2002; pp. 100–102. [Google Scholar]
- Van den Top, J.G.B.; De Heer, N.; Klein, W.R.; Ensink, J.M. Penile and preputial tumours in the horse: A retrospective study of 114 affected horses. J. Equine Vet. 2008, 40, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Hudson, R.S.; Wolfe, D.F. Physical defects which limit breeding soundness of bulls. In American Association of Bovine Practitioners Conference Proceedings, Oklahoma City, OK, USA, 28 November–1 December 1983; American Association of Bovine Practitioners: Ashland, OH, USA, 1983. [Google Scholar]
- Ashdown, R.R. Persistence of the penile frenulum in young bulls. Vet. Rec. 1962, 74, 1464–1468. [Google Scholar]
- Elmore, R.G.; Breuer, J.; Youngquist, R.S.; Lasley, J.F.; Bierschwald, C.J. Breeding soundness examinations in 18 closely related inbred Angus bull. Theriogenology 1978, 10, 355–363. [Google Scholar] [CrossRef]
- Elmore, R.G. Surgical repair of bovine persistent penile frenulum. Vet. Med. Small Anim. Clin. 1981, 76, 701–704. [Google Scholar]
- Fesseha, H. Acute paraphimosis in bull and its surgical management. Prac. Clin. Investig. 2020, 3, 68–71. [Google Scholar]
- Reynard, J.M.; Barau, J.M. Reduction of paraphimosis the simple way: The Dundee technique. Br. J. Urol. Intern. 1999, 83, 859–860. [Google Scholar] [CrossRef]
- Choe, J.M. Paraphimosis: Current treatment options. Am. Fam. Physician 2000, 62, 2623–2626. [Google Scholar]
- Adeola, B.S.; Enobong, H. Surgical management of paraphimosis in dog: A case report. Glob. Vet. 2016, 16, 49–51. [Google Scholar]
- Fossum, T. Small Animal Surgery, 3rd ed.; Elsevier: Philadelphia, PA, USA, 2007; 1632p. [Google Scholar]
- Hopper, R.M. Management of male reproductive tract injuries and disease. Vet. Clin. N. Am. Food. Anim. Pract. 2016, 32, 497–510. [Google Scholar] [CrossRef]
- McDiarmid, J.J. “Corkscrew penis” and other breeding abnormalities in beef bulls. N. Z. Vet. J. 2011, 23, 35–36. [Google Scholar] [CrossRef]
- Javdani Gandomani, M.; Dehghani Nazhvani, S.; Raayat Jahromi, A.R. Congenital penile urethral aplasia in a 4-day-old bull calf. Iran J. Vet. Res. 2009, 10, 87–89. [Google Scholar]
- Gilbert, R.O. The diagnosis of short penis as a cause of impotentia coeundi in bulls. Theriogenology 1989, 32, 805–815. [Google Scholar] [CrossRef]
- Young, S.; Hudson, R.; Walker, D.F. Impotence in bulls due to vascular shuns from the corpus cavernosum penis. J. Am. Vet. Med. Assoc. 1977, 171, 643–648. [Google Scholar] [CrossRef]
- Hopper, R.M.; Wolfe, D.F. Restorative surgery of the penis. In Bovine Reproduction, 2nd ed.; Hopper, R.M., Ed.; John Wiley and Sons Ltd.: Starkville, MS, USA, 2021; pp. 210–229. [Google Scholar]
- Moll, H.D.; Wolfe, D.F.; Hathcock, J.T. Caversonography for diagnosis of erection failure in bulls. Compend. Contin. Educ. Vet. 1993, 161, 1160–1164. [Google Scholar]
- Lima, U.A.; Freire, L.Q.B.; Lima, W.S.M.; Sancler-Silva, Y.F.R.; Mesquita, E.P.; Oliveira, D.; Torres, M.B.A.M.; Carneiro, G.F. Characterization of vesicular glands, study of vesiculitis and its association with other accessory sex glands in bulls. Arq. Bras. Med. Vet. Zootec. 2024, 76, 7–15. [Google Scholar] [CrossRef]
- Sánchez, J.M.; Rabaglino, M.B.; Bagés-Arnal, S.; McDonald, M.; Behura, S.K.; Spencer, T.E.; Longeran, P.; Fernandez-Fuertes, B. Sperm exposure to accessory gland secretions alters the transcriptomic response of the endometrium in cattle. Theriogenology 2024, 218, 26–34. [Google Scholar] [CrossRef]
- Sethi, M.; Mohanty, T.K.; Shah, N.; Bhakat, M.; Kumar, N.; Baithalu, R.K. Understanding the Crucial Role of Seminal Plasma Exosomes in Bull Fertility: A Review. Reprod. Domest. Anim. 2024, 59, e70000. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.S.; Palomares, R.; Heins, B.; Xavier, P.; Fyke, H.; Hurley, D.J.; Gordon, J. Analytical and clinical validation of diagnostic tests for the detection of leucospermia in beef bulls. Theriogenology 2024, 230, 46–53. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Veterinary Gross Anatomical Nomenclature. In Nomina Anatomica Veterinária, 6th ed.; Editorial Committee: Hanover, Germany, 2017.
- McGowan, M.; Holland, M.K.; Boe-Hansen, G. Review: Ontology and endocrinology of the reproductive system of bulls from fetus to maturity. Animal 2018, 12, 19–26. [Google Scholar] [CrossRef]
- Campero, C.M.; Bagshaw, P.A.; Ladds, P.W. Lesions of presumed congenital origin in the accessory sex glands of bulls. Aust. Vet. J. 1989, 66, 80–85. [Google Scholar]
- Bagshaw, P.A.; Ladds, P.W. A study of the accessory sex glands of bulls in abattoirs in Northern Australia. Aust. Vet. J. 1974, 50, 489–495. [Google Scholar]
- Campero, C.M.; Ladds, P.W.; Thomas, A.D. Pathological findings in the bulbourethral glands of bulls. Aust. Vet. J. 1988, 65, 241–244. [Google Scholar] [CrossRef]
- Rovay, H.; Barth, A.D.; Chirino-Trejo, M.; Martínez, M.F. Update on treatment of vesiculitis in bulls. Theriogenology 2008, 70, 495–503. [Google Scholar] [CrossRef]
- Martínez, M.F.; Barth, A.D. Early detection and treatment of vesicular adenitis in bulls. Anim. Reprod. Sci. 2007, 101, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.F.; Arteaga, A.A.; Barth, A.D. Intraglandular injection of antibiotics for the treatment of vesicular adenitis in bulls. Anim. Reprod. Sci. 2008, 104, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Severo, N.C.; de Assumpção, T.I.; Peixer, M.A.S.; da Cunha Xavier, M.; Malard, P.F.; Brunel, H.D.S.S.; Lançoni, R. Effectiveness of intraglandular allogeneic mesenchymal stem cell administration for treating chronic vesicular adenitis in bulls. Theriogenology 2025, 241, 117419. [Google Scholar] [CrossRef]
- Shah, B.A.; Hopwood, M.L.; Faulkner, L.C. Seminal vesiculectomy in bulls. J. Reprod. Fertil. 1968, 16, 171–177. [Google Scholar]
- Ahola, J.K.; Foster, H.A.; VanOverbeke, D.L.; Jensen, K.S.; Wilson, R.L.; Glaze, J.B.; Fife, T.E.; Gray, C.W.; Nash, S.A.; Panting, R.R.; et al. Survey of quality defects in market beef and dairy cows and bulls sold through livestock auction markets in the Western United States: I. Incidence rates. J. Anim. Sci. 2011, 89, 1474–1483. [Google Scholar] [CrossRef]
- Bartels, J.E. Femoral-tibial osteoarthrosis in the bull: I. Clinical survey and radiological interpretation. J. Am. Vet. Rad. Soc. 1975, 16, 159–173. [Google Scholar] [CrossRef]
- Berry, S.L. Diseases of the digital soft tissues. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, F.M.; Spitzer, J.C. The new Society for Theriogenology breeding soundness evaluation system. Vet. Clin. N. Am. Food Anim. Pract. 1997, 13, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Desrochers, A. Diagnosis and prognosis of common disorders involving the proximal limb. Vet. Clin. N. Am. Food Anim. Pract. 2017, 33, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, N.G. Stifle injuries in cattle. Vet. Clin. N. Am. Food Anim. Pract. 1996, 12, 59–84. [Google Scholar] [CrossRef]
- Frithian, D.C.; Kelly, M.A.; Mow, V.C. Material properties and structure- function relationships in the menisci. Clin. Orthop. Relat. Res. 1990, 252, 19–31. [Google Scholar] [CrossRef]
- Gentile, A.; Testoni, S. Inherited diseases of cattle: A selected review. Slov. Vet. Zb. 2006, 43, 17–29. [Google Scholar]
- Greenough, P.R. Lameness in Cattle, 3rd ed.; W.B. Saunders: Philadelphia, PA, USA, 1972; 336p. [Google Scholar]
- Greenough, P. Pododermatitis circumscripta (ulceration of the sole) in cattle. Agri Prac. 1987, 6, 17–22. [Google Scholar]
- Hoblet, K.H.; Weiss, W. Metabolic hoof horn disease claw horn disruption. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 111–127. [Google Scholar] [CrossRef]
- Lischer, C.; Ossent, P.; Raben, M.; Geyer, H. Suspensory structures and supporting structures of the third phalanx of cows and their relevance to development of typical sole ulcers (Rusterholz ulcers). Vet. Rec. 2002, 151, 694–698. [Google Scholar]
- Nelson, D.R.; Huhn, J.C.; Knelle, S.K. Peripheral detachment of the medial meniscus with injury to the medial collateral ligament in 50 cattle. Vet. Rec. 1990, 127, 59–66. [Google Scholar]
- Pentecost, R.; Niehaus, A. Cranial cruciate ligament, meniscus, upward fixation of the patella. Vet. Clin. N. Am. Food Anim. Pract. 2014, 30, 265–281. [Google Scholar]
- Persson, Y.; Söderquist, L.; Ekman, S. Joint disorder; a contributory cause to reproductive failure in beef bulls? Acta Vet. Scand. 2007, 49, 31. [Google Scholar] [CrossRef] [PubMed]
- Purohit, R.C.; Hudson, R.S.; Riddell, M.G.; Carson, R.L.; Wolfe, D.F.; Walker, D.F. Thermography of the bovine scrotum. Am. J. Vet. Res. 1985, 46, 2388–2392. [Google Scholar] [CrossRef]
- Reiland, S.; Stromberg, B.; Olsson, S.E.; Dreimanis, I.; Olsson, I.G. Osteochondrosis in growing bulls. Pathology, frequency and severity on different feedings. Acta Radiol. 1978, 358, 179–196. [Google Scholar]
- Weisbrode, S.E.; Monke, D.R.; Dodaro, S.T.; Hull, B.L. Osteochondrosis, degenerative joint disease, and vertebral osteophytosis in middle-aged bulls. J. Am. Vet. Med. Assoc. 1982, 181, 700–705. [Google Scholar] [CrossRef]

| Pathology | Etiological Factor | Type of Defect | Treatment | Estimated Frequency [%] |
|---|---|---|---|---|
| Orchitis | Infectious, autoimmune, allergic, mechanical trauma | Acquired | Antibiotic therapy | 1.94–7.1% |
| Hypoplasia testis | BVD virus, genetic mutation | Congenital. acquired | Cull, vaccines | 0.2–3.6% |
| Degeneratio testis | Trauma, tumours, deficiency of nutrients, Trypanosoma vivax, idiopathic | Acquired | Cull, GnRH | 8.1% |
| Hydrocele testis | Trauma, tumours, orchitis, epididymitis | Acquired | Surgical, self-treatment | 1% |
| Cryptochidism | Genetic mutation | Congenital | Surgical | 2.6% |
| Epididymitis | Infectious, autoimmune, allergic, mechanical trauma, chlamydophilia, | Acquired | Antibiotic therapy, cull | 3.7% |
| Hernia scrotalis | ? | Congenital. acquired | Surgical | 0.87% |
| Penile papillomatosis | BPV virus | Acquired | Surgical, vaccines | ? |
| Penile hematoma | Trauma | Acquired | Antibiotic therapy, surgical | 7–14.9% |
| Penile hair ring | Trauma | Acquired | Self-treatment, surgical | ? |
| Frenulum preputii persistens | Genetic mutation | Congenital | Surgical | 0.5% |
| Phimosis and paraphimosis | Trauma, genetic mutation, paralysis | Congenital. acquired | Self-treatment, surgical | ? |
| Pathology | Country/Region | Breed | Frequency (%) | Authors |
|---|---|---|---|---|
| Hypoplasia | Cameroon | Local breeds | 5.8 | Kouamo & Nyonga 2022 [7] |
| Algeria | Local breeds | 0.66 | Bousmaha & Khoudja 2012 [8] | |
| Mexico | Brahman | 3.45 | Silva et al., 2008 [9] | |
| Mexico | Nelore | 1.87 | Silva et al., 2008 [9] | |
| Mexico | Brown Swiss | 3.1 | Silva et al., 2008 [9] | |
| Australia | Santa Gertrudis | 1.4 | McGowan et al., 2002 [10] | |
| Australia | Brown Swiss | 3.1 | McGowan et al., 2002 [10] | |
| Ethiopia | Local breeds | 3.6 | Migbaru et al., 2014 [11] | |
| Ethiopia | Local breeds | 18.8 | Gemeda 2017 [12] | |
| Cameroon | Local breeds | 8.9 | Kouamo & Eta 2021 [13] | |
| Turkey | Mixed | 1.67 | Uyar et al., 2019 [14] | |
| Testicular degeneration | Cameroon | Local breeds | 2.5 | Kouamo & Nyonga 2022 [7] |
| Ethiopia | Local breeds | 6.5 | Gemeda 2017 [12] | |
| Ethiopia | Local breeds | 8.1 | Migbaru et al., 2014 [11] | |
| Cameroon | Local breeds | 1.0 | Kouamo & Eta 2021 [13] | |
| Cryptorchidism | Cameroon | Local breeds | 1.3 | Kouamo & Nyonga 2022 [7] |
| Ethiopia | Local breeds | 1.0 | Gemeda 2017 [12] | |
| Mexico | Brahman | 0.49 | Silva et al., 2008 [9] | |
| Mexico | Brown Swiss | 0.92 | Silva et al., 2008 [9] | |
| Ethiopia | Local breeds | 2.6 | Migbaru et al., 2014 [11] | |
| Turkey | Mixed | 0.44 | Uyar et al., 2019 [14] | |
| Hematoma (testicles/penile) | Cameroon | Local breeds | 1.9 | Kouamo & Nyonga 2022 [7] |
| Cameroon | Local breeds | 14.9 | Kouamo & Eta 2021 [13] | |
| Ethiopia | Local breeds | 9.0 | Gemeda 2017 [12] | |
| Ethiopia | Local breeds | 2.1 | Migbaru et al., 2014 [11] | |
| Orchitis | Cameroon | Local breeds | 0.5 | Kouamo & Nyonga 2022 [7] |
| Cameroon | Local breeds | 7.0 | Kouamo & Eta 2021 [13] | |
| Ethiopia | Local breeds | 4.4 | Migbaru et al., 2014 [11] | |
| Turkey | Mixed | 1.23 | Uyar et al., 2019 [14] | |
| Epididymitis | Cameroon | Local breeds | 0.2 | Kouamo & Nyonga 2022 [7] |
| Cameroon | Local breeds | 2.0 | Kouamo & Eta 2021 [13] | |
| Ethiopia | Local breeds | 3.4 | Migbaru et al., 2014 [11] | |
| Australia | Santa Gertrudis | 3.0 | McGowan et al., 2002 [10] | |
| Ethiopia | Local breeds | 2.5 | Gemeda 2017 [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butkiewicz, A.F.; Zdun, M.; Jaśkowski, J.M. Selected Pathologies of the Male Genital Organs in Bulls, Including Frequency, Significance, and Risk Factors: A Review. Animals 2025, 15, 2804. https://doi.org/10.3390/ani15192804
Butkiewicz AF, Zdun M, Jaśkowski JM. Selected Pathologies of the Male Genital Organs in Bulls, Including Frequency, Significance, and Risk Factors: A Review. Animals. 2025; 15(19):2804. https://doi.org/10.3390/ani15192804
Chicago/Turabian StyleButkiewicz, Aleksander F., Maciej Zdun, and Jędrzej M. Jaśkowski. 2025. "Selected Pathologies of the Male Genital Organs in Bulls, Including Frequency, Significance, and Risk Factors: A Review" Animals 15, no. 19: 2804. https://doi.org/10.3390/ani15192804
APA StyleButkiewicz, A. F., Zdun, M., & Jaśkowski, J. M. (2025). Selected Pathologies of the Male Genital Organs in Bulls, Including Frequency, Significance, and Risk Factors: A Review. Animals, 15(19), 2804. https://doi.org/10.3390/ani15192804






