Simultaneous Detection and Differentiation of Four Eimeria Species in Chickens (E. tenella, E. maxima, E. necatrix, and E. acervulina) Using a Multiplex TaqMan-MGB qPCR Assay
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasite Strains and DNA Samples
2.2. Clinical Samples
2.3. Genomic DNA Preparation
2.4. Primers and Probes for Multiplex qPCR
2.5. Construction of Standard Plasmids
2.6. Establishment of the Multiplex TaqMan qPCR Reaction System
2.7. Standard Curve Creation
2.8. Sensitivity, Specificity, and Reproducibility Evaluations
2.9. Evaluation of Clinical Samples by Quadruplex TaqMan Real-Time PCR and Conventional PCR
2.10. Data and Statistical Analysis
3. Results
3.1. Establishment and Optimization of the Multiplex Real-Time TaqMan-MGB PCR Assay
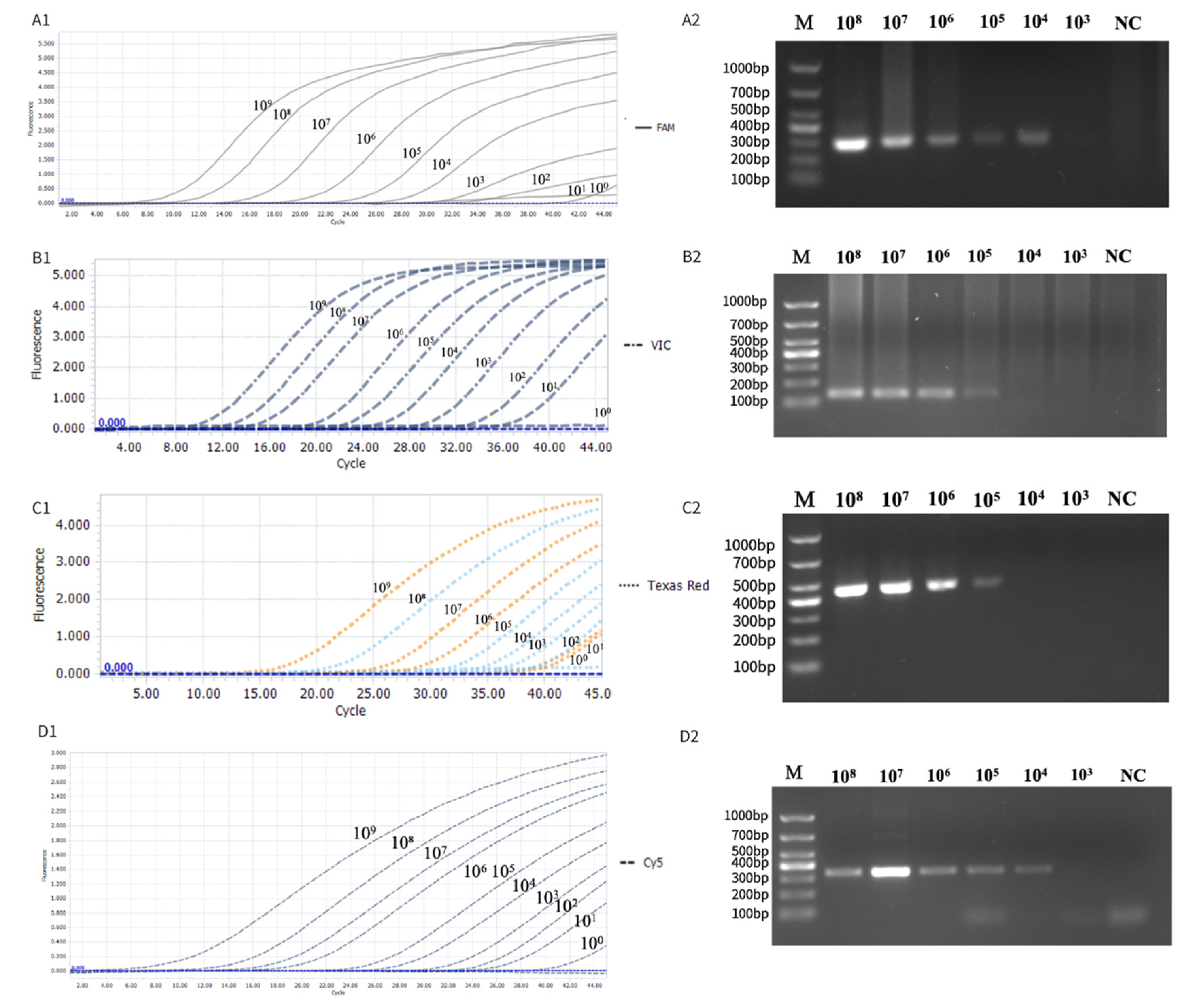
3.2. Standard Curve Preparation and Evaluation
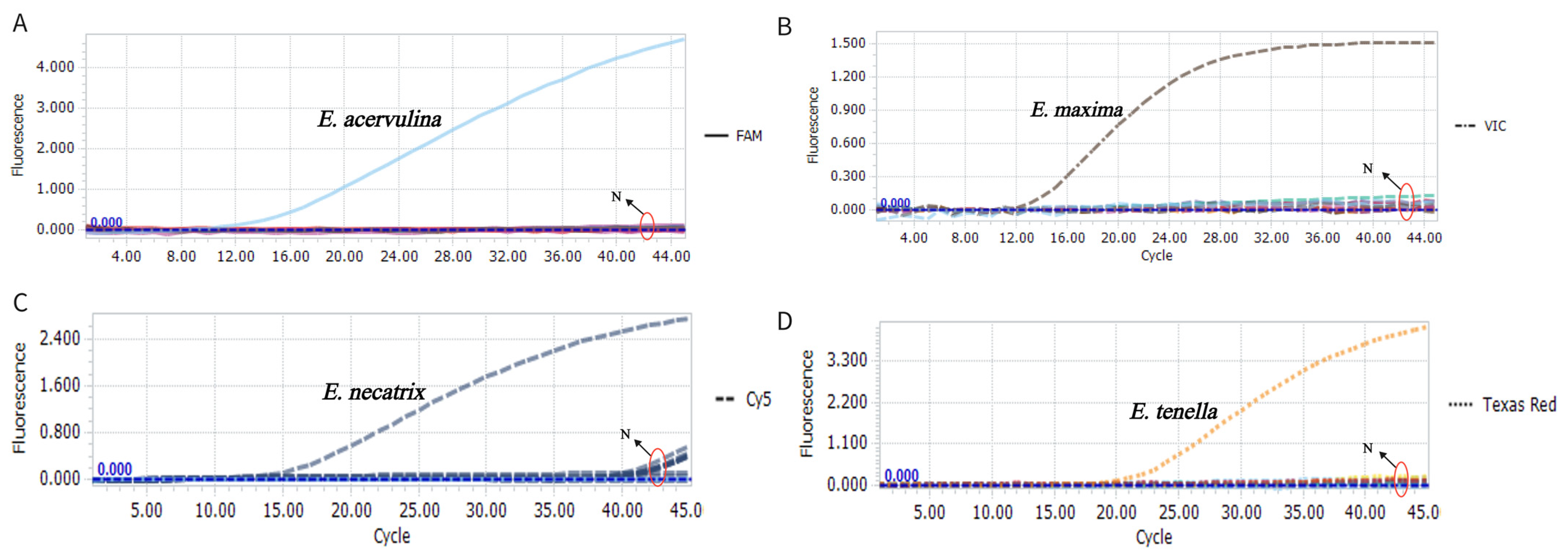
3.3. Specificity Evaluation
3.4. Sensitivity Evaluation
3.5. Reproducibility of the Multiplex TaqMan-MGB qPCR Assay
3.6. Detection of Clinical Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fatoba, A.J.; Adeleke, M.A. Transgenic Eimeria Parasite: A Potential Control Strategy for Chicken Coccidiosis. Acta Trop. 2020, 205, 105417. [Google Scholar] [CrossRef]
- Kadykalo, S.V.; Roberts, T.; Thompson, M.; Wilson, J.; Lang, M.; Espeisse, O.; Jenkins, M. The Value of Anticoccidials for Sustainable Global Poultry Production. Int. J. Antimicrob. Agents 2018, 51, 304–310. [Google Scholar] [CrossRef]
- Tomazic, M.L.; Florentin, G.; Vera, R.; Pinedo, M.E.; Gómez-Osorio, L.M. Chicken Coccidiosis in Peri-Urban Family Far-ming in Two South American Countries: Prevalence and Circulating Eimeria spp. Animals 2025, 15, 982. [Google Scholar] [CrossRef]
- Badri, M.; Olfatifar, M.; Hayati, A.; Bijani, B.; Samimi, R.; Abdoli, A.; Nowak, O.; Diaz, D.; Eslahi, A.V. The global prevalence and associated risk factors of Eimeria infection in domestic chickens: A systematic review and meta-analysis. Vet. Med. Sci. 2024, 10, e1469. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-Calculating the Cost of Coccidiosis in Chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef]
- Blake, D.P.; Vrba, V.; Xia, D.; Jatau, I.D.; Spiro, S.; Nolan, M.J.; Underwood, G.; Tomley, F.M. Genetic and Biological Characterisation of Three Cryptic Eimeria Operational Taxonomic Units That Infect Chickens (Gallus gallus domesticus). Int. J. Parasitol. 2021, 51, 621–634. [Google Scholar] [CrossRef]
- Kucukkara, Z.; Ozkan, I.A.; Tasdemir, S.; Ceylan, O. Classification of chicken Eimeria species through deep transfer learning models: A comparative study on model efficacy. Vet. Parasitol. 2025, 334, 110400. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, Q.; Wang, D.; Zheng, G.; Wang, S.; Han, Y.; Xu, Y.; He, H. Differential Expression of Toll-like Receptors and Associated Cytokines in the Bursa of Eimeria tenella-Infected Chickens. Res. Vet. Sci. 2025, 188, 105607. [Google Scholar] [CrossRef]
- Thenmozhi, V.; Veerakumari, L.; Raman, M. Preliminary Genetic Diversity Study on Different Isolates of Eimeria tenella from South India. Int. J. Adv. Vet. Sci. Technol. 2014, 3, 114–118. [Google Scholar] [CrossRef]
- Blake, D.P.; Clark, E.L.; Macdonald, S.E.; Thenmozhi, V.; Kundu, K.; Garg, R.; Jatau, I.D.; Ayoade, S.; Kawahara, F.; Moftah, A.; et al. Population, Genetic, and Antigenic Diversity of the Apicomplexan Eimeria tenella and Their Relevance to Vaccine Development. Proc. Natl. Acad. Sci. USA 2015, 112, E5343–E5350. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xiang, Q.; Li, M.; Sun, X.; Lu, M.; Yan, R.; Song, X.; Li, X. Pathogenic Effects of Single or Mixed Infections of Eimeria mitis, Eimeria necatrix, and Eimeria tenella in Chickens. Vet. Sci. 2022, 9, 657. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, T.; Zahid, R.; Imran, M.; Abbas, A.; Butt, A.; Aslam, S.; Ahmad, J. Vaccines against Chicken Coccidiosis with Particular Reference to Previous Decade: Progress, Challenges, and Opportunities. Parasitol. Res. 2022, 121, 2749–2763. [Google Scholar] [CrossRef]
- Liana, Y.A.; Swai, M.C. Mathematical Modeling of Coccidiosis Dynamics in Chickens with Some Control Strategies. Abstr. Appl. Anal. 2024, 2024, 1072681. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Wang, L.; Li, H.; Chen, J.; Liu, Q. Advancements in Understanding Chicken Coccidiosis: From Eimeria Biology to Innovative Control Strategies. Anim. Dis. 2024, 2, 39. [Google Scholar] [CrossRef]
- Kassaw, S.; Abdela, S.; Berihun, A.M. Investigation of Eimeria Species in Chicken: Coprological Prevalence, Gross Pathological Lesion and Pathoanatomical Species Identification in South Gondar Zone, Ethiopia. Acta Parasitol. 2025, 70, 70. [Google Scholar] [CrossRef] [PubMed]
- Amina, K.; Sadek, M.; Mustapha, O.; Moussadak, T. Prevalence of Coccidiosis in Broiler Chickens in Medea, Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2025, 118, 102323. [Google Scholar] [CrossRef]
- Godwin, R.M.; Morgan, J.A.T. A Molecular Survey of Eimeria in Chickens across Australia. Vet. Parasitol. 2015, 214, 16–21. [Google Scholar] [CrossRef]
- Andreopoulou, M.; Chaligiannis, I.; Sotiraki, S.; Vasileiou, N.G.C.; Papadopoulos, E. Prevalence and Molecular Detection of Eimeria Species in Different Types of Poultry in Greece and Associated Risk Factors. Parasitol. Res. 2022, 121, 2051–2063. [Google Scholar] [CrossRef]
- Liao, S.; Lin, X.; Zhou, Q.; Wang, Z.; Yan, Z.; Wang, D.; Su, G.; Li, J.; Lv, M.; Hu, J.; et al. Epidemiological Investigation of Coccidiosis and Associated Risk Factors in Broiler Chickens Immunized with Live Anticoccidials in China. Front. Vet. Sci. 2024, 11, 1375026. [Google Scholar] [CrossRef]
- Joseph, A.; Adekunle, M. Diagnosis and Control of Chicken Coccidiosis: A Recent Update. J. Parasitol. Dis. 2018, 42, 483–493. [Google Scholar] [CrossRef]
- Kumar, S.; Garg, R.; Moftah, A.; Clark, E.L.; Macdonald, S.E.; Chaudhry, A.S.; Sparagano, O.; Banerjee, P.S.; Kundu, K.; Tomley, F.M.; et al. An Optimised Protocol for Molecular Identification of Eimeria from Chickens. Vet. Parasitol. 2014, 199, 24–31. [Google Scholar] [CrossRef]
- Singla, L.D.; Gupta, S.K. Advances in Diagnosis of Coccidiosis in Poultry. In Veterinary Diagnostics: Current Trends; Gupta, R.P., Garg, S.R., Nehra, V., Lather, D., Eds.; Satish Serial Publishing House: Delhi, India, 2012; pp. 615–628. [Google Scholar]
- Hamidinejat, H.; Shapouri, M.S.; Mayahi, M.; Borujeni, M.P. Characterization of Eimeria Species in Commercial Broilers by PCR Based on ITS1 Regions of rDNA. Iran. J. Parasitol. 2010, 5, 48. [Google Scholar]
- Haug, A.; Gjevre, A.G.; Thebo, P.; Mattsson, J.G.; Kaldhusdal, M. Coccidial Infections in Commercial Broilers: Epidemiological Aspects and Comparison of Eimeria Species Identification by Morphometric and Polymerase Chain Reaction Techniques. Avian Pathol. 2008, 37, 161–170. [Google Scholar] [CrossRef]
- Fernandez, S.; Costa, A.C.; Katsuyama, A.M.; Madeira, A.M.B.N.; Gruber, A. A Survey of the Inter- and Intraspecific RAPD Markers of Eimeria spp. of the Domestic Fowl and the Development of Reliable Diagnostic Tools. Parasitol. Res. 2003, 89, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Fernández, S.; Pagotto, A.H.; Furtado, M.M.; Katsuyama, A.M.; Madeira, A.M.B.N.; Gruber, A. A multiplex PCR assay for the simultaneous detection and discrimination of the seven Eimeria species that infect domestic fowl. Parasitology 2003, 127, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Kundu, K.; Kumar, S.; Sarathi, P.; Garg, R. Quantification of Eimeria necatrix, E. acervulina and E. maxima Genomes in Commercial Chicken Farms by Quantitative Real-Time PCR. J. Parasitol. Dis. 2020, 44, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Barkway, C.P.; Pocock, R.L.; Vrba, V.; Blake, D.P. Loop-Mediated Isothermal Amplification (LAMP) Assays for the Species-Specific Detection of Eimeria That Infect Chickens. BMC Vet. Res. 2011, 7, 67. [Google Scholar] [CrossRef]
- Woods, W.G.; Richards, G.; Whithear, K.G.; Anderson, G.R.; Jorgensen, W.K.; Gasser, R.B. High-Resolution Electrophoretic Procedures for the Identification of Five Eimeria Species from Chickens, and Detection of Population Variation. Electrophoresis 2000, 21, 3558–3563. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Chen, J.; Wang, W.; Yu, H.; Gao, C.; An, T.; Wang, Y.; Chen, H.; Zhu, L.; et al. Development and Application of a Quadruplex TaqMan Real-Time Fluorescence Quantitative PCR Assay for Four Porcine Digestive Pathogens. Front. Cell. Infect. Microbiol. 2024, 14, 1468783. [Google Scholar] [CrossRef]
- Xue, B.; Li, Y.; Wang, X.; Li, R.; Zeng, X.; Yang, M.; Xu, X.; Ye, T.; Bao, L.; Huang, Y.; et al. TaqMan-MGB Probe Quantitative PCR Assays to Genotype the Three Primary LHON mtDNA Mutations. Sci. Rep. 2020, 10, 12241. [Google Scholar] [CrossRef]
- Khalifa, M.M.; Abushahba, M.F.N.; El-Saber Batiha, G.; El-Deeb, M.S. Smart Application of Silver Nanoparticles in the Treatment of Chicken Coccidiosis in Combination with Special Supplement to Alleviate Its Toxicity. Vet. Parasitol. 2025, 336, 110440. [Google Scholar] [CrossRef]
- Mesa-Pineda, C.; Navarro-Ruíz, L.; López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Gómez-Osorio, L.M. Chicken Coccidiosis: From the Parasite Lifecycle to Control of the Disease. Front. Vet. Sci. 2021, 8, 787653. [Google Scholar] [CrossRef]
- El-Shall, N.A.; Abd El-Hack, M.E.; Albaqami, N.M.; Khafaga, A.F.; Taha, A.E.; Swelum, A.A.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; AbuQamar, S.F.; et al. Phytochemical Control of Poultry Coccidiosis: A Review. Poult. Sci. 2022, 101, 101542. [Google Scholar] [CrossRef] [PubMed]
- Britez, J.D.; Rodriguez, A.E.; Di Ciaccio, L.; Marugán-Hernandez, V.; Tomazic, M.L. What Do We Know about Surface Proteins of Chicken Parasites Eimeria? Life 2023, 13, 1295. [Google Scholar] [CrossRef] [PubMed]
- Sultan, R.; Aslam, A.; Tipu, M.Y.; Rehman, H.U.; Anjum, A.; Krull, W.; Kumosani, T.; Shaib, H.; Barbour, E.K. Appraisal of a New Patented Method for Control of Chicken Coccidiosis. J. Appl. Anim. Res. 2019, 47, 573–581. [Google Scholar] [CrossRef]
- Geng, T.; Ye, C.; Lei, Z.; Shen, B.; Fang, R.; Hu, M.; Zhao, J.; Zhou, Y. Prevalence of Eimeria Parasites in the Hubei and Henan Provinces of China. Parasitol. Res. 2021, 120, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Lin, X.; Zhou, Q.; Yan, Z.; Wu, C.; Li, J.; Lv, M.; Hu, J.; Cai, H.; Song, Y. Prevalence and Geographic Distribution of Eimeria Species on Commercial Broiler Farms in Guangdong, China. BMC Vet. Res. 2024, 20, 171. [Google Scholar] [CrossRef]
- Huang, Y.; Ruan, X.; Li, L.; Zeng, M. Prevalence of Eimeria Species in Domestic Chickens in Anhui Province, China. J. Parasitol. Dis. 2017, 41, 1014–1019. [Google Scholar] [CrossRef]
- Flores, R.A.; Nguyen, B.T.; Cammayo, P.T.; Kim, S.; Kim, J.; Lillehoj, H.S. Epidemiological Investigation and Drug Resistance of Eimeria Species in Korean Chicken Farms. BMC Vet. Res. 2022, 18, 277. [Google Scholar] [CrossRef]
- Mesa, C.; Gómez-Osorio, L.M.; López-Osorio, S. Survey of Coccidia on Commercial Broiler Farms in Colombia: Frequency of Eimeria Species, Anticoccidial Sensitivity, and Histopathology. Poult. Sci. 2021, 100, 101239. [Google Scholar] [CrossRef]
- Blake, D.P.; Qin, Z.; Cai, J.; Smith, A.L.; Murray, G. Development and Validation of Real-Time Polymerase Chain Reaction Assays Specific to Four Species of Eimeria. Avian Pathol. 2008, 37, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.; Venkatesan, L.; Aadimoolam, R.; Tirunelveli, H.; Sriraman, R. Sequence Diversity of Internal Transcribed Spacer-1 (ITS-1) Region of Eimeria Infecting Chicken and Its Relevance in Species Identification from Indian Field Samples. Parasitol. Res. 2010, 106, 513–521. [Google Scholar] [CrossRef]
- Morgan, J.A.T.; Morris, G.M.; Wlodek, B.M.; Byrnes, R.; Jenner, M.; Constantinoiu, C.C.; Anderson, G.R. Real-Time Polymerase Chain Reaction (PCR) Assays for the Specific Detection and Quantification of Seven Eimeria Species That Cause Coccidiosis in Chickens. Mol. Cell. Probes 2009, 23, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, U.C.; Fraga, J.S.; Licois, D.; Pakandl, M.; Gruber, A. Development of Molecular Assays for the Identification of the 11 Eimeria Species of the Domestic Rabbit (Oryctolagus cuniculus). Vet. Parasitol. 2011, 176, 275–280. [Google Scholar] [CrossRef]
- Muñoz-Gómez, V.; Furrer, R.; Yin, J.; Shaw, A.P.M.; Rasmussen, P.; Torgerson, P.R. Prediction of Coccidiosis Prevalence in Extensive Backyard Chickens in Countries and Regions of the Horn of Africa. Vet. Parasitol. 2024, 327, 110143. [Google Scholar] [CrossRef]
- Blake, D.P. Eimeria of chickens: The changing face of an old foe. Avian Pathol. 2025, 54, 267–278. [Google Scholar] [CrossRef]
- Ola-Fadunsin, S.D. Investigations on the Occurrence and Associated Risk Factors of Avian Coccidiosis in Osun State, Nigeria. J. Parasitol. Res. 2017, 2017, 9264191. [Google Scholar] [CrossRef] [PubMed]


| Primes | Sequence (5′ → 3′) | Target Gene | Product Size (bp) |
|---|---|---|---|
| acer-F | AAGCATCATTGCCACCT | Eimeria acervulina AF446055 ITS-1 | 140 |
| acer-R | TGCCAGGGTCACATGT | ||
| acer-probe | FAM-CGGCGCATGCACCGCT-MGB | ||
| max-F | ATCATTGAATCCCTTTCA | Eimeria maxima AF446060 ITS-1 | 105 |
| max-R | ACCCTTCTAAAGAGC | ||
| max-probe | VIC-ATTAAGGACACAAACAATGCCTA-MGB | ||
| tene-F | TTATGAGAGGAGAAGACG | Eimeria tenella AF446074 ITS-1 | 111 |
| tene-R | AGACAGAACGCACACA | ||
| tene-probe | TXR-ATGCAGAGCGCTCGCGGCTC-MGB | ||
| nec-F | ACACAGTTTGTACGCCT | Eimeria necatrix AF446069 ITS-1 | 60 |
| nec-R | AAGCTGACGCTTGAAAC | ||
| nec-probe | CY5-AGAACGCGCTGCTGCTG-MGB |
| Pathogens | Primers | Sequence (5′ → 3′) | Product Size (bp) | Accession No. |
|---|---|---|---|---|
| Eimeria acervulina | F | ACGACGCATTTTTGTG | 214 | AF446055 |
| R | GCTATGGGTGCTCATC | |||
| Eimeriamaxima | F | AGAACTAGCCTAACCC | 138 | AF446060 |
| R | ATGCAAGAGGACATC | |||
| Eimeria tenella | F | GTGGAACCTCTCAAGA | 431 | AF446074 |
| R | TGATCCTGCGTTGTGA | |||
| Eimeria necatrix | F | AGTAGAAGAGCCTATCA | 292 | AF446069 |
| R | TCATTCACACAGTTTGTAC |
| Plasmid Standards | Concentration of Template (Copies/μL) | Intra-Coefficient of Variation | Inter-Coefficient of Variation | ||
|---|---|---|---|---|---|
| X ± SD | CV (%) | X ± SD | CV (%) | ||
| p-acer | 107 | 14.527 ± 0.270 | 1.86 | 14.680 ± 0.264 | 1.80 |
| 106 | 18.660 ± 0.235 | 1.26 | 18.630 ± 0.350 | 1.88 | |
| 105 | 22.776 ± 0.287 | 1.26 | 22.753 ± 0.371 | 1.63 | |
| p-max | 107 | 13.79 ± 0.199 | 1.45 | 13.833 ± 0.220 | 1.59 |
| 106 | 18.567 ± 0.255 | 1.38 | 18.500 ± 0.266 | 1.22 | |
| 105 | 23.247 ± 0.291 | 1.25 | 23.150 ± 0.260 | 1.13 | |
| p-ten | 107 | 21.547 ± 0.192 | 0.89 | 21.733 ± 0.380 | 1.75 |
| 106 | 25.573 ± 0.276 | 1.08 | 25.427 ± 0.387 | 1.52 | |
| 105 | 29.607 ± 0.248 | 0.84 | 30.073 ± 0.318 | 1.06 | |
| p-nec | 107 | 14.257 ± 0.261 | 1.83 | 14.543 ± 0.215 | 1.48 |
| 106 | 17.523 ± 0.163 | 0.93 | 17.556 ± 0.261 | 1.49 | |
| 105 | 22.827 ± 0.172 | 0.76 | 23.000 ± 0.209 | 0.91 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Chen, X.-L.; Wu, S.-H.; Cai, X.; Jiang, B.; You, W.; Zheng, M. Simultaneous Detection and Differentiation of Four Eimeria Species in Chickens (E. tenella, E. maxima, E. necatrix, and E. acervulina) Using a Multiplex TaqMan-MGB qPCR Assay. Animals 2025, 15, 2792. https://doi.org/10.3390/ani15192792
Lin L, Chen X-L, Wu S-H, Cai X, Jiang B, You W, Zheng M. Simultaneous Detection and Differentiation of Four Eimeria Species in Chickens (E. tenella, E. maxima, E. necatrix, and E. acervulina) Using a Multiplex TaqMan-MGB qPCR Assay. Animals. 2025; 15(19):2792. https://doi.org/10.3390/ani15192792
Chicago/Turabian StyleLin, Lin, Xiao-Li Chen, Sheng-Hui Wu, Xi Cai, Bin Jiang, Wei You, and Min Zheng. 2025. "Simultaneous Detection and Differentiation of Four Eimeria Species in Chickens (E. tenella, E. maxima, E. necatrix, and E. acervulina) Using a Multiplex TaqMan-MGB qPCR Assay" Animals 15, no. 19: 2792. https://doi.org/10.3390/ani15192792
APA StyleLin, L., Chen, X.-L., Wu, S.-H., Cai, X., Jiang, B., You, W., & Zheng, M. (2025). Simultaneous Detection and Differentiation of Four Eimeria Species in Chickens (E. tenella, E. maxima, E. necatrix, and E. acervulina) Using a Multiplex TaqMan-MGB qPCR Assay. Animals, 15(19), 2792. https://doi.org/10.3390/ani15192792






