Alterations in Lipid Metabolism and Hepatopancreatic Lipidomics Induced by Microcystin-LR Exposure in Common Carp (Cyprinus carpio)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Design
2.3. Histological Observation
2.4. Gene Expression Measurement
2.5. Biochemical Assay
2.6. Western Blot
2.7. Lipids Extraction
2.8. LC-MS/MS Methodology
2.9. Statistical Analysis
3. Results
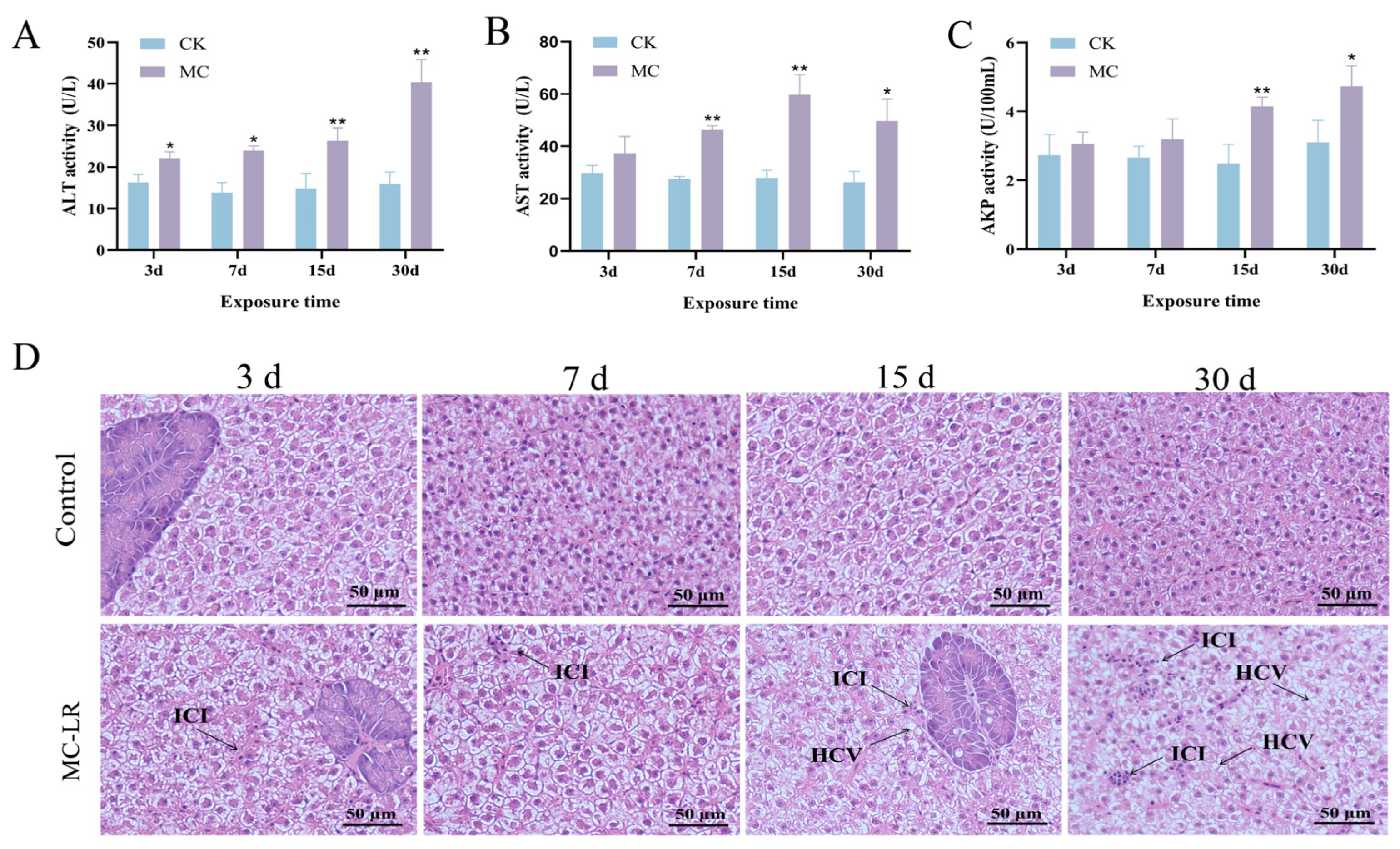
3.1. Increased Serum Enzyme Activity and Hepatopancreas Injury
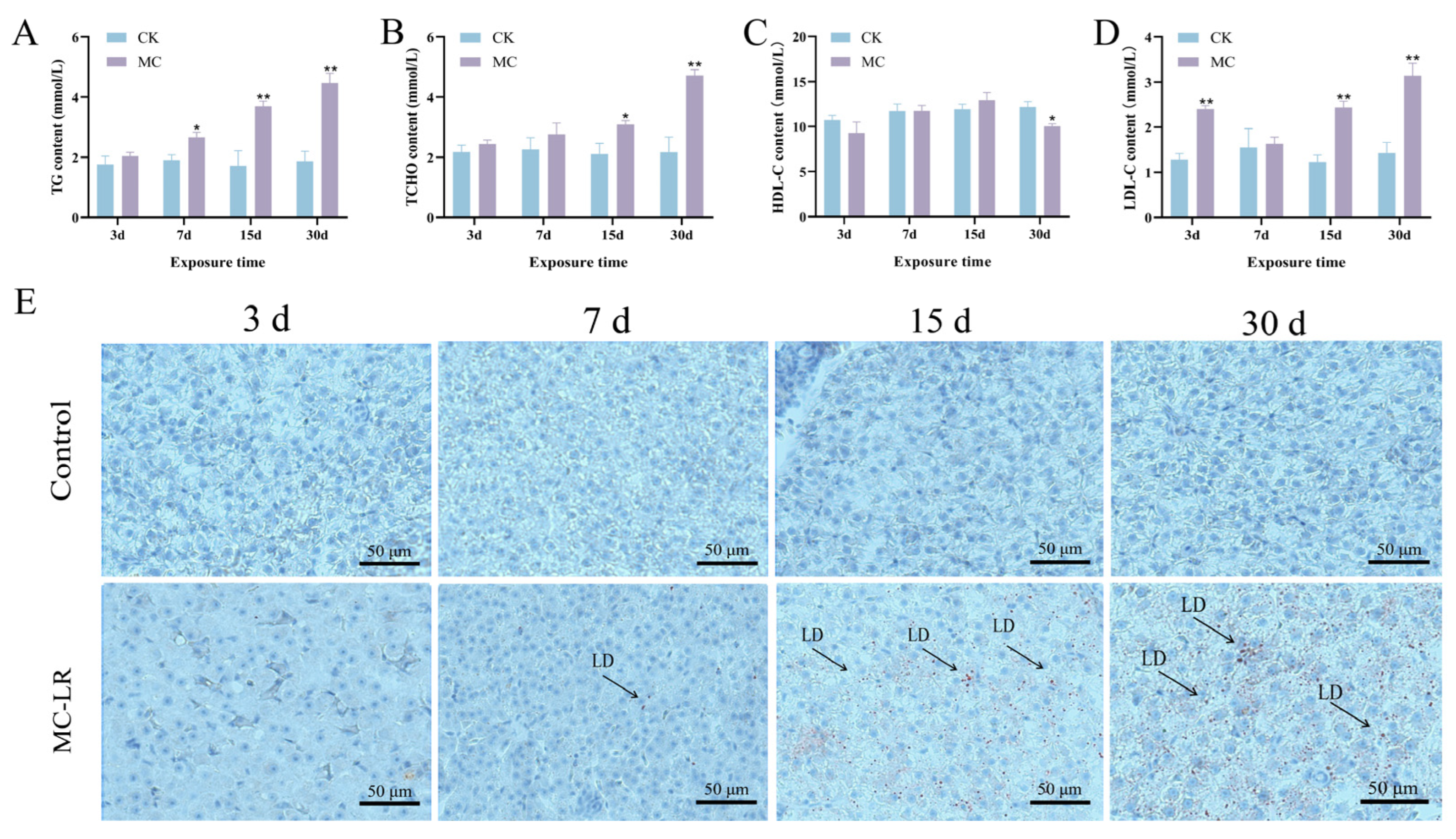
3.2. Altered Serum Lipid Levels and Hepatic Steatosis
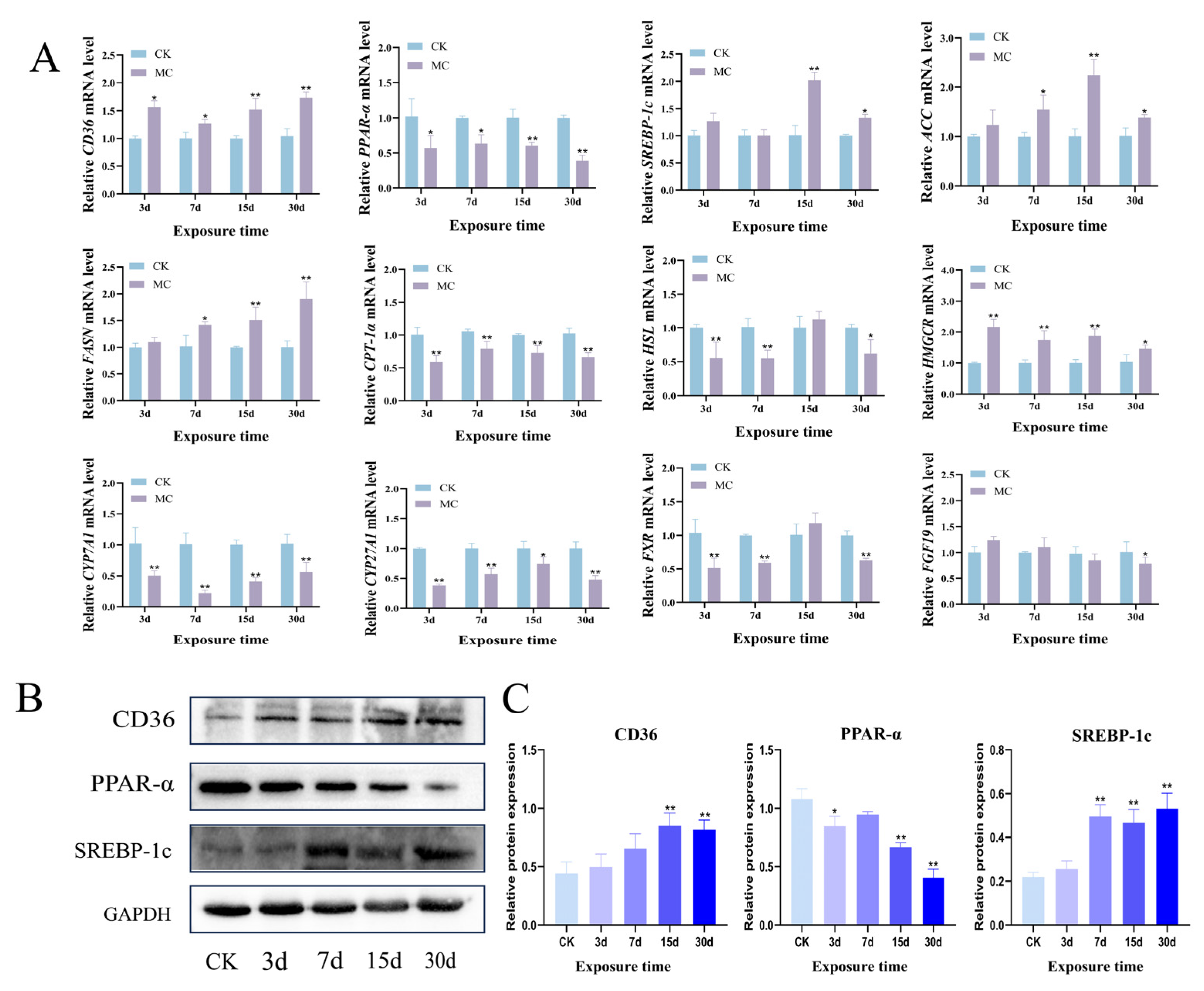
3.3. Lipid Metabolism Disorder in Hepatopancreas
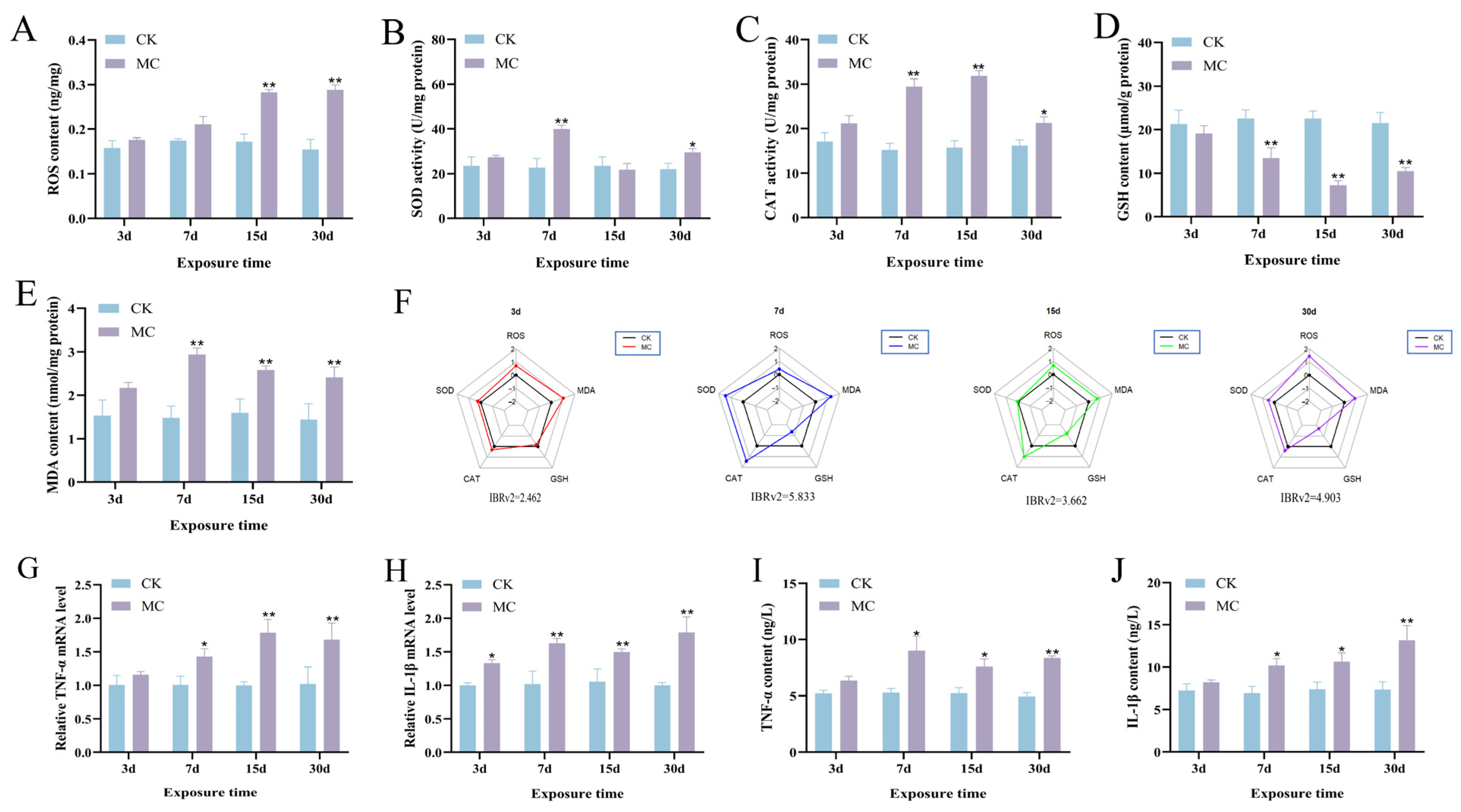
3.4. Oxidative Stress and Inflammatory Response in Hepatopancreas
3.5. Lipidomic Variations of Hepatopancreas
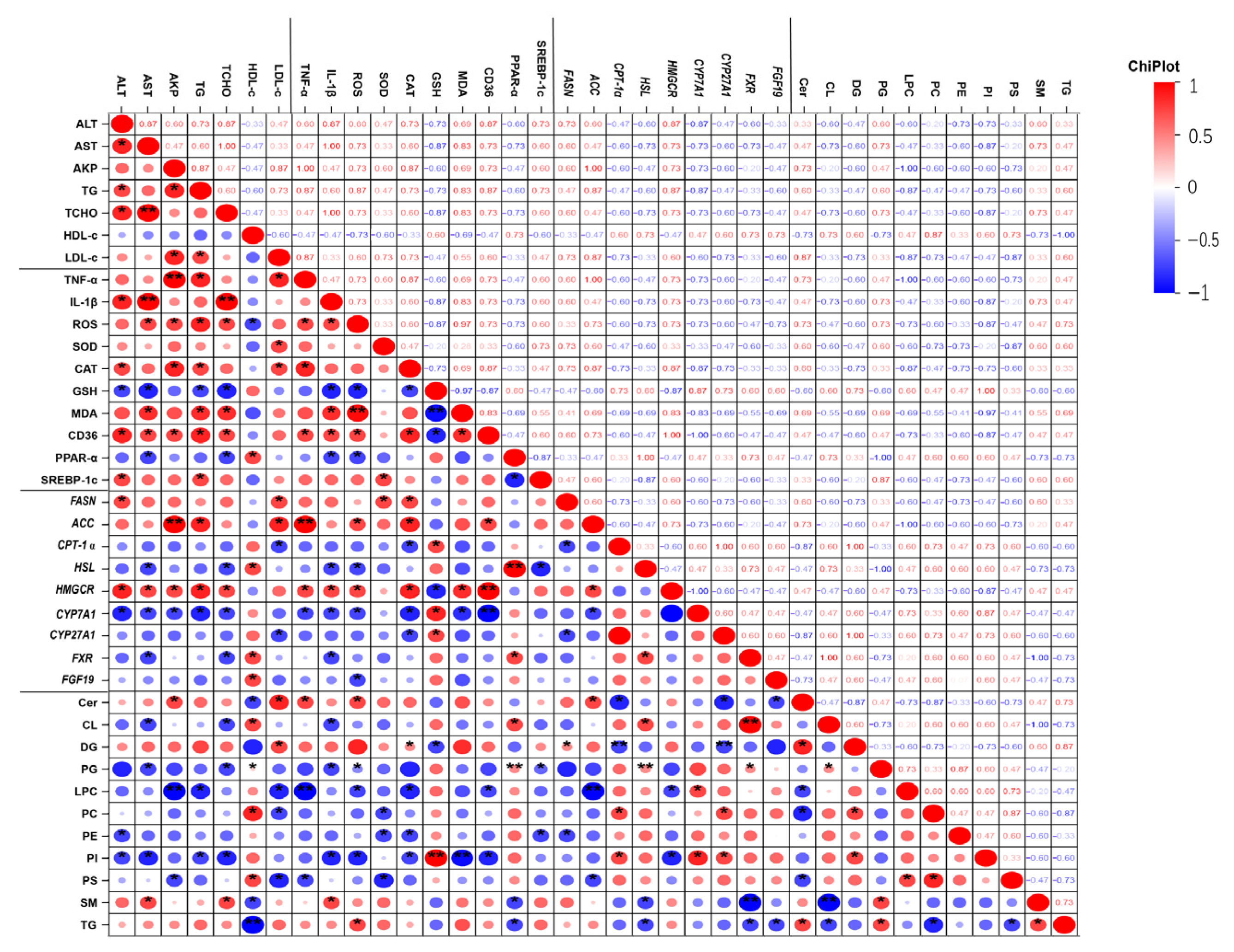
3.6. Correlation Between Altered Lipids and Biochemical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glibert, P.M. Eutrophication, harmful algae and biodiversity—Challenging paradigms in a world of complex nutrient changes. Mar. Pollut. Bull. 2017, 124, 591–606. [Google Scholar] [CrossRef]
- Sehnal, L.; Procházková, T.; Smutná, M.; Kohoutek, J.; Lepšová-Skácelová, O.; Hilscherová, K. Widespread occurrence of retinoids in water bodies associated with cyanobacterial blooms dominated by diverse species. Water Res. 2019, 156, 136–147. [Google Scholar] [CrossRef]
- Amorim, C.A.; Moura, A.D.N. Ecological impacts of freshwater algal blooms on water quality, plankton biodiversity, structure, and ecosystem functioning. Sci. Total Environ. 2021, 758, 143605. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qu, G.; Gan, Y.; Zhang, Z.; Li, R.; Wang, T. Elimination of Microcystis aeruginosa in water via dielectric barrier discharge plasma: Efficacy, mechanism and toxin release. J. Hazard. Mater. 2022, 422, 126956. [Google Scholar] [CrossRef]
- Preece, E.P.; Moore, B.C.; Hardy, F.J. Transfer of microcystin from freshwater lakes to Puget Sound, WA and toxin accumulation in marine mussels (Mytilus trossulus). Ecotoxicol. Environ. Saf. 2015, 122, 98–105. [Google Scholar] [CrossRef]
- Masango, M.G.; Myburgh, J.G.; Labuschagne, L.; Govender, D.; Bengis, R.G.; Naicker, D. Assessment of microcystis bloom toxicity associated with wildlife mortality in the Kruger National Park, South Africa. J. Wildl. Dis. 2010, 46, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Niu, Y.; Xie, P.; Chen, J.; Ma, Z.; Tao, M.; Qi, M.; Wu, L.; Guo, L. Factors affecting temporal and spatial variations of microcystins in Gonghu Bay of Lake Taihu, with potential risk of microcystin contamination to human health. Sci. World J. 2010, 10, 1795–1809. [Google Scholar] [CrossRef]
- Li, X.; Cheng, R.; Shi, H.; Tang, B.; Xiao, H.; Zhao, G. A simple highly sensitive and selective aptamer-based colorimetric sensor for environmental toxins microcystin-LR in water samples. J. Hazard. Mater. 2016, 304, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Shan, K.; Shang, M.; Zhou, B.; Li, L.; Wang, X.; Yang, H.; Song, L. Application of Bayesian network including Microcystis morphospecies for microcystin risk assessment in three cyanobacterial bloom-plagued lakes, China. Harmful Algae 2019, 83, 14–24. [Google Scholar] [CrossRef]
- Wei, L.; Liu, Y.; Zhong, S.; Wu, H.; Ruan, J.; Liu, M.; Zhou, Q.; Zhong, Q. Transcriptome analysis of grass carp provides insights into the immune-related genes and pathways in response to MC-LR induction. Aquaculture 2018, 488, 207–216. [Google Scholar] [CrossRef]
- Bouaïcha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural Diversity, Characterization and Toxicology of Microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef]
- Arman, T.; Clarke, J.D. Microcystin Toxicokinetics, Molecular Toxicology, and Pathophysiology in Preclinical Rodent Models and Humans. Toxins 2021, 13, 537. [Google Scholar] [CrossRef]
- Chen, L.; Xie, P. Mechanisms of microcystin-induced cytotoxicity and apoptosis. Mini Rev. Med. Chem. 2016, 16, 1018. [Google Scholar] [CrossRef]
- Du, C.; Zheng, S.; Yang, Y.; Feng, X.; Chen, J.; Tang, Y.; Wang, H.; Yang, F. Chronic exposure to low concentration of MC-LR caused hepatic lipid metabolism disorder. Ecotoxicol. Environ. Saf. 2022, 239, 113649. [Google Scholar] [CrossRef]
- He, J.; Shu, Y.; Dai, Y.; Gao, Y.; Liu, S.; Wang, W.; Jiang, H.; Zhang, H.; Hong, P.; Wu, H. Microcystin-leucine arginine exposure induced intestinal lipid accumulation and MC-LR efflux disorder in Lithobates catesbeianus tadpoles. Toxicology 2022, 465, 153058. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, Y.; Xie, L.; Wang, L.; He, Y.; Wan, X.; Xue, Q. Long-term environmental exposure to microcystins increases the risk of nonalcoholic fatty liver disease in humans: A combined fisher-based investigation and murine model study. Environ. Int. 2020, 138, 105648. [Google Scholar] [CrossRef] [PubMed]
- Aviram, R.; Manella, G.; Kopelman, N.; Neufeld-Cohen, A.; Zwighaft, Z.; Elimelech, M.; Adamovich, Y.; Golik, M.; Wang, C.; Han, X.; et al. Lipidomics analyses reveal temporal and spatial lipid organization and uncover daily oscillations in intracellular organelles. Mol. Cell 2016, 62, 636–648. [Google Scholar] [CrossRef]
- Lin, L.; Ding, Y.; Wang, Y.; Wang, Z.; Yin, X.; Yan, G.; Zhang, L.; Yang, P.; Shen, H. Functional lipidomics: Palmitic acid impairs hepatocellular carcinoma development by modulating membrane fluidity and glucose metabolism. Hepatology 2017, 66, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Vinaixa, M.; Schymanski, E.L.; Neumann, S.; Navarro, M.; Salek, R.M.; Yanes, O. Mass spectral databases for LC/MS- and GC/MS-based metabolomics: State of the field and future prospects. Trends Anal. Chem. 2016, 78, 23–35. [Google Scholar] [CrossRef]
- Tahir, R.; Samra; Ghaffar, A.; Afzal, F.; Qazi, I.H.; Zhao, L.; Yan, H.; Kuo, H.; Khan, H.; Yang, S. Chronic cypermethrin induced toxicity and molecular fate assessment within common carp (Cyprinus carpio) using multiple biomarkers approach and its novel therapeutic detoxification. Chemosphere 2024, 357, 142096. [Google Scholar] [CrossRef]
- Fischer, W.J.; Dietrich, D.R. Pathological and biochemical characterization of microcystin-induced hepatopancreas and kidney damage in carp (Cyprinus carpio). Toxicol. Appl. Pharmacol. 2000, 164, 73–81. [Google Scholar] [CrossRef]
- Shi, L.; Du, X.; Liu, H.; Chen, X.; Ma, Y.; Wang, R.; Tian, Z.; Zhang, S.; Guo, H.; Zhang, H. Update on the adverse effects of microcystins on the liver. Environ. Res. 2021, 195, 110890. [Google Scholar] [CrossRef] [PubMed]
- Painefilú, J.C.; González, C.; Cárcamo, J.G.; Bianchi, V.A.; Luquet, C.M. Microcystin-LR modulates multixenobiotic resistance proteins in the middle intestine of rainbow trout, Oncorhynchus mykiss. Aquat. Toxicol. 2022, 253, 106327. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, K.; Nan, X.; Ding, W.; Ma, J.; Li, X. Hepatocyte apoptosis is triggered by hepatic inflammation in common carp acutely exposed to microcystin-LR or chronically exposed to Microcystis. Ecotoxicol. Environ. Saf. 2024, 286, 117230. [Google Scholar] [CrossRef]
- Miao, Z.; Miao, Z.; Wang, S.; Wu, H.; Xu, S. Exposure to imidacloprid induce oxidative stress, mitochondrial dysfunction, inflammation, apoptosis and mitophagy via NF-kappaB/JNK pathway in grass carp hepatocytes. Fish Shellfish Immunol. 2022, 120, 674–685. [Google Scholar] [CrossRef]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef]
- Shi, C.; Guo, H.; Wu, T.; Tao, N.; Wang, X.; Zhong, J. Effect of three types of thermal processing methods on the lipidomics profile of tilapia fillets by UPLC-Q-Extractive Orbitrap mass spectrometry. Food Chem. 2019, 298, 125029. [Google Scholar] [CrossRef]
- Taguchi, R.; Ishikawa, M. Precise and global identification of phospholipid molecular species by an Orbitrap mass spectrometer and automated search engine Lipid Search. J. Chromatogr. A 2010, 1217, 4229–4239. [Google Scholar] [CrossRef] [PubMed]
- Nandini, S.; Sánchez-Zamora, C.; Sarma, S.S.S. Toxicity of cyanobacterial blooms from the reservoir Valle de Bravo (Mexico): A case study on the rotifer Brachionus calyciflorus. Sci. Total Environ. 2019, 688, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Jiang, G.; Dai, Y.; Huang, Y.; Wang, X.; Xiao, K.; Wang, M.; Abasubong, K.; Xu, Y.; Zhang, D.; et al. Effect of dietary cholesterol on growth performance, cholesterol deposition, and lipid metabolism in adult Chinese mitten crab (Eriocheir sinensis). Aquac. Nutr. 2022, 2022, 2012958. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Wu, M.; Zhang, C.; Che, Y.; Li, W.; Li, X. Serum immune responses in common carp (Cyprinus carpio L.) to paraquat exposure: The traditional parameters and circulating microRNAs. Fish Shellfish Immunol. 2018, 76, 133–142. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, W.; Liu, Y.; Guo, H.; Wang, L.; Yang, L.; Li, L.; Li, D.; Tang, R. Chronic microcystin-LR exposure induces abnormal lipid metabolism via endoplasmic reticulum stress in male zebrafish. Toxins 2020, 12, 107. [Google Scholar] [CrossRef]
- Osuna-Jiménez, I.; Abril, N.; Vioque-Fernández, A.; Gómez-Ariza, J.L.; Prieto-Álamo, M.; Pueyo, C. The environmental quality of Doñana surrounding areas affects the immune transcriptional profile of inhabitant crayfish Procambarus clarkii. Fish Shellfish Immunol. 2014, 40, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Chen, Y.; Ren, J.; Jiang, S.; Zhang, S.; Xu, H.; Li, Y. Lipidomics and transcriptomics analysis revealed the role of the spleen of Nile tilapia (Oreochromis niloticus) in lipid metabolism. Aquaculture 2024, 592, 741173. [Google Scholar] [CrossRef]
- Carroll, R.G.; Zasłona, Z.; Galván-Peña, S.; Koppe, E.L.; Sévin, D.C.; Angiari, S.; Triantafilou, M.; Triantafilou, K.; Modis, L.K.; O Neill, L.A. An unexpected link between fatty acid synthase and cholesterol synthesis in proinflammatory macrophage activation. J. Biol. Chem. 2018, 293, 5509–5521. [Google Scholar] [CrossRef]
- Bu, K.; Kim, M.; Shin, M.K.; Lee, S.; Sung, J. Regulation of Benzo[a]pyrene-Induced Hepatic Lipid Accumulation through CYP1B1-Induced mTOR-Mediated Lipophagy. Int. J. Mol. Sci. 2024, 25, 1324. [Google Scholar] [CrossRef]
- Salie, M.J.; Thelen, J.J. Regulation and structure of the heteromeric acetyl-CoA carboxylase. BBA Mol. Cell Biol. Lipids 2016, 1861, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Glatz, J.F.C.; Luiken, J.J.F.P. From fat to FAT (CD36/SR-B2): Understanding the regulation of cellular fatty acid uptake. Biochimie 2017, 136, 21–26. [Google Scholar] [CrossRef]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.N.; Ables, G.P.; Otlivanchik, O.A.; Schoiswohl, G.; Zechner, R.; Blaner, W.S.; Goldberg, I.J.; Schwabe, R.F.; Chua, S.C.; Huang, L. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J. Biol. Chem. 2008, 283, 13087–13099. [Google Scholar] [CrossRef]
- Rizzolo, D.; Kong, B.; Taylor, R.E.; Brinker, A.; Goedken, M.; Buckley, B.; Guo, G.L. Bile acid homeostasis in female mice deficient in Cyp7a1 and Cyp27a1. Acta Pharm. Sin. B 2021, 11, 3847–3856. [Google Scholar] [CrossRef]
- Cai, S.; He, H.; Nguyen, T.; Mennone, A.; Boyer, J.L. Retinoic acid represses CYP7A1 expression in human hepatocytes and HepG2 cells by FXR/RXR-dependent and independent mechanisms. J. Lipid Res. 2010, 51, 2265–2274. [Google Scholar] [CrossRef]
- Chen, J.; Sun, D.; Cui, H.; Rao, C.; Li, L.; Guo, S.; Yang, S.; Zhang, Y.; Cao, X. Toxic effects of carbon quantum dots on the gut-liver axis and gut microbiota in the common carp. Environ. Sci. Nano 2022, 9, 173–188. [Google Scholar] [CrossRef]
- Cao, L.; Shao, N.; Du, J.; Zhu, H.; Gao, J.; Li, Q.; Sun, Y.; Hu, J.; Yin, G.; Xu, G. Involvement of reactive oxygen species (ROS) in the hepatopancreatic cytotoxicity, oxidative stress, and apoptosis induced by microcystin-LR in Eriocheir sinensis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 276, 109801. [Google Scholar] [CrossRef]
- Pu, C.; Liu, Y.; Zhu, J.; Ma, J.; Cui, M.; Mehdi, O.M.; Wang, B.; Wang, A.; Zhang, C. Mechanisms insights into bisphenol S-induced oxidative stress, lipid metabolism disruption, and autophagy dysfunction in freshwater crayfish. J. Hazard. Mater. 2024, 479, 135704. [Google Scholar] [CrossRef]
- Adeoye, O.; Olawumi, J.; Opeyemi, A.; Christiania, O. Review on the role of glutathione on oxidative stress and infertility. JBRA Assist. Reprod. 2018, 22, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Labine, M.; Gong, Y.; Minuk, G.Y. Long-term, low-dose exposure to microcystin-LR does not cause or increase the severity of liver disease in rodents. Ann. Hepatol. 2017, 16, 959–965. [Google Scholar] [CrossRef]
- Dang, E.V.; McDonald, J.G.; Russell, D.W.; Cyster, J.G. Oxysterol restraint of cholesterol synthesis prevents AIM2 inflammasome activation. Cell 2017, 171, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Rod-In, W.; Jung, J.J.; Jung, S.K.; Lee, S.; Park, W.J. Lipids extracted from Aptocyclus ventricosus eggs possess immunoregulatory effects on RAW264.7 cells by activating the MAPK and NF-κB signaling pathways. Mar. Drugs 2024, 22, 368. [Google Scholar] [CrossRef]
- Ren, H.; Huang, G.; Liu, W.; Zhang, W.; Liu, Y.; Su, G.; Shen, D. IL-1β induced RXRα overexpression through activation of NF-κB signaling in gastric carcinoma. Biomed. Pharmacother. 2016, 78, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Beigi, T.; Safi, A.; Satvati, M.; Kalantari-Hesari, A.; Ahmadi, R.; Meshkibaf, M. Protective role of ellagic acid and taurine against fluoxetine induced hepatotoxic effects on biochemical and oxidative stress parameters, histopathological changes, and gene expressions of IL-1β, NF-κB, and TNF-α in male Wistar rats. Life Sci. 2022, 304, 120679. [Google Scholar] [CrossRef]
- Kartsoli, S.; Kostara, C.E.; Tsimihodimos, V.; Bairaktari, E.T.; Christodoulou, D.K. Lipidomics in non-alcoholic fatty liver disease. World J. Hepatol. 2020, 12, 436–450. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Quan, J.; Lu, J.; Zhao, G.; Sun, J. Metabonomics analysis reveals the protective effect of nano-selenium against heat stress of rainbow trout (Oncorhynchus mykiss). J. Proteom. 2022, 259, 104545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, H.; Wang, C.; Wang, Y.; Wei, Z.; Xue, C.; Mao, X.; Zhang, T. Targeted lipidomics reveal the effects of different phospholipids on the phospholipid profiles of hepatic mitochondria and endoplasmic reticulum in high-fat/high-fructose-diet-induced nonalcoholic fatty liver disease mice. J. Agric. Food Chem. 2022, 70, 3529–3540. [Google Scholar] [CrossRef] [PubMed]
- Bandu, R.; Mok, H.J.; Kim, K.P. Phospholipids as cancer biomarkers: Mass spectrometry-based analysis. Mass Spectrom. Rev. 2018, 37, 107–138. [Google Scholar] [CrossRef]
- Hollie, N.I.; Cash, J.G.; Matlib, M.A.; Wortman, M.; Basford, J.E.; Abplanalp, W.; Hui, D.Y. Micromolar changes in lysophosphatidylcholine concentration cause minor effects on mitochondrial permeability but major alterations in function. BBA Mol. Cell Biol. Lipids 2014, 1841, 888–895. [Google Scholar] [CrossRef]
- Maud, L.; Vlad, R.; Kim, M.; Maachi, M.; Dominique, W.; François, P.; Jean, P.B.; Raoul, P.; Chantal, H.; Jacqueline, C.; et al. Serum adipokine levels predictive of liver injury in patients with non-alcoholic fatty liver disease. Liver Int. 2009, 29, 1431–1438. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 14205–14218. [Google Scholar] [CrossRef]
- Liu, Y.; Zang, X.; Feng, K.; Liu, S.; Zhang, J.; Lv, Z.; Xin, Y.; Yu, M. Lipidomic determination of serum lipids by ultra-high performance liquid chromatography—Mass spectrometry (UPLC-MS) for the characterization of nonalcoholic fatty liver disease (NAFLD). Anal. Lett. 2022, 55, 879–890. [Google Scholar] [CrossRef]
- Chaurasia, B.; Summers, S.A. Ceramides—Lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab. 2015, 26, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Meng, F.; Yao, L. Hepatotoxicity and immunotoxicity of MC-LR on silver carp. Ecotoxicol. Environ. Saf. 2019, 169, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Le Manach, S.; Huet, H.; Duvernois-Berthet, E.; Chaouch, S.; Duval, C.; Sotton, B.; Ponger, L.; Marie, A.; Mathéron, L.; et al. An integrated omic analysis of hepatic alteration in medaka fish chronically exposed to cyanotoxins with possible mechanisms of reproductive toxicity. Environ. Pollut. 2016, 219, 119–131. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Lou, M.; Liu, X.; Wen, W.; Li, X. Alterations in Lipid Metabolism and Hepatopancreatic Lipidomics Induced by Microcystin-LR Exposure in Common Carp (Cyprinus carpio). Animals 2025, 15, 2803. https://doi.org/10.3390/ani15192803
Zhao H, Lou M, Liu X, Wen W, Li X. Alterations in Lipid Metabolism and Hepatopancreatic Lipidomics Induced by Microcystin-LR Exposure in Common Carp (Cyprinus carpio). Animals. 2025; 15(19):2803. https://doi.org/10.3390/ani15192803
Chicago/Turabian StyleZhao, Haoyang, Mengya Lou, Xin Liu, Wenjun Wen, and Xiaoyu Li. 2025. "Alterations in Lipid Metabolism and Hepatopancreatic Lipidomics Induced by Microcystin-LR Exposure in Common Carp (Cyprinus carpio)" Animals 15, no. 19: 2803. https://doi.org/10.3390/ani15192803
APA StyleZhao, H., Lou, M., Liu, X., Wen, W., & Li, X. (2025). Alterations in Lipid Metabolism and Hepatopancreatic Lipidomics Induced by Microcystin-LR Exposure in Common Carp (Cyprinus carpio). Animals, 15(19), 2803. https://doi.org/10.3390/ani15192803




