Physiological and Behavioral Responses of Stabled Horses (Equus caballus) to Three Types of Environmental Enrichment
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Experimental Design
2.3. Dependent Variables
2.4. Statistical Analysis
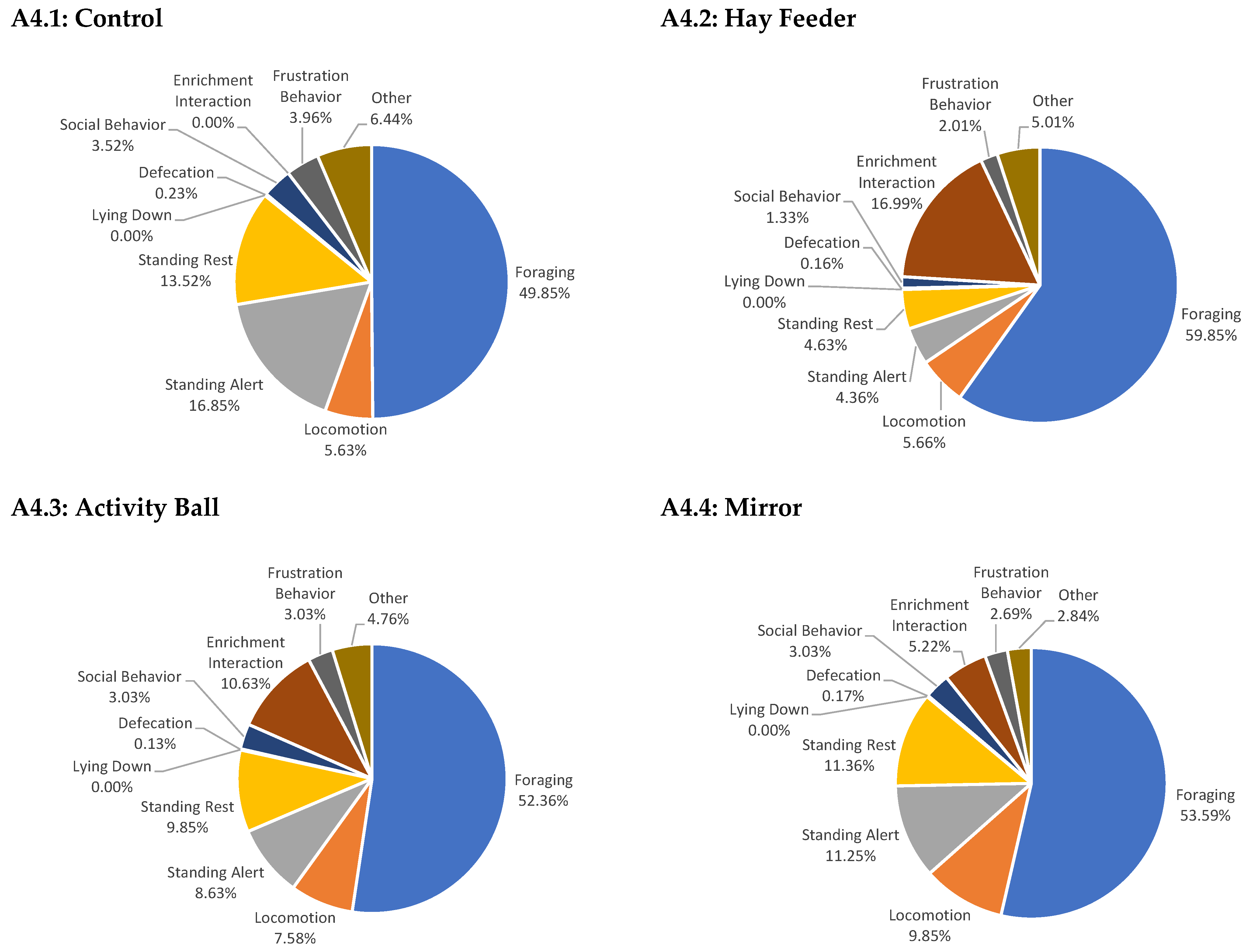
3. Results
3.1. Physiology
3.1.1. Heart Rate
3.1.2. Respiration Rate
3.2. Behavior
3.2.1. Enrichment Interaction
3.2.2. Foraging
3.2.3. Locomotion
3.2.4. Frustration Behavior
3.2.5. Other Behaviors
4. Discussion
4.1. Engagement with Enrichment
4.2. Physiological and Behavioral Effects
4.3. Time-of-Day Effects
4.4. Overall Evaluation
4.5. Other Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HF | Hay feeder |
| AB | Activity ball |
| MIR | Mirror |
| CON | Control |
Appendix A
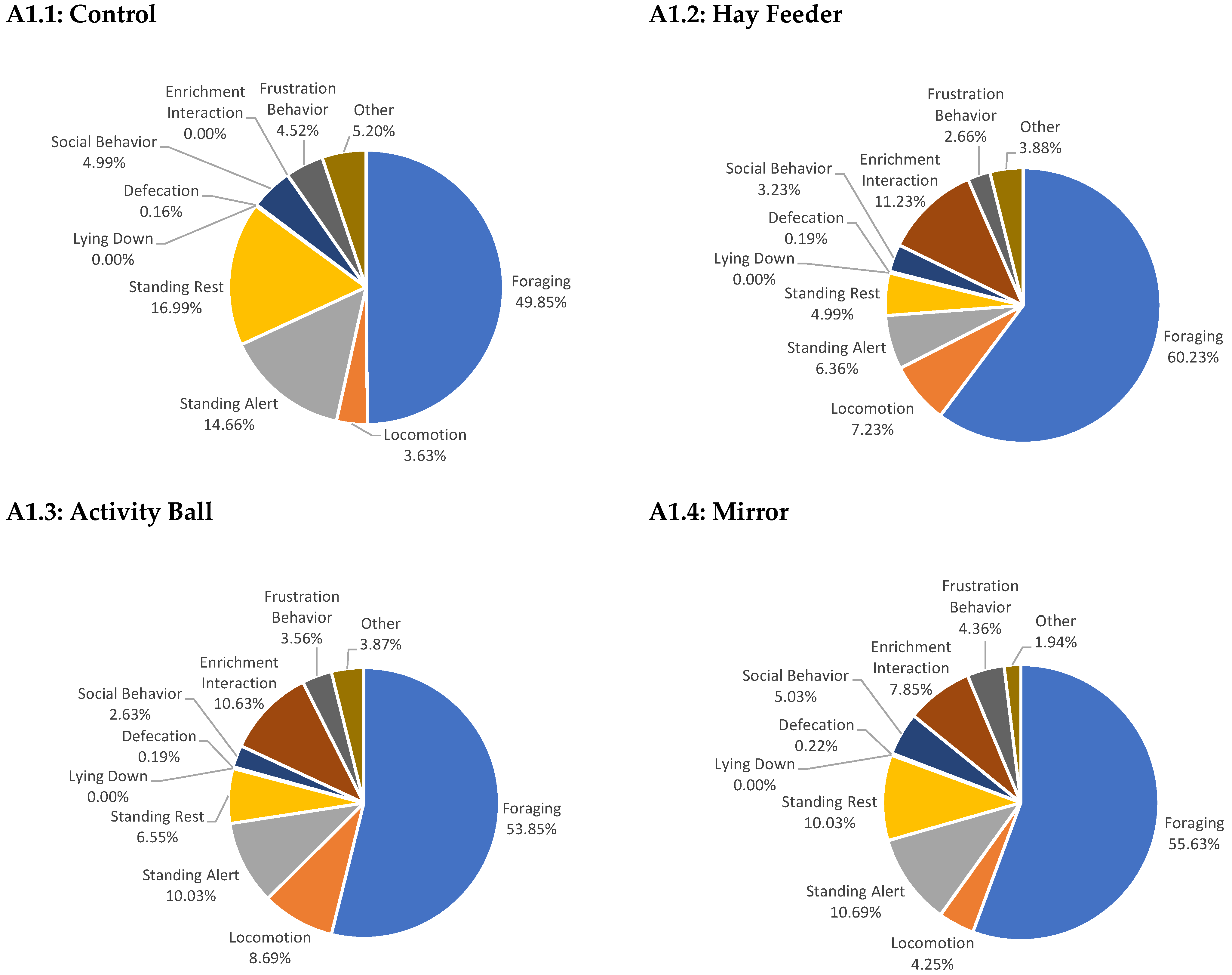
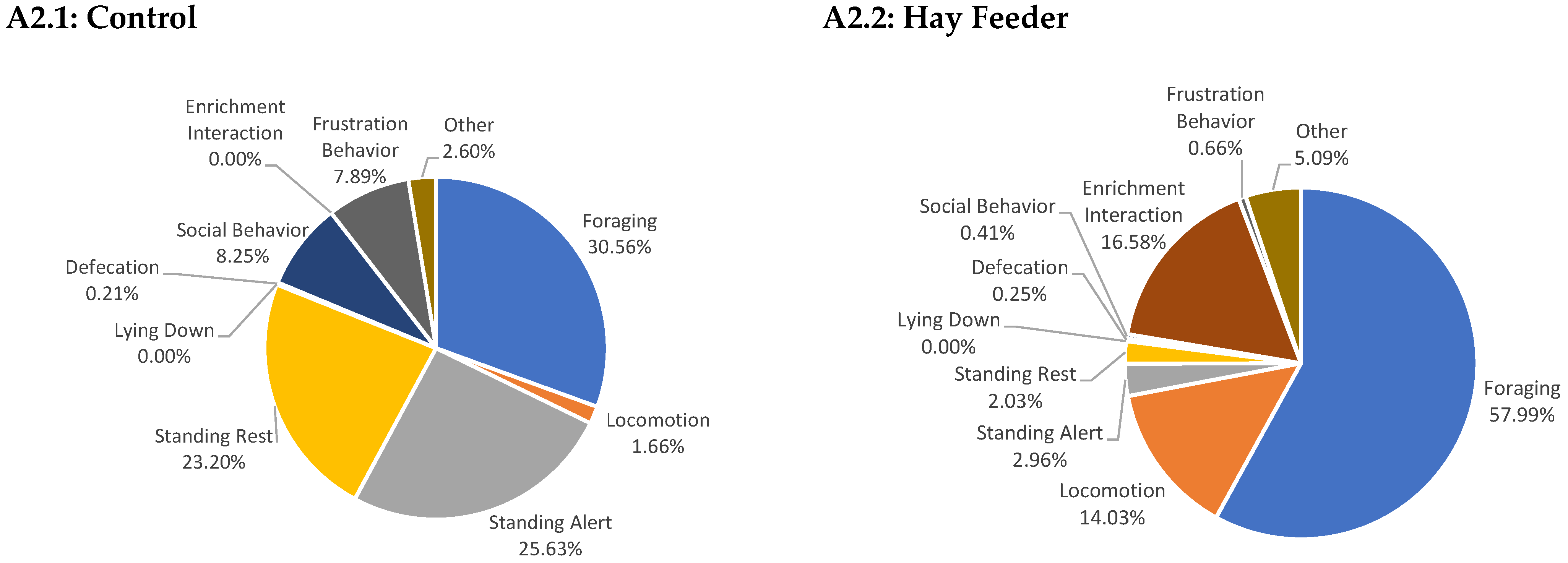
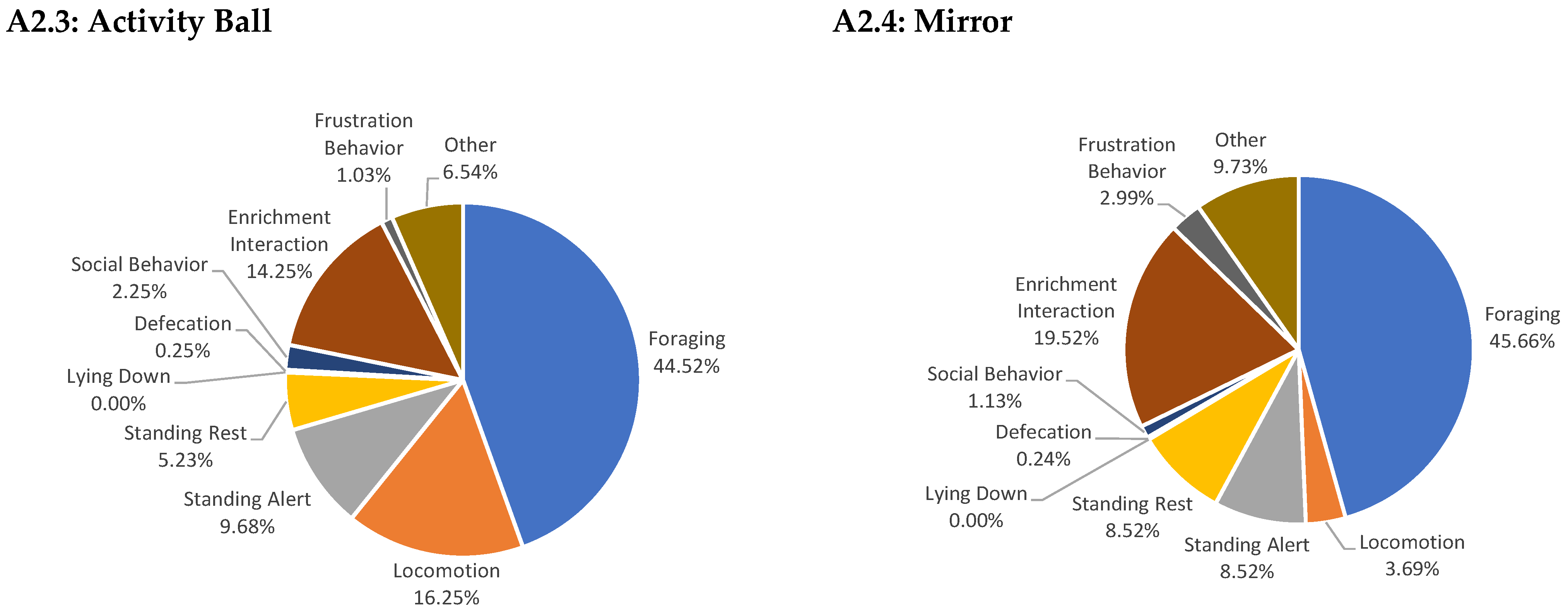
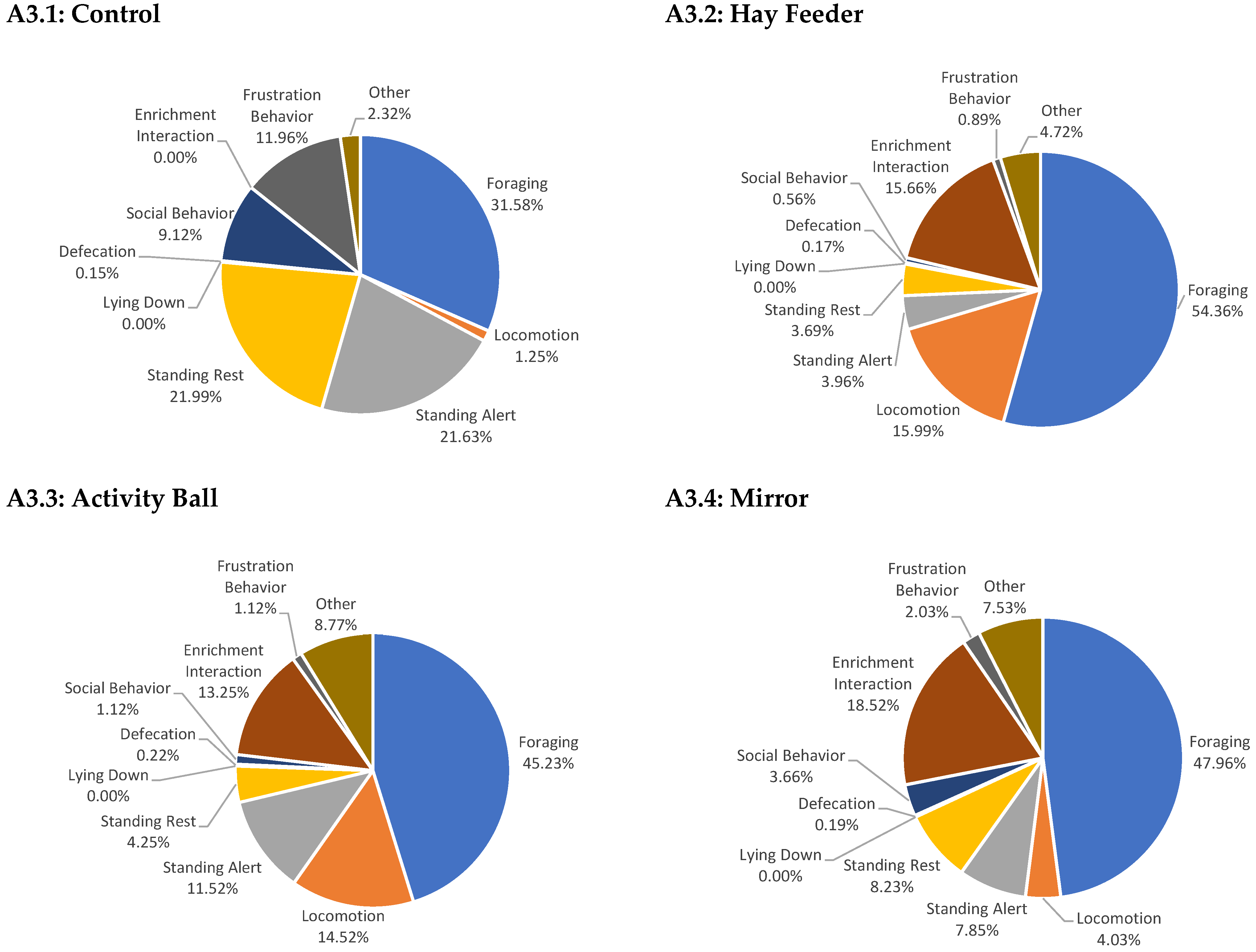
References
- Dawkins, M.S. Evolution and Animal Welfare. Q. Rev. Biol. 1998, 73, 305–328. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.; Weary, D.M.; Pajor, E.A.; Milligan, B.N. A scientific conception of animal welfare that reflects ethical concerns. Anim. Welf. 1997, 6, 187–205. [Google Scholar] [CrossRef]
- Fraser, D. Understanding animal welfare. Acta Vet. Scand. 2008, 50, S1. [Google Scholar] [CrossRef]
- Broom, D.M. Animal welfare: Concepts and measurement. J. Anim. Sci. 1991, 69, 4167–4175. [Google Scholar] [CrossRef]
- McPhee, M.E.; Carlstead, K. The importance of maintaining natural behaviors in captive mammals. In Wild Mammals in Captivity: Principles and Techniques for Zoo Management, 2nd ed.; Kleiman, D., Thompson, K., Baer, K., Eds.; University of Chicago Press: Chicago, IL, USA, 2010; pp. 303–313. [Google Scholar]
- Jørgensen, G.H.M.; Bøe, K.E. A note on the effect of daily exercise and paddock size on the behaviour of domestic horses (Equus caballus). Appl. Anim. Behav. Sci. 2007, 107, 166–173. [Google Scholar] [CrossRef]
- Hemsworth, L.M.; Jongman, E.C.; Coleman, G.J. The human-horse relationship: Identifying the antecedents of horse owner attitudes towards horse husbandry and management behaviour. Animals 2021, 11, 278. [Google Scholar] [CrossRef]
- Martinson, K.; Hathaway, M.; Wilson, J.H.; Gilkerson, B.; Peterson, P.R.; Del Vecchio, R. University of Minnesota horse owner survey: Building an equine extension program. J. Ext. 2006, 44, 6RIB4. Available online: https://open.clemson.edu/joe/vol44/iss6/13/ (accessed on 7 April 2023).
- Ross, M.; Proudfoot, K.; Merkies, K.; Elsohaby, I.; Mills, M.; Macmillan, K.; Mckenna, S.; Ritter, C. Horse housing on Prince Edward Island, Canada: Attitudes and experiences related to keeping horses outdoors and in groups. Animals 2023, 13, 275. [Google Scholar] [CrossRef]
- Jørgensen, G.H.M.; Bøe, K.E. Individual paddocks versus social enclosure for horses. EAAP Sci. Ser. 2007, 122, 79–83. [Google Scholar] [CrossRef]
- Lewis, H.E. The Basics of Horse Stall Design. Stable Management. 2021. Available online: https://stablemanagement.com/barns-grounds/the-basics-of-horse-stall-design/ (accessed on 24 October 2023).
- Hotchkiss, J.W.; Reid, S.W.J.; Christley, R.M. A survey of horse owners in Great Britain regarding horses in their care. Part 1: Horse demographic characteristics and management. Equine Vet. J. 2007, 39, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.V. Don’t fence me in: Managing psychological well-being for elite performance horses. J. Appl. Anim. Welf. Sci. 2007, 10, 309–329. [Google Scholar] [CrossRef]
- Werhahn, H.; Hessel, E.F.; Van den Weghe, H.F.A. Competition horses housed in single stalls (II): Effects of free exercise on the behavior in the stable, the behavior during training, and the degree of stress. J. Equine Vet. Sci. 2012, 32, 22–31. [Google Scholar] [CrossRef]
- McGreevy, P. Equine Behavior: A Guide for Veterinarians and Equine Scientists; Saunders Ltd.: Uckfield, UK, 2004. [Google Scholar]
- Goodwin, D. Horse behaviour: Evolution, domestication and feralisation. In The Welfare of Horses; Waran, N., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 1–18. [Google Scholar]
- Boyd, L.E.; Carbonaro, D.A.; Houpt, K.A. The 24-hour time budget of Przewalski horses. Appl. Anim. Behav. Sci. 1988, 21, 5–17. [Google Scholar] [CrossRef]
- Ransom, J.I.; Cade, B.S. Quantifying equid behavior: A research ethogram for free-roaming feral horses. In US Geological Survey Techniques and Methods 2-A9; United States Geological Survey: Reston, VA, USA, 2009; p. 23. [Google Scholar] [CrossRef]
- Ogilvie-Graham, T.S. Time Budget Studies in Stalled Horses. Ph.D. Dissertation, University of Edinburgh. Edinburgh Research Archive, Edinburgh, UK, 1994. Available online: http://hdl.handle.net/1842/29924 (accessed on 27 October 2023).
- Gellman, K.; Ruina, A. Standing horse posture: A longer stance is more stable. Biol. Open 2022, 11, bio059139. [Google Scholar] [CrossRef]
- Harris, P.A. How understanding the digestive process can help minimise digestive disturbances due to diet and feeding practices. In Proceedings of the BEVA Specialist Days on Nutrition and Behaviour; Harris, P.A., Gomarsall, G., Davidson, H.P.B., Green, R., Eds.; Equine Veterinary Journal Ltd.: Fordham, UK, 1999; pp. 45–50. [Google Scholar]
- Campbell-Thompson, M.L.; Merritt, A.M. Effect of ranitidine on gastric acid secretion in young male horses. Am. J. Vet. Res. 1987, 48, 1511–1515. [Google Scholar] [CrossRef]
- McClure, S.R. Equine Gastric Ulcers: Special Care and Nutrition. American Association of Equine Practitioners. 2016. Available online: https://aaep.org/horsehealth/equine-gastric-ulcers-special-care-and-nutrition (accessed on 23 October 2023).
- Orsini, J. Gastric ulceration in the mature horse: A review. Equine Vet. Educ. 2000, 12, 24–27. [Google Scholar] [CrossRef]
- Murray, M.J.; Schusser, G. Application of gastric pH-metry in horses: Measurement of 24 hour gastric pH in horses fed, fasted, and treated with ranitidine. J. Vet. Intern. Med. 1993, 6, 133. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Cripps, P.J.; French, N.P.; Green, L.E.; Nicol, C.J. Management factors associated with stereotypic and redirected behaviour in the thoroughbred horse. Equine Vet. J. 1995, 27, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.J.; McGreevy, P.D. Stereotypic behavior in the stabled horse: Cause, effects, and prevention without compromising horse welfare. In The Welfare of Horses; Waran, N., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 99–124. [Google Scholar]
- McBride, S.D.; Hemmings, A. A neurologic perspective of equine stereotypy. J. Equine Vet. Sci. 2009, 29, 10–16. [Google Scholar] [CrossRef]
- Newberry, R.C. Environmental enrichment: Increasing the biological relevance of captive environments. Appl. Anim. Behav. Sci. 1995, 44, 229–243. [Google Scholar] [CrossRef]
- Shepherdson, D. Tracing the path of environmental enrichment in zoos. In Second Nature: Environmental Enrichment for Captive Animals; Shepherdson, D., Mellen, J.D., Hutchins, M., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1998; pp. 1–13. [Google Scholar]
- Taylor, P.S.; Schrobback, P.; Verdon, M.; Lee, C. An effective environmental enrichment framework for the continual improvement of production animal welfare. Anim. Welf. 2022, 32, e14. [Google Scholar] [CrossRef] [PubMed]
- Alligood, C.; Leighty, K. Putting the “E” in SPIDER: Evolving trends in the evaluation of environmental enrichment efficacy in zoological settings. Anim. Behav. Cogn. 2015, 2, 200–217. [Google Scholar] [CrossRef]
- Mellen, J.; MacPhee, M.S. Philosophy of environmental enrichment: Past, present, and future. Zoo Biol. 2001, 20, 211–226. [Google Scholar] [CrossRef]
- Decker, S.; Lavery, J.M.; Mason, G.J. Don’t use it? Don’t lose it! Why active use if not required for stimuli, resources or “enrichments” to have welfare value. Zoo Biol. 2023, 42, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A. A circumplex model of affect. J. Personal. Soc. Psychol. 1980, 39, 1161–1178. [Google Scholar] [CrossRef]
- Endler, N.S.; Edwards, J.M.; Vitelli, R.; Parker, J.D.A. Assessment of state and trait anxiety: Endler multidimensional anxiety scales. Anxiety Res. 1989, 2, 1–14. [Google Scholar] [CrossRef]
- Horback, K.M.; Parsons, T.D. Judgement bias testing in group-housed gestating sows. Behav. Process. 2019, 159, 86–92. [Google Scholar] [CrossRef]
- Leiner, L.; Fendt, M. Behavioural fear and heart rate responses of horses after exposure to novel objects: Effects of habituation. Appl. Anim. Behav. Sci. 2011, 131, 104–109. [Google Scholar] [CrossRef]
- Benhajali, H.; Richard-Yris, M.A.; Ezzaouia, M.; Charfi, F.; Hausberger, M. Foraging opportunity: A crucial criterion for horse welfare? Animal 2009, 3, 1308–1312. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.D.; Redgate, S.; Zinchenko, S.; Owen, H.; Barfoot, C.; Harris, P. The effect of presenting forage in multi-layered haynets and at multiple sites on night time budgets of stabled horses. Appl. Anim. Behav. Sci. 2015, 171, 108–116. [Google Scholar] [CrossRef]
- Goodwin, D.; Davidson, H.P.B.; Harris, P. Foraging enrichment for stabled horses: Effects on behaviour and selection. Equine Vet. J. 2002, 34, 686–691. [Google Scholar] [CrossRef]
- Henderson, J.V.; Waran, N.K. Reducing equine stereotypies using an EquiballTM. Anim. Welf. 2001, 10, 73–80. [Google Scholar] [CrossRef]
- Jørgensen, G.H.M.; Liestøl, S.H.O.; Bøe, K.E. Effects of enrichment items on activity and social interactions in domestic horses (Equus caballus). Appl. Anim. Behav. Sci. 2011, 129, 100–110. [Google Scholar] [CrossRef]
- Kenney, T. The Effects of Enrichment Items on Cribbing in Equus caballus. Bachelor’s Thesis, Alfred University. AURA: Alfred University Research & Archives, Alfred, NY, USA, 2018. Available online: http://hdl.handle.net/10829/8208 (accessed on 6 October 2021).
- Rochais, C.; Henry, S.; Hausberger, M. “Hay-bags” and “slow feeders”: Testing their impact on horse behaviour and welfare. Appl. Anim. Behav. Sci. 2018, 198, 52–59. [Google Scholar] [CrossRef]
- Thorne, J.; Goodwin, D.; Kennedy, M.; Davidson, H.; Harris, P. Foraging enrichment for individually housed horses: Practicality and effects on behaviour. Appl. Anim. Behav. Sci. 2005, 94, 149–164. [Google Scholar] [CrossRef]
- Winskill, L.C.; Waran, N.K.; Young, R.J. The effect of a foraging device (a modified ‘Edinburgh Foodball’) on the behaviour of the stabled horse. Appl. Anim. Behav. Sci. 1996, 48, 25–35. [Google Scholar] [CrossRef]
- Grime, E.; Northrop, A.; Rosbotham, M.E.; Howells, K.L. The effect of mirrors for the control of stereotypic weaving in the stabled horse. BSAP Occas. Publ. 2004, 32, 185–186. [Google Scholar] [CrossRef]
- McAfee, L.M.; Mills, D.S.; Cooper, J.J. The use of mirrors for the control of stereotypic weaving behaviour in the stabled horse. Appl. Anim. Behav. Sci. 2002, 78, 159–173. [Google Scholar] [CrossRef]
- Mills, D.; Davenport, K. The effect of a neighbouring conspecific versus the use of a mirror for the control of stereotypic weaving behaviour in the stabled horse. Anim. Sci. 2002, 74, 95–101. [Google Scholar] [CrossRef]
- Bulens, A.; Dams, A.; Beirendonck, S.V.; Thielen, J.V.; Driessen, B. A preliminary study on the long-term interest of horses in ropes and Jolly Balls. J. Vet. Behav. 2015, 10, 83–86. [Google Scholar] [CrossRef]
- Bulens, A.; Beirendonck, S.V.; Thielen, J.V.; Driessen, B. The enriching effect of non-commercial items in stabled horses. Appl. Anim. Behav. Sci. 2013, 143, 46–51. [Google Scholar] [CrossRef]
- Huo, X.; Yaemklang, S.; Pimmai, P.; Kupittayanant, P.; Na-Lampang, P. A preliminary study of the effects of enrichment on stereotypic and non-stereotypic horses. Vet. Integr. Sci. 2021, 19, 581–590. [Google Scholar] [CrossRef]
- Kędzierski, W.; Janczarek, I.; Stachurska, A.; Wilk, I. Comparison of effects of different relaxing massage frequencies and different music hours on reducing stress level in race horses. J. Equine Vet. Sci. 2017, 53, 100–107. [Google Scholar] [CrossRef]
- Kędzierski, W.; Janczarek, I.; Stachurska, A.; Wilk, I. Massage or music meant to be relaxing, result in lowering salivary cortisol concentration in race horses. Pferdeheilkunde Equine Med. 2017, 33, 146–151. [Google Scholar] [CrossRef]
- Neveux, C.; Ferard, M.; Dickel, L.; Bouet, V.; Petit, O.; Valenchon, M. Classical music reduces acute stress of domestic horses. J. Vet. Behav. 2016, 15, 81. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Janczarek, I.; Wilk, I.; Wnuk-Pawlak, E. Use of music therapy in aiding the relaxation of geriatric horses. J. Equine Vet. Sci. 2019, 78, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef]
- World Weather. Weather in Davis (California) in April 2022. April 2022. Available online: https://world-weather.info/forecast/usa/davis/april-2022/ (accessed on 23 July 2023).
- Protequus. Nightwatch® Smart Halter™ [Equipment and Software]; Protequus: Austin, TX, USA, 2021; Available online: https://smarthalter.com (accessed on 4 April 2022).
- Friard, O.; Gamba, M. Behavioral Observation Research Interactive Software, BORIS; Version 7.13.6. [Computer Software]. Protequus: Austin, TX, USA, 2022. Available online: https://www.boris.unito.it/ (accessed on 8 July 2022).
- Waring, G.H. Horse Behavior; Noyes Publications/William Andrew Publishing: Norwich, NY, USA, 2003. [Google Scholar]
- Hox, J.J.; Moerbeek, M.; Kluytmans, A.; van de Schoot, R. Analyzing indirect effects in cluster randomized trials. The effect of estimation method, number of groups and group sizes on accuracy and power. Front. Psychol. 2014, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Song, X.Y. Evaluation of the Bayesian and maximum likelihood approaches in analyzing structural equation models with small sample sizes. Multivar. Behav. Res. 2004, 39, 653–686. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 3.3.1. [Computer Software]. R Foundation for Statistical Computing: Vienna, Austria, 2013. Available online: http://www.R-project.org/ (accessed on 26 May 2023).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. J. Math. Methods Biosci. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- European Respiratory Society. Your lungs and exercise. Breathe 2016, 12, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Art, T.; Desmecht, D.; Amory, H.; Lekeux, P. Synchronization of locomotion and respiration in trotting ponies. J. Vet. Med. 1990, 37, 97–103. [Google Scholar] [CrossRef]
- Franklin, S.H.; Van Erck-Westergren, E.; Bayly, W.M. Respiratory responses to exercise in the horse. Equine Vet. J. 2012, 44, 726–732. [Google Scholar] [CrossRef]
- Safryghin, A.; Hebesberger, D.V.; Wascher, C.A. Testing for behavioral and physiological responses of domestic horses (Equus caballus) across different contexts–consistency over time and effects of context. Front. Psychol. 2019, 10, 849. [Google Scholar] [CrossRef]
- Baumgartner, M.; Boisson, T.; Erhard, M.H.; Zeitler-Feicht, M.H. Common feeding practices pose a risk to the welfare of horses when kept on non-edible bedding. Animals 2020, 10, 411. [Google Scholar] [CrossRef]
- Garner, J.P. Stereotypies and other abnormal repetitive behaviors: Potential impact on validity, reliability, and replicability of scientific outcomes. Inst. Lab. Anim. Res. J. 2005, 46, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, G.H.M.; Liestøl, S.H.O.; Bøe, K.E. Use of different items of “enrichment” for individual and group kept horses. J. Vet. Behav. 2010, 5, 216. [Google Scholar] [CrossRef]
- Browning, H. The natural behavior debate: Two conceptions of animal welfare. J. Appl. Anim. Welf. Sci. 2020, 23, 325–337. [Google Scholar] [CrossRef]
- Gomez, P.; Stahel, W.A.; Danuser, B. Respiratory responses during affective picture viewing. Biol. Psychol. 2004, 67, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Stachurska, A.; Janczarek, I.; Wilk, I.; Kędzierski, W. Does music influence emotional state in race horses? J. Equine Vet. Sci. 2015, 35, 650–656. [Google Scholar] [CrossRef]
| Item | Color | Size | |
|---|---|---|---|
 | Hay feeder: High Country Plastics (Caldwell, ID, USA) Hay Play™ Slow-feeding forage ball | Red | 16 in. diameter |
 | Activity ball: Horsemen’s Pride (Streetsboro, OH, USA) mega ball | Red | 25 in. diameter |
 | Mirror: Marketing Holders (Mims, FL, USA) acrylic mirror sheet | N/a | 24 in. × 24 in. |
| Behavior | Description |
|---|---|
| Foraging | The horse is searching for and/or grazing/chewing/eating food that did not directly come from the hay feeder enrichment item; the horse may be moving a around the stall with its head below the withers and its muzzle close to the ground or shifting through the substrate, and may or may not be actively chewing; or the horse may be standing stationary with its head either below or above the withers and is chewing food. |
| Locomotion | The horse has taken at least two footfalls or more forward or backward, at any pace, and is moving around the stall without interacting with the enrichment item. |
| Frustration behavior | The horse expresses frustration in one of the following ways:
|
| Enrichment interaction | The horse is interacting with the enrichment item in one of the following ways:
|
| Other | Any behavior not otherwise listed (e.g., drinking, urinating, grooming, and rolling). |
| Out of view | The horse’s head is lowered and completely hidden from view behind the stall wall and its behavior is unable to be accurately recorded. |
| Time | Hay Feeder (HF) | Activity Ball (AB) | Mirror (MIR) | Control (CON) | p-Value (HF vs. AB) | p-Value (HF vs. MIR) | p-Value (HF vs. CON) | p-Value (AB vs. MIR) | p-Value (AB vs. CON) | p-Value (MIR vs. CON) |
|---|---|---|---|---|---|---|---|---|---|---|
| 08:00 h | 70.58 ± 3.52 A | 53.58 ± 3.22 B | 64.25 ± 5.06 Bx | 29.52 ± 3.88 C | 0.028 | 0.028 | 0.027 | - | 0.027 | 0.022 |
| 12:00 h | 71.96 ± 4.25 A | 62.52 ± 4.25 A | 55.85 ± 6.52 Bx | 35.56 ± 4.58 C | - | 0.036 | 0.022 | 0.029 | 0.028 | 0.019 |
| 16:00 h | 65.58 ± 6.52 A | 54.22 ± 4.20 B | 53.41 ± 4.58 Bx | 37.89 ± 4.02 C | 0.032 | 0.032 | 0.036 | - | 0.027 | 0.024 |
| 20:00 h | 75.85 ± 3.25 A | 46.52 ± 3.85 B | 42.52 ± 5.52 By | 30.22 ± 3.99 C | 0.039 | 0.041 | 0.038 | - | 0.031 | 0.036 |
| Time | Hay Feeder (HF) | Activity Ball (AB) | Mirror (MIR) | Control (CON) | p-Value (HF vs. AB) | p-Value (HF vs. MIR) | p-Value (HF vs. CON) | p-Value (AB vs. MIR) | p-Value (AB vs. CON) | p-Value (MIR vs. CON) |
|---|---|---|---|---|---|---|---|---|---|---|
| 08:00 h | 60.23 ± 5.25 A | 53.85 ± 3.89 A | 55.63 ± 6.03 A | 49.85 ± 5.63 Bx | - | - | 0.035 | - | 0.038 | 0.041 |
| 12:00 h | 57.99 ± 4.89 A | 44.52 ± 4.25 B | 45.66 ± 4.25 B | 30.56 ± 4.58 Cy | 0.036 | 0.029 | 0.029 | - | 0.036 | 0.032 |
| 16:00 h | 54.36 ± 3.55 A | 45.23 ± 5.12 A | 47.96 ± 7.52 A | 31.58 ± 6.99 By | - | - | 0.025 | - | 0.023 | 0.039 |
| 20:00 h | 59.85 ± 5.63 | 52.36 ± 6.63 | 53.59 ± 2.36 | 49.85 ± 6.87 x | - | - | - | - | - | - |
| Time | Hay Feeder (HF) | Activity Ball (AB) | Mirror (MIR) | Control (CON) | p-Value (HF vs. AB) | p-Value (HF vs. MIR) | p-Value (HF vs. CON) | p-Value (AB vs. MIR) | p-Value (AB vs. CON) | p-Value (MIR vs. CON) |
|---|---|---|---|---|---|---|---|---|---|---|
| 08:00 h | 7.23 ± 1.03 Ay | 8.69 ± 1.56 Ay | 4.25 ± 1.01 B | 3.63 ± 1.03 B | - | 0.039 | 0.031 | 0.043 | 0.019 | - |
| 12:00 h | 14.03 ± 2.96 Ax | 16.25 ± 2.52 Ax | 3.69 ± 0.96 B | 1.66 ± 0.69 B | - | 0.026 | 0.042 | 0.023 | 0.031 | - |
| 16:00 h | 15.99 ± 3.66 Ax | 14.52 ± 3.33 Ax | 4.03 ± 1.12 B | 1.25 ± 0.23 B | - | 0.032 | 0.025 | 0.037 | 0.036 | - |
| 20:00 h | 5.66 ± 1.03 y | 7.58 ± 1.01 y | 9.85 ± 1.33 | 5.63 ± 1.03 | - | - | - | - | - | - |
| Time | Hay Feeder (HF) | Activity Ball (AB) | Mirror (MIR) | Control (CON) | p-Value (HF vs. AB) | p-Value (HF vs. MIR) | p-Value (HF vs. CON) | p-Value (AB vs. MIR) | p-Value (AB vs. CON) | p-Value (MIR vs. CON) |
|---|---|---|---|---|---|---|---|---|---|---|
| 08:00 h | 2.66 ± 0.23 | 3.56 ± 0.22 | 4.36 ± 1.01 | 4.52 ± 1.12 | - | - | - | - | - | - |
| 12:00 h | 0.66 ± 0.06 B | 1.03 ± 0.12 B | 2.99 ± 0.63 B | 7.89 ± 1.13 A | - | - | 0.035 | - | 0.041 | 0.027 |
| 16:00 h | 0.89 ± 0.09 B | 1.12 ± 0.13 B | 2.03 ± 0.16 B | 11.96 ± 1.69 A | - | - | 0.031 | - | 0.041 | 0.043 |
| 20:00 h | 2.01 ± 0.63 | 3.03 ± 0.96 | 2.69 ± 0.25 | 3.96 ± 0.98 | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brauns, M.; Ali, A.; Berger, J.; McLean, A. Physiological and Behavioral Responses of Stabled Horses (Equus caballus) to Three Types of Environmental Enrichment. Animals 2025, 15, 2779. https://doi.org/10.3390/ani15192779
Brauns M, Ali A, Berger J, McLean A. Physiological and Behavioral Responses of Stabled Horses (Equus caballus) to Three Types of Environmental Enrichment. Animals. 2025; 15(19):2779. https://doi.org/10.3390/ani15192779
Chicago/Turabian StyleBrauns, Miranda, Ahmed Ali, Jeannine Berger, and Amy McLean. 2025. "Physiological and Behavioral Responses of Stabled Horses (Equus caballus) to Three Types of Environmental Enrichment" Animals 15, no. 19: 2779. https://doi.org/10.3390/ani15192779
APA StyleBrauns, M., Ali, A., Berger, J., & McLean, A. (2025). Physiological and Behavioral Responses of Stabled Horses (Equus caballus) to Three Types of Environmental Enrichment. Animals, 15(19), 2779. https://doi.org/10.3390/ani15192779






