Saccharomyces cerevisiae Fermentation-Derived Postbiotics Supplementation to Dairy Calves: Effects on Growth, Metabolism, Immune Status and Preliminary First Lactation Outcomes
Simple Summary
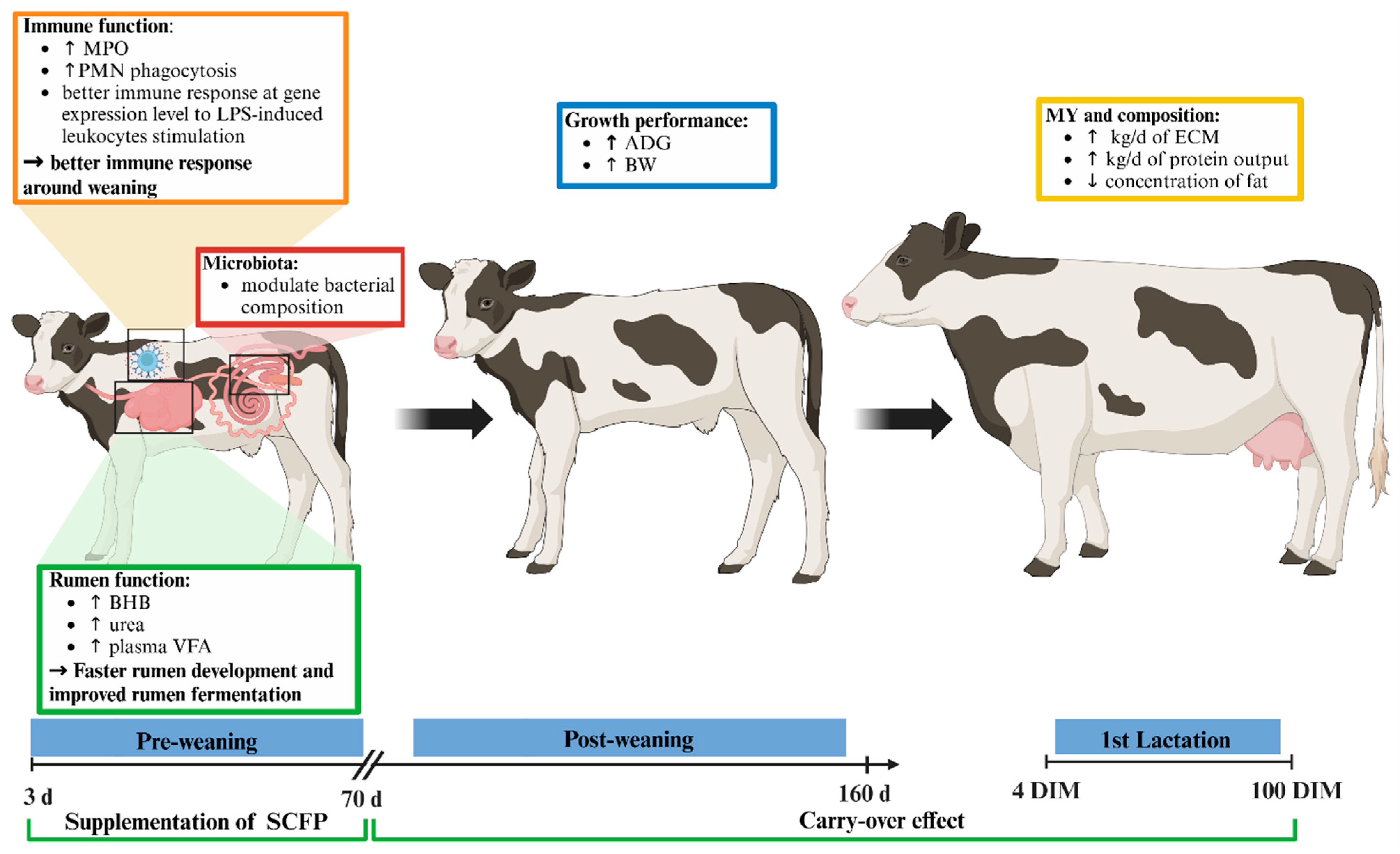
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Management and Experimental Design
2.2. Health Status, Feed Intake and Body Measurements
2.3. Blood Samples and Analysis
2.4. Blood Leucocytes Gene Expression After Whole-Blood Ex Vivo LPS Challenge
2.5. Phagocytosis Capacity of Polymorphonuclear Neutrophils (PMN)
2.6. Fecal and Rumen Samples
2.7. Gas Chromatography for VFA Analysis
2.8. Fecal DNA Extraction, 16S rRNA Gene Amplification, and Illumina Sequencing
2.9. Management of Heifers in Their Post-Experiment and First Lactation
2.10. Statistical Analysis
3. Results
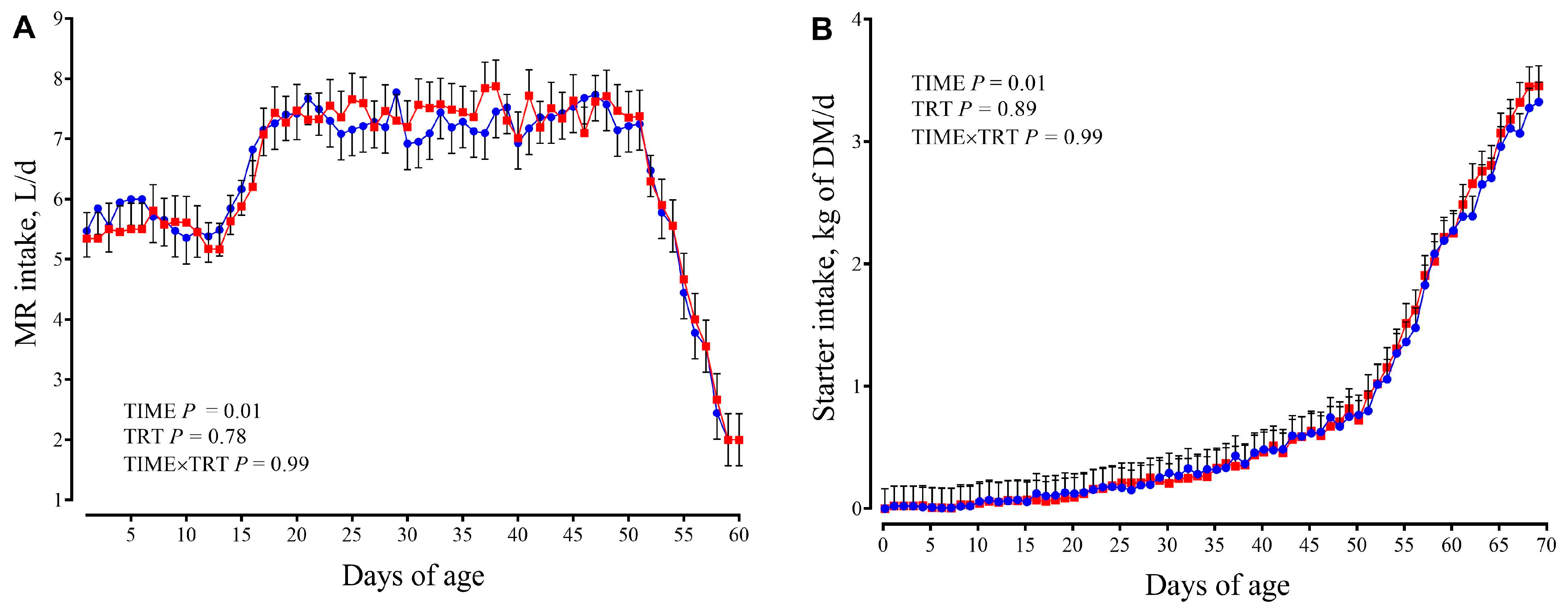
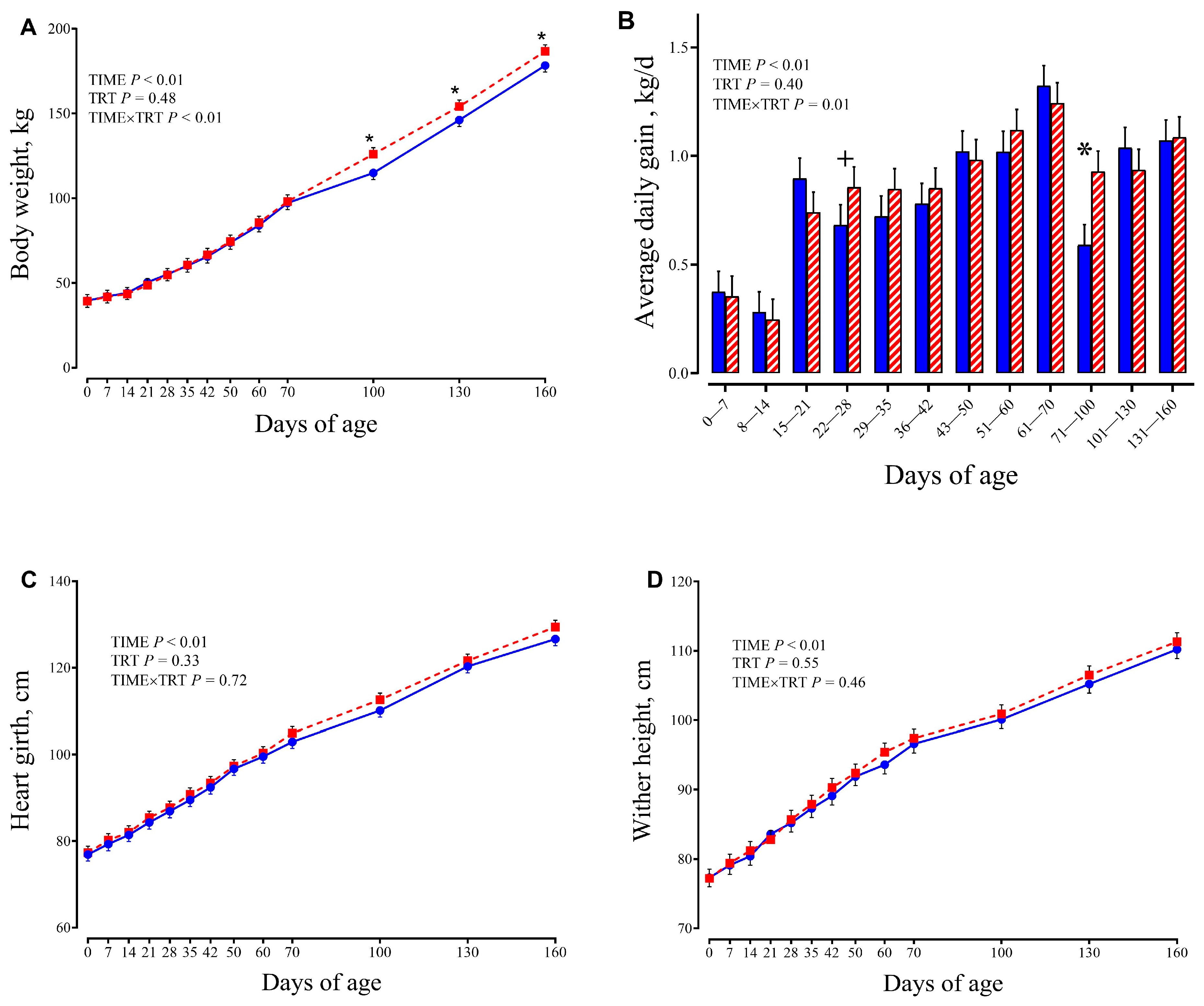
3.1. Feed Intake and Growth Performance
3.2. Blood Metabolites
3.3. Volatile Fatty Acids in Plasma
3.4. White Blood Cell Differential Count
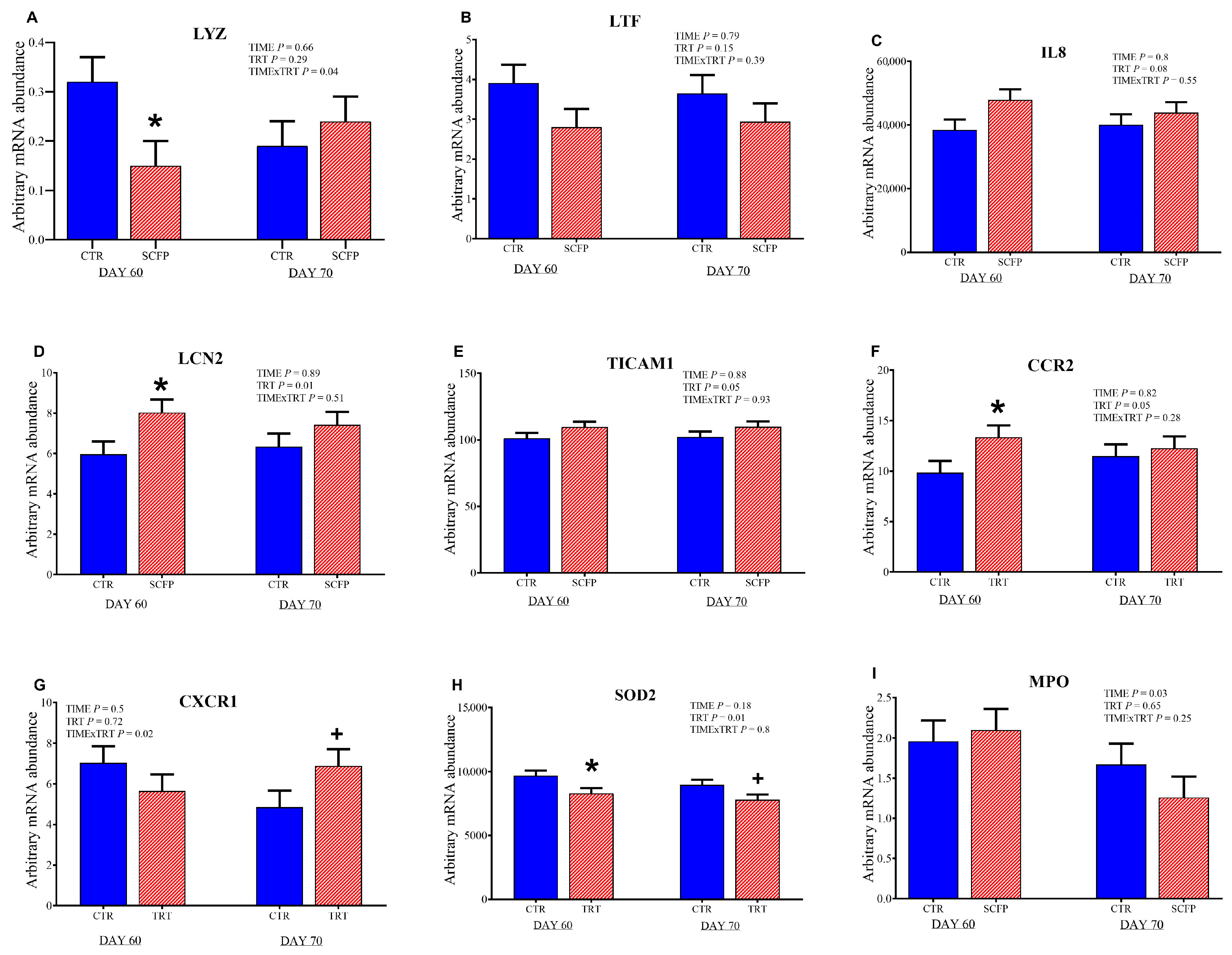
3.5. Gene Expression of Blood Leucocytes After Whole-Blood Ex Vivo LPS Challenge
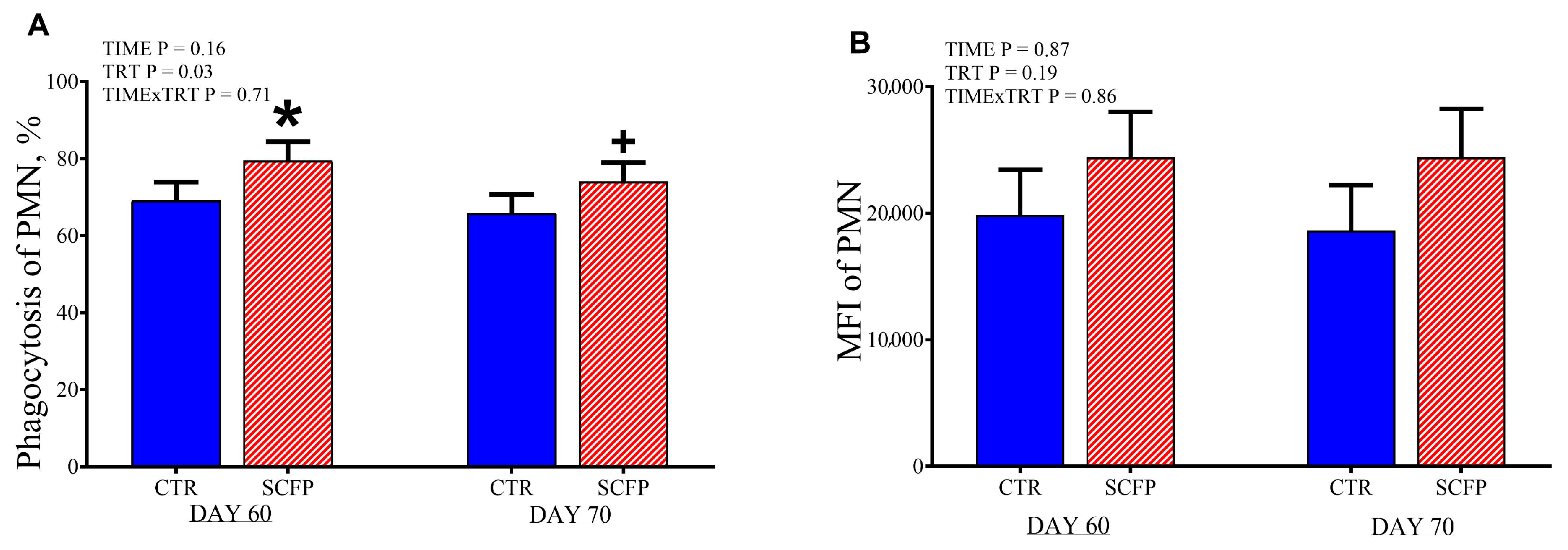
3.6. Phagocytosis Capacity of PMN
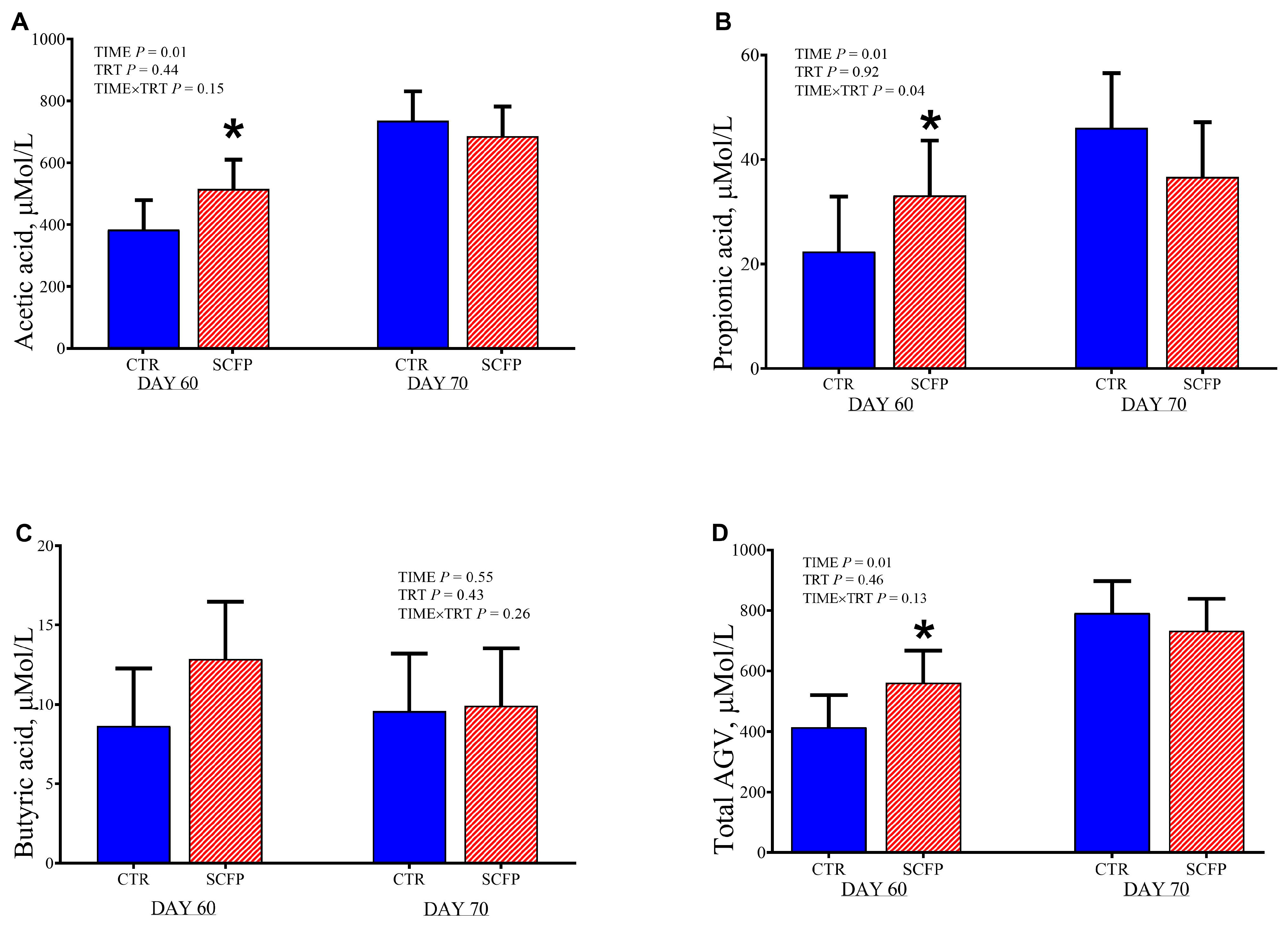
3.7. Fecal and Rumen Fluid Analysis
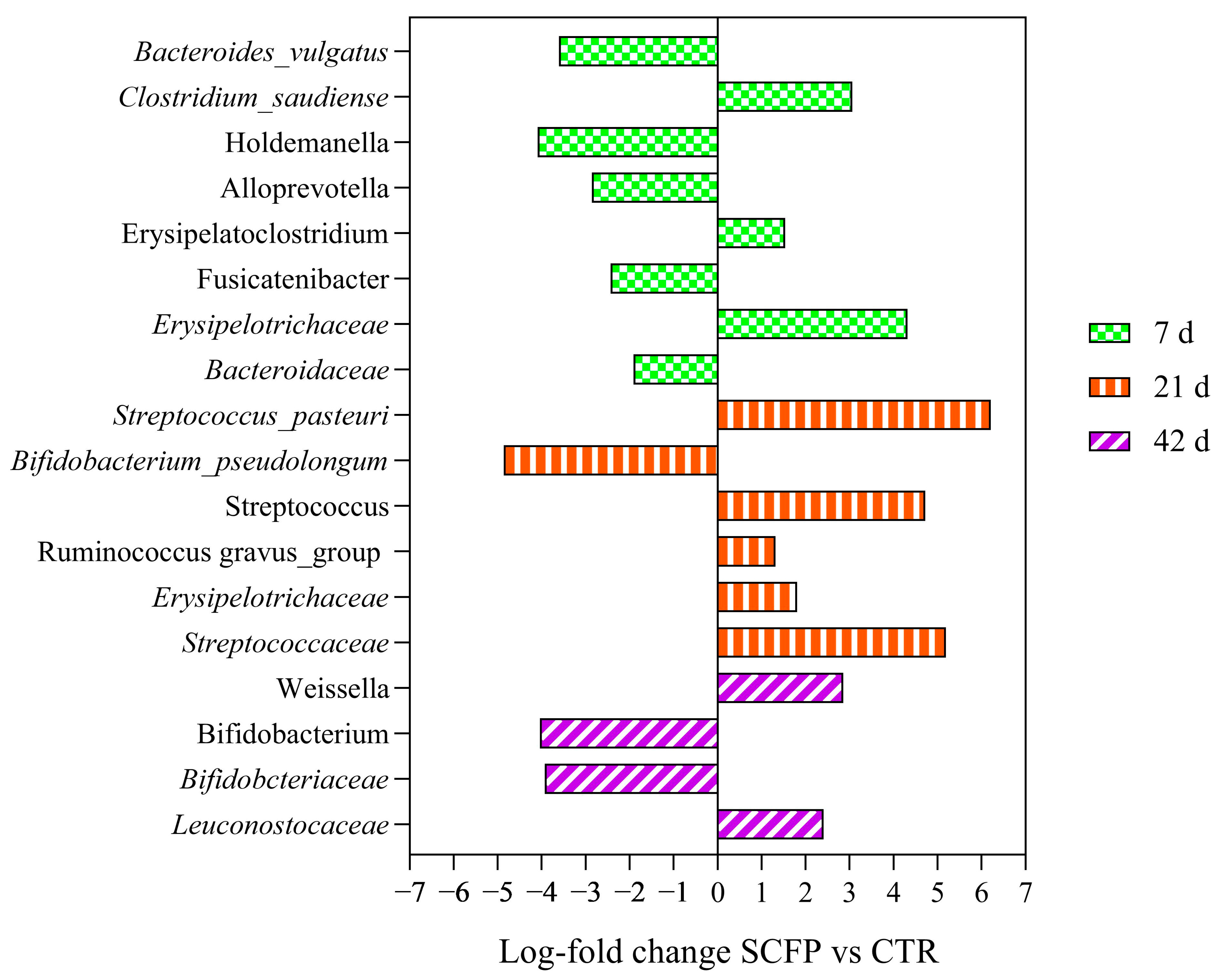
3.8. Metagenomic Analysis of Fecal Microbiota
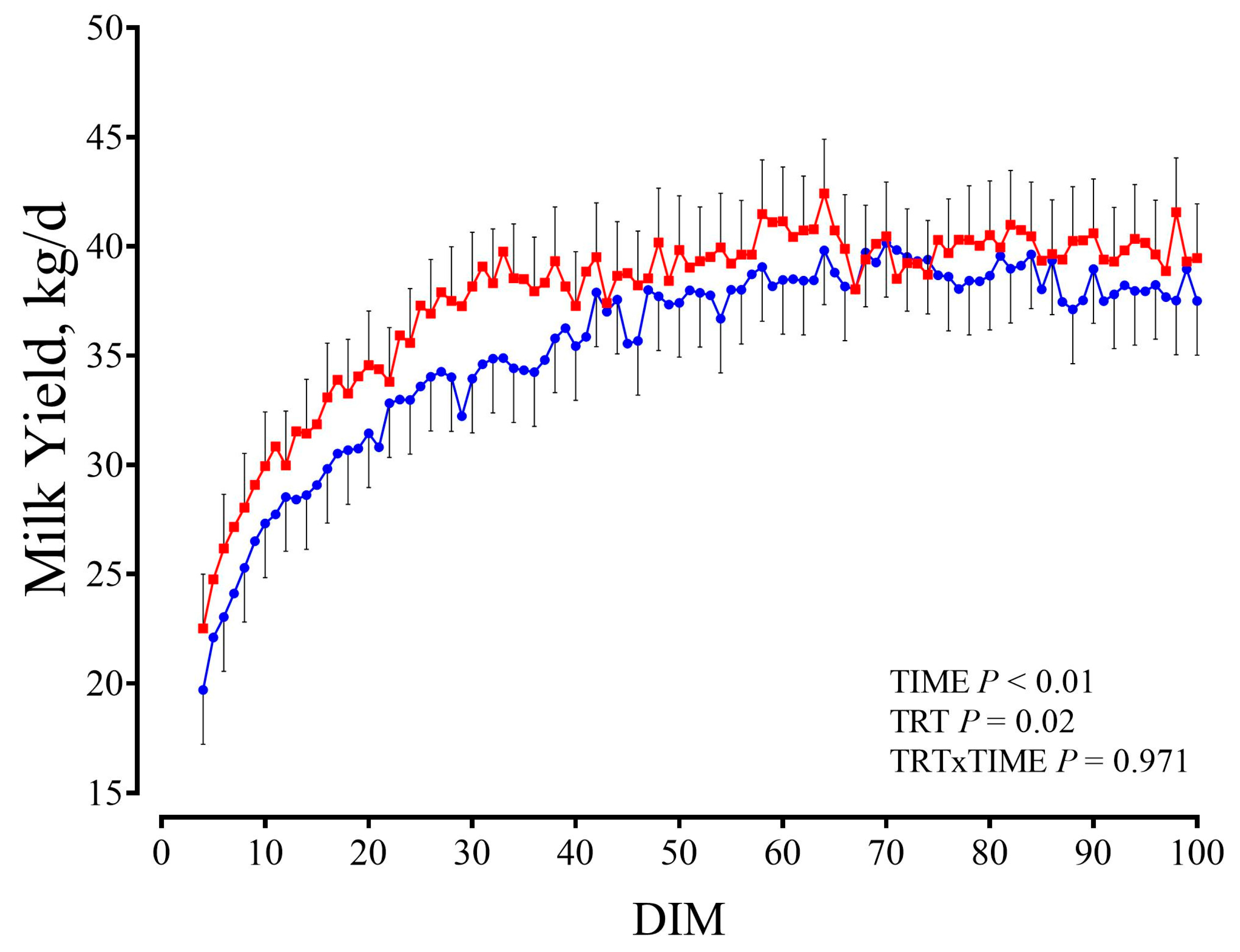
3.9. First Lactation Performance
4. Discussion
4.1. Effect on Performance, Metabolism and Rumen Development in Calves
4.2. Effect on Health Status and Immune System in Calves
4.3. Effect on Fecal Microbiota Composition
4.4. Post-Experiment Performance in First Lactation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| (C2 + C3)/C4 | sum of acetic acid and propionic acid to butyric acid |
| ADG | average daily gain |
| AI | artificial insemination |
| AICC | Akaike information criterion corrected |
| ALP | alkaline phosphatase |
| AOPP | advanced oxidation protein products |
| AST/GOT | aspartate-aminotransferase |
| BHB | beta-hydroxybutyrate |
| BW | body weight |
| CTR | control group |
| C2/C3 | ratios of acetic acid to propionic acid |
| DIM | days in milk |
| DM | dry matter |
| DMI | dry matter intake |
| ECM | energy corrected milk |
| EDTA | ethylenediaminetetraacetic acid |
| FRAP | ferric reducing antioxidant power |
| GGT | γ-glutamyl transferase |
| GPFT | genomic productivity, functionality and type index |
| HG | heart girth |
| LPS | lipopolysaccharide |
| LSM | least squares means |
| MFI | mean fluorescent intensity |
| MPO | myeloperoxidase |
| MR | milk replacer |
| NEFA | nonesterified fatty acids |
| PBS | phosphate-buffered saline |
| PMN | polymorphonuclear neutrophils |
| PON | paraoxonase |
| qPCR | quantitative PCR |
| ROM | total reactive oxygen metabolites |
| SCFP | Saccharomyces cerevisiae fermentation-derived postbiotic |
| TMR | total mixed ration |
| TRT | treatment |
| VFA | volatile fatty acids |
| WBA | whole blood stimulation assay |
| WBC | total white blood cells |
| WH | wither height |
References
- Heinrichs, A.J.; Heinrichs, B.S. A Prospective Study of Calf Factors Affecting First-Lactation and Lifetime Milk Production and Age of Cows When Removed from the Herd. J. Dairy Sci. 2011, 94, 336–341. [Google Scholar] [CrossRef]
- Jasper, J.; Weary, D.M. Effects of Ad Libitum Milk Intake on Dairy Calves. J. Dairy Sci. 2002, 85, 3054–3058. [Google Scholar] [CrossRef]
- Rooke, J. Basic Animal Nutrition and Feeding, 4th Edition, by W. G. Pond, D.C. Church & K. R. Pond. Viii + 615 Pp. Chichester: John Wiley & Sons (1995). £19.50 (Paperback). ISBN 0 471 30864 1. J. Agric. Sci. 1996, 126, 375–376. [Google Scholar] [CrossRef]
- Chaucheyras-Durand, F.; Fonty, G. Establishment of Cellulolytic Bacteria and Development of Fermentative Activities in the Rumen of Gnotobiotically-Reared Lambs Receiving the Microbial Additive Saccharomyces cerevisiae CNCM I-1077. Reprod. Nutr. Dev. 2001, 41, 57–68. [Google Scholar] [CrossRef]
- Ridge, J.P.; Fuchs, E.J.; Matzinger, P. Neonatal Tolerance Revisited: Turning on Newborn T Cells with Dendritic Cells. Science 1996, 271, 1723–1726. [Google Scholar] [CrossRef] [PubMed]
- Nagahata, H.; Kojima, N.; Higashitani, I.; Ogawa, H.; Noda, H. Postnatal Changes in Lymphocyte Function of Dairy Calves. J. Vet. Med. Ser. B 1991, 38, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Godden, S. Colostrum Management for Dairy Calves. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 19–39. [Google Scholar] [CrossRef]
- Stanton, A.L.; Kelton, D.F.; LeBlanc, S.J.; Wormuth, J.; Leslie, K.E. The Effect of Respiratory Disease and a Preventative Antibiotic Treatment on Growth, Survival, Age at First Calving, and Milk Production of Dairy Heifers. J. Dairy Sci. 2012, 95, 4950–4960. [Google Scholar] [CrossRef] [PubMed]
- Eckles, C.H.; Williams, V.M.; Wilbur, J.W.; Palmer, L.S.; Harshaw, H.M. Yeast as a Supplementary Feed for Calves. J. Dairy Sci. 1924, 7, 421–439. [Google Scholar] [CrossRef]
- Maina, T.W.; McDonald, P.O.; Rani Samuel, B.E.; Sardi, M.I.; Yoon, I.; Rogers, A.; McGill, J.L. Feeding Saccharomyces cerevisiae Fermentation Postbiotic Products Alters Immune Function and the Lung Transcriptome of Preweaning Calves with an Experimental Viral-Bacterial Coinfection. J. Dairy Sci. 2024, 107, 2253–2267. [Google Scholar] [CrossRef]
- Reed, G.; Nagodawithana, T.W. Yeast Technology; Springer: Dordrecht, The Netherlands, 1990; ISBN 978-94-011-9773-1. [Google Scholar]
- Newman, K. Mannan-Oligosaccharides: Natural Polymers with Significant Impact on the Gastrointestinal Microflora and the Immune System. In Proceedings of the 10th Annual Symposium; Nottingham University Press: Nottingham, UK, 1994; pp. 167–174. [Google Scholar]
- Robinson, P.H.; Erasmus, L.J. Effects of Analyzable Diet Components on Responses of Lactating Dairy Cows to Saccharomyces cerevisiae Based Yeast Products: A Systematic Review of the Literature. Anim. Feed Sci. Technol. 2009, 149, 185–198. [Google Scholar] [CrossRef]
- Zaworski, E.M.; Shriver-Munsch, C.M.; Fadden, N.A.; Sanchez, W.K.; Yoon, I.; Bobe, G. Effects of Feeding Various Dosages of Saccharomyces cerevisiae Fermentation Product in Transition Dairy Cows. J. Dairy Sci. 2014, 97, 3081–3098. [Google Scholar] [CrossRef] [PubMed]
- Knoblock, C.E.; Shi, W.; Yoon, I.; Oba, M. Effects of Supplementing a Saccharomyces cerevisiae Fermentation Product during the Periparturient Period on the Immune Response of Dairy Cows Fed Fresh Diets Differing in Starch Content. J. Dairy Sci. 2019, 102, 6199–6209. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E.S.; Martin, S.A. Effects of a Saccharomyces cerevisiae Culture on Ruminal Bacteria That Utilize Lactate and Digest Cellulose. J. Dairy Sci. 1997, 80, 2035–2044. [Google Scholar] [CrossRef]
- Lesmeister, K.E.; Heinrichs, A.J.; Gabler, M.T. Effects of Supplemental Yeast (Saccharomyces cerevisiae) Culture on Rumen Development, Growth Characteristics, and Blood Parameters in Neonatal Dairy Calves. J. Dairy Sci. 2004, 87, 1832–1839. [Google Scholar] [CrossRef]
- Berge, A.C.B.; Moore, D.A.; Besser, T.E.; Sischo, W.M. Targeting Therapy to Minimize Antimicrobial Use in Preweaned Calves: Effects on Health, Growth, and Treatment Costs. J. Dairy Sci. 2009, 92, 4707–4714. [Google Scholar] [CrossRef]
- Lopreiato, V.; Ghaffari, M.H.; Cattaneo, L.; Ferronato, G.; Alharthi, A.S.; Piccioli-Cappelli, F.; Loor, J.J.; Trevisi, E.; Minuti, A. Suitability of Rumination Time during the First Week after Calving for Detecting Metabolic Status and Lactation Performance in Simmental Dairy Cows: A Cluster-Analytic Approach. Ital. J. Anim. Sci. 2021, 20, 1909–1923. [Google Scholar] [CrossRef]
- Jahan, N.; Minuti, A.; Trevisi, E. Assessment of Immune Response in Periparturient Dairy Cows Using Ex Vivo Whole Blood Stimulation Assay with Lipopolysaccharides and Carrageenan Skin Test. Vet. Immunol. Immunopathol. 2015, 165, 119–126. [Google Scholar] [CrossRef]
- Floridia, V.; Sfulcini, M.; D’Alessandro, E.; Cattaneo, L.; Mezzetti, M.; Liotta, L.; Trevisi, E.; Lopreiato, V.; Minuti, A. Effect of Different Anticoagulant Agents on Immune-Related Genes in Leukocytes Isolated from the Whole-Blood of Holstein Cows. Genes 2023, 14, 406. [Google Scholar] [CrossRef]
- Hulbert, L.E.; Carroll, J.A.; Burdick, N.C.; Randel, R.D.; Brown, M.S.; Ballou, M.A. Innate Immune Responses of Temperamental and Calm Cattle after Transportation. Vet. Immunol. Immunopathol. 2011, 143, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Scatà, M.C.; Alhussien, M.N.; Grandoni, F.; Reale, A.; Zampieri, M.; Hussen, J.; De Matteis, G. Hyperthermia-Induced Changes in Leukocyte Survival and Phagocytosis: A Comparative Study in Bovine and Buffalo Leukocytes. Front. Vet. Sci. 2024, 10, 1327148. [Google Scholar] [CrossRef]
- Ahmed, S.; Minuti, A.; Bani, P. In Vitro Rumen Fermentation Characteristics of Some Naturally Occurring and Synthetic Sugars. Ital. J. Anim. Sci. 2013, 12, e57. [Google Scholar] [CrossRef]
- Patrone, V.; Minuti, A.; Lizier, M.; Miragoli, F.; Lucchini, F.; Trevisi, E.; Rossi, F.; Callegari, M.L. Differential Effects of Coconut versus Soy Oil on Gut Microbiota Composition and Predicted Metabolic Function in Adult Mice. BMC Genom. 2018, 19, 808. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for Comprehensive Statistical, Functional, and Meta-Analysis of Microbiome Data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle; National Academies Press: Washington, DC, USA, 2021; ISBN 978-0-309-67777-6. [Google Scholar]
- Klopp, R.N.; Yoon, I.; Eicher, S.; Boerman, J.P. Effects of Feeding Saccharomyces cerevisiae Fermentation Products on the Health of Holstein Dairy Calves Following a Lipopolysaccharide Challenge. J. Dairy Sci. 2022, 105, 1469–1479. [Google Scholar] [CrossRef]
- Littell, R.C.; Henry, P.R.; Ammerman, C.B. Statistical Analysis of Repeated Measures Data Using SAS Procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [CrossRef] [PubMed]
- Quigley, J.D.; Wallis, L.B.; Dowlen, H.H.; Heitmann, R.N. Sodium Bicarbonate and Yeast Culture Effects on Ruminal Fermentation, Growth, and Intake in Dairy Calves. J. Dairy Sci. 1992, 75, 3531–3538. [Google Scholar] [CrossRef]
- Magalhães, V.J.A.; Susca, F.; Lima, F.S.; Branco, A.F.; Yoon, I.; Santos, J.E.P. Effect of Feeding Yeast Culture on Performance, Health, and Immunocompetence of Dairy Calves. J. Dairy Sci. 2008, 91, 1497–1509. [Google Scholar] [CrossRef]
- Galvão, K.N.; Santos, J.E.P.; Coscioni, A.; Villaseñor, M.; Sischo, W.M.; Anna Catharina, B. Berge Effect of Feeding Live Yeast Products to Calves with Failure of Passive Transfer on Performance and Patterns of Antibiotic Resistance in Fecal Escherichia coli. Reprod. Nutr. Dev. 2005, 45, 427–440. [Google Scholar] [CrossRef]
- Klopp, R.N.; Centeno-Martinez, R.E.; Yoon, I.; Johnson, T.A.; Boerman, J.P. Effects of Feeding Saccharomyces cerevisiae Fermentation Products on the Health and Growth Performance of Holstein Dairy Calves. JDS Commun. 2022, 3, 174–179. [Google Scholar] [CrossRef]
- Arshad, M.A.; Hassan, F.; Rehman, M.S.; Huws, S.A.; Cheng, Y.; Din, A.U. Gut Microbiome Colonization and Development in Neonatal Ruminants: Strategies, Prospects, and Opportunities. Anim. Nutr. 2021, 7, 883–895. [Google Scholar] [CrossRef]
- Olagaray, K.E.; Sivinski, S.E.; Saylor, B.A.; Mamedova, L.K.; Sauls-Hiesterman, J.A.; Yoon, I.; Bradford, B.J. Effect of Saccharomyces cerevisiae Fermentation Product on Feed Intake Parameters, Lactation Performance, and Metabolism of Transition Dairy Cattle. J. Dairy Sci. 2019, 102, 8092–8107. [Google Scholar] [CrossRef]
- Quigley, J.D.; Caldwell, L.A.; Sinks, G.D.; Heitmann, R.N. Changes in Blood Glucose, Nonesterified Fatty Acids, and Ketones in Response to Weaning and Feed Intake in Young Calves. J. Dairy Sci. 1991, 74, 250–257. [Google Scholar] [CrossRef]
- Alugongo, G.M.; Xiao, J.; Wu, Z.; Li, S.; Wang, Y.; Cao, Z. Review: Utilization of Yeast of Saccharomyces cerevisiae Origin in Artificially Raised Calves. J. Anim. Sci. Biotechnol. 2017, 8, 34. [Google Scholar] [CrossRef]
- Melo, L.Q.; Costa, S.F.; Lopes, F.; Guerreiro, M.C.; Armentano, L.E.; Pereira, M.N. Rumen Morphometrics and the Effect of Digesta PH and Volume on Volatile Fatty Acid Absorption1. J. Anim. Sci. 2013, 91, 1775–1783. [Google Scholar] [CrossRef]
- Goff, J.P. Pathophysiology of Calcium and Phosphorus Disorders. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Reber, A.J.; Lockwood, A.; Hippen, A.R.; Hurley, D.J. Colostrum Induced Phenotypic and Trafficking Changes in Maternal Mononuclear Cells in a Peripheral Blood Leukocyte Model for Study of Leukocyte Transfer to the Neonatal Calf. Vet. Immunol. Immunopathol. 2006, 109, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Chase, C.C.L.; Hurley, D.J.; Reber, A.J. Neonatal Immune Development in the Calf and Its Impact on Vaccine Response. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.H.A.; Slate, J.R.; Hong, S.; Yoon, I.; McGill, J.L. Supplementing a Saccharomyces cerevisiae Fermentation Product Modulates Innate Immune Function and Ameliorates Bovine Respiratory Syncytial Virus Infection in Neonatal Calves. J. Anim. Sci. 2020, 98, skaa252. [Google Scholar] [CrossRef]
- Sanchez, N.C.B.; Young, T.R.; Carroll, J.A.; Corley, J.R.; Rathmann, R.J.; Johnson, B.J. Yeast Cell Wall Supplementation Alters the Metabolic Responses of Crossbred Heifers to an Endotoxin Challenge. Innate Immun. 2014, 20, 104–112. [Google Scholar] [CrossRef]
- Murphy, E.A.; Davis, J.M.; Brown, A.S.; Carmichael, M.D.; Ghaffar, A.; Mayer, E.P. Oat β-Glucan Effects on Neutrophil Respiratory Burst Activity Following Exercise. Med. Sci. Sports Exerc. 2007, 39, 639–644. [Google Scholar] [CrossRef]
- Jensen, G.S.; Hart, A.N.; Schauss, A.G. An Antiinflammatory Immunogen from Yeast Culture Induces Activation and Alters Chemokine Receptor Expression on Human Natural Killer Cells and B Lymphocytes in Vitro. Nutr. Res. 2007, 27, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, R. The Effect of Leiber Beta-S on Selected Immunity Indicators in Calves. Acta Vet. Brno 2014, 83, 113–118. [Google Scholar] [CrossRef]
- Engstad, R.E.; Robertsen, B. Recognition of Yeast Cell Wall Glucan by Atlantic Salmon (Salmo salar L.) Macrophages. Dev. Comp. Immunol. 1993, 17, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J. Myeloperoxidase. Proc. Assoc. Am. Physicians 1999, 111, 383–389. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Owens, S.-E.; Turner, M.L. Innate Immunity and the Sensing of Infection, Damage and Danger in the Female Genital Tract. J. Reprod. Immunol. 2017, 119, 67–73. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Liang, G.; Griebel, P.J.; Guan, L.L. Taxonomic and Functional Compositions of the Small Intestinal Microbiome in Neonatal Calves Provide a Framework for Understanding Early Life Gut Health. Appl. Environ. Microbiol. 2019, 85, e02534-18. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, G.; Teixeira, A.G.V.; Foditsch, C.; Bicalho, M.L.; Machado, V.S.; Bicalho, R.C. Fecal Microbial Diversity in Pre-Weaned Dairy Calves as Described by Pyrosequencing of Metagenomic 16S RDNA. Associations of Faecalibacterium Species with Health and Growth. PLoS ONE 2013, 8, e63157. [Google Scholar] [CrossRef]
- Centeno-Martinez, R.E.; Dong, W.; Klopp, R.N.; Yoon, I.; Boerman, J.P.; Johnson, T.A. Effects of Feeding Saccharomyces cerevisiae Fermentation Postbiotic on the Fecal Microbial Community of Holstein Dairy Calves. Anim. Microbiome 2023, 5, 13. [Google Scholar] [CrossRef]
- Ma, T.; Villot, C.; Renaud, D.; Skidmore, A.; Chevaux, E.; Steele, M.; Guan, L.L. Linking Perturbations to Temporal Changes in Diversity, Stability, and Compositions of Neonatal Calf Gut Microbiota: Prediction of Diarrhea. ISME J. 2020, 14, 2223–2235. [Google Scholar] [CrossRef]
- Tun, H.M.; Li, S.; Yoon, I.; Meale, S.J.; Azevedo, P.A.; Khafipour, E.; Plaizier, J.C. Saccharomyces cerevisiae Fermentation Products (SCFP) Stabilize the Ruminal Microbiota of Lactating Dairy Cows during Periods of a Depressed Rumen PH. BMC Vet. Res. 2020, 16, 237. [Google Scholar] [CrossRef]
- Du, Y.; Gao, Y.; Hu, M.; Hou, J.; Yang, L.; Wang, X.; Du, W.; Liu, J.; Xu, Q. Colonization and Development of the Gut Microbiome in Calves. J. Anim. Sci. Biotechnol. 2023, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, S.; Wang, H.; Wang, M.; Yu, L. Relative Significances of PH and Substrate Starch Level to Roles of Streptococcus bovis S1 in Rumen Acidosis. AMB Express 2016, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a More Comprehensive Concept for Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic Distribution of Three Pathways for Propionate Production within the Human Gut Microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of Human Colonic Butyrate-Producing Bacteria Revealed by Analysis of the Butyryl-CoA:Acetate CoA-Transferase Gene. Environ. Microbiol. 2010, 12, 304–314. [Google Scholar] [CrossRef]
- Zanton, G.I.; Heinrichs, A.J. Meta-Analysis to Assess Effect of Prepubertal Average Daily Gain of Holstein Heifers on First-Lactation Production. J. Dairy Sci. 2005, 88, 3860–3867. [Google Scholar] [CrossRef]
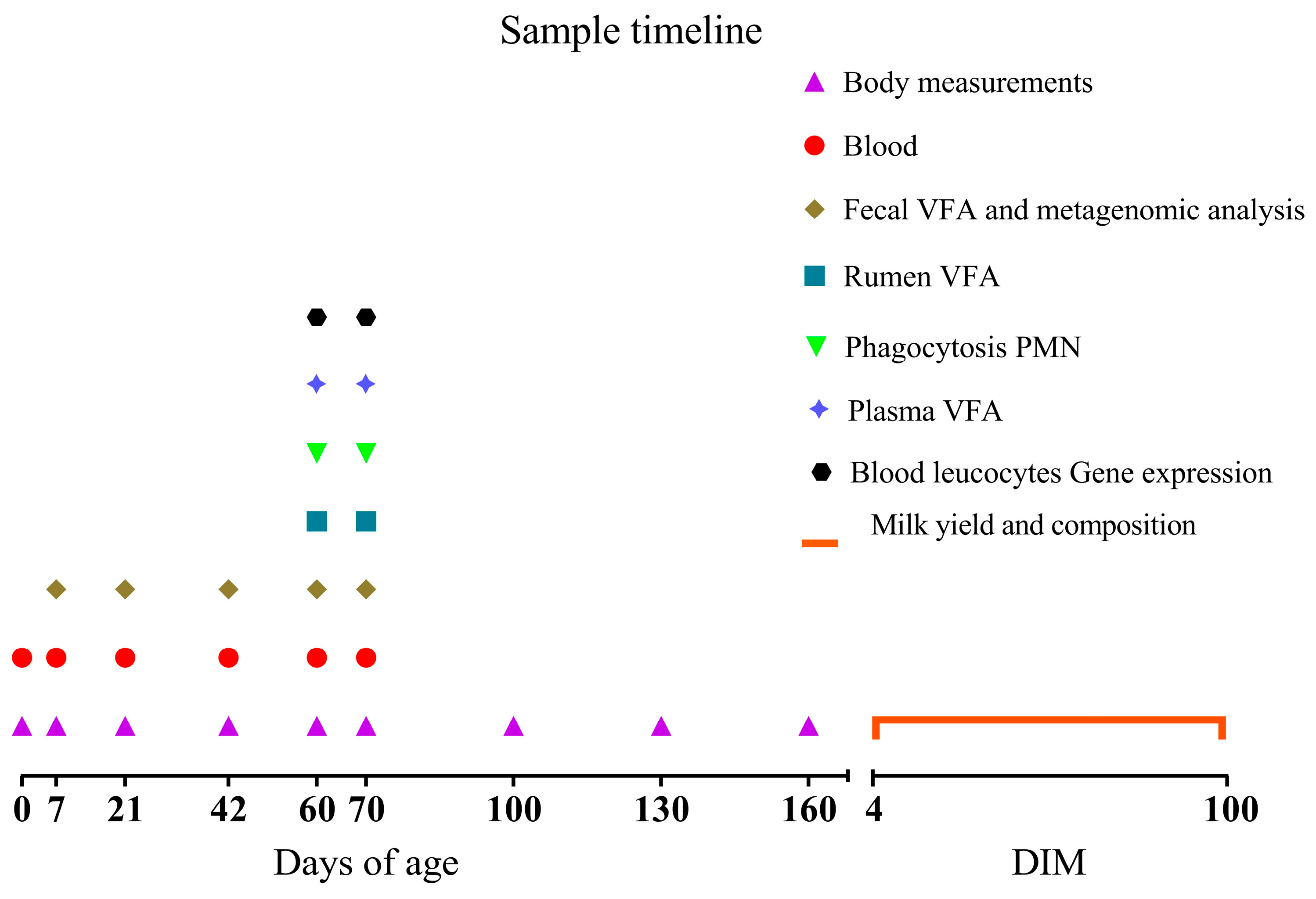


| Item | MR 1 | Calf Starter 2 Until 70 d | Concentrate 3 From 70 to 160 d |
|---|---|---|---|
| CP, % | 22.5 | 16.3 | 18 |
| Crude fat, % | 18 | 3.5 | 2.5 |
| Crude fiber, % | 0.0 | 14.94 | 14.5 |
| Ash, % | 8.4 | 7.84 | 8 |
| Na, % | 0.71 | 0.39 | |
| Ca, % | 0.84 | ||
| P, % | 0.64 |
| Item | Hay Mix | TMR | ||
|---|---|---|---|---|
| Heifer | Heifer from −60 d to Calving | Lactation | ||
| Ingredients | ||||
| Corn silage | 29.8 | |||
| Concentrate mix 1 | 3.03 | 3.65 | 25.8 | |
| Alfalfa hay | 34.0 | 25.6 | 26.4 | |
| Grass hay | 33.0 | |||
| Barley silage | 41.3 | 49.7 | ||
| Barley straw | 33.0 | 15.9 | 29.8 | 2.84 |
| Soybean meal | 7.09 | 8.85 | 8.10 | |
| Sunflower meal | 6.41 | 7.59 | 4.20 | |
| Hydrogenated fats | 0.88 | |||
| Vitamin and mineral premix 2 | 0.67 | 0.41 | 1.22 | |
| Limestone | 0.36 | |||
| Sodium bicarbonate | 0.36 | |||
| Sodium Chloride | 0.04 | |||
| Nutrient composition, % of DM 3 | ||||
| Starch | 9.80 | 11.3 | 27.4 | |
| CP | 8.90 | 14.1 | 12.3 | 15.7 |
| Metabolizable protein (MP) | 8.49 | 8.44 | 10.0 | |
| Lysine (% MP) | 0.32 | 4.7 | 3.8 | 6.57 |
| Methionine (% MP) | 0.07 | 1.4 | 1.9 | 2.15 |
| NDF | 60.7 | 49.2 | 52.3 | 31.8 |
| ADF | 38.6 | 31.2 | 32.2 | 19.9 |
| Crude fat (EE) | 1.28 | 2.59 | 2.65 | 3.61 |
| Ash | 8.30 | 8.13 | 6.60 | 7.44 |
| Ca | 1.18 | 0.64 | 0.31 | 1.21 |
| P | 0.14 | 0.40 | 0.36 | 0.37 |
| Mg | 0.23 | 0.25 | 0.23 | 0.27 |
| K | 0.58 | 1.31 | 0.86 | 0.78 |
| Na | 0.06 | 0.09 | 0.09 | 0.23 |
| Energy, Mcal/kg of DM | ||||
| ME | 1.78 | 2.34 | 1.94 | 2.62 |
| NEM | 0.92 | 1.53 | 1.28 | 1.79 |
| NEL | 0.34 | 1.57 | ||
| CTR | SCFP | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| TIME | TRT | TIME × TRT | ||||
| Milk yield, kg/d | 35.45 | 37.62 | 0.65 | <0.01 * | 0.02 * | 0.971 |
| Fat, % | 3.97 | 3.78 | 0.05 | <0.01 * | 0.01 * | 0.27 |
| Fat yield kg/d | 1.39 | 1.41 | 0.03 | <0.01 * | 0.68 | 0.29 |
| Protein, % | 3.49 | 3.49 | 0.01 | 0.2 | 0.94 | 0.8 |
| Protein yield, kg/d | 1.24 | 1.31 | 0.02 | <0.01 * | 0.01 * | 0.77 |
| Lactose, % | 4.74 | 4.76 | 0.04 | 0.04 * | 0.71 | <0.01 * |
| Milk conductibility, mS/cm | 8.95 | 9.03 | 0.04 | 0.17 | 0.12 | 0.29 |
| ECM, kg/d | 43.54 | 45.42 | 0.65 | <0.01 * | 0.04 * | 0.81 |
| FCM, kg/d | 35.04 | 36.1 | 0.59 | <0.01 * | 0.21 | 0.41 |
| Body weight 1, kg | 567.99 | 580.17 | 11.95 | 0.90 | 0.48 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfulcini, M.; Lopreiato, V.; Piccioli-Cappelli, F.; Patrone, V.; Bisaschi, M.; Yoon, I.; Zontini, A.M.; Barbato, M.; Cattaneo, L.; Archetti, I.; et al. Saccharomyces cerevisiae Fermentation-Derived Postbiotics Supplementation to Dairy Calves: Effects on Growth, Metabolism, Immune Status and Preliminary First Lactation Outcomes. Animals 2025, 15, 2728. https://doi.org/10.3390/ani15182728
Sfulcini M, Lopreiato V, Piccioli-Cappelli F, Patrone V, Bisaschi M, Yoon I, Zontini AM, Barbato M, Cattaneo L, Archetti I, et al. Saccharomyces cerevisiae Fermentation-Derived Postbiotics Supplementation to Dairy Calves: Effects on Growth, Metabolism, Immune Status and Preliminary First Lactation Outcomes. Animals. 2025; 15(18):2728. https://doi.org/10.3390/ani15182728
Chicago/Turabian StyleSfulcini, Marta, Vincenzo Lopreiato, Fiorenzo Piccioli-Cappelli, Vania Patrone, Marta Bisaschi, Ilkyu Yoon, Alessandro Maria Zontini, Mario Barbato, Luca Cattaneo, Ivonne Archetti, and et al. 2025. "Saccharomyces cerevisiae Fermentation-Derived Postbiotics Supplementation to Dairy Calves: Effects on Growth, Metabolism, Immune Status and Preliminary First Lactation Outcomes" Animals 15, no. 18: 2728. https://doi.org/10.3390/ani15182728
APA StyleSfulcini, M., Lopreiato, V., Piccioli-Cappelli, F., Patrone, V., Bisaschi, M., Yoon, I., Zontini, A. M., Barbato, M., Cattaneo, L., Archetti, I., Trevisi, E., & Minuti, A. (2025). Saccharomyces cerevisiae Fermentation-Derived Postbiotics Supplementation to Dairy Calves: Effects on Growth, Metabolism, Immune Status and Preliminary First Lactation Outcomes. Animals, 15(18), 2728. https://doi.org/10.3390/ani15182728







