Housing in a Large Open Cage Did Not Affect the Phenotypic Traits of Obese Male Zucker fa/fa Rats When Compared to IVC-Housed Rats, but Improved the Rats’ Well-Being
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Design
2.2.1. Experiments 1 and 2 (IVC)
2.2.2. Experiment 3 (LOC)
2.3. Assessment of Animal Welfare
2.4. Analyses
2.5. Outcome Measurements
2.6. Statistical Analyses
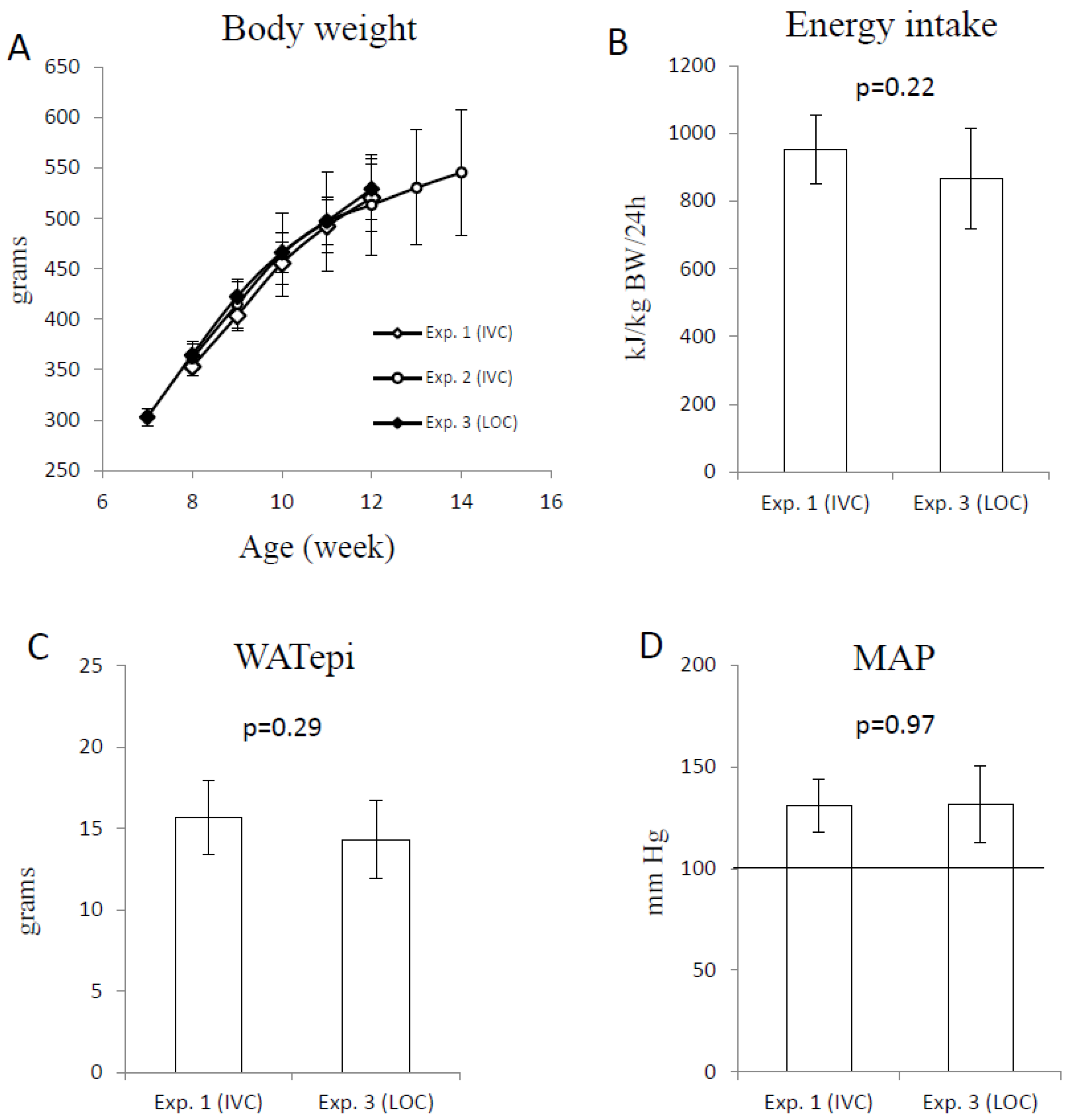
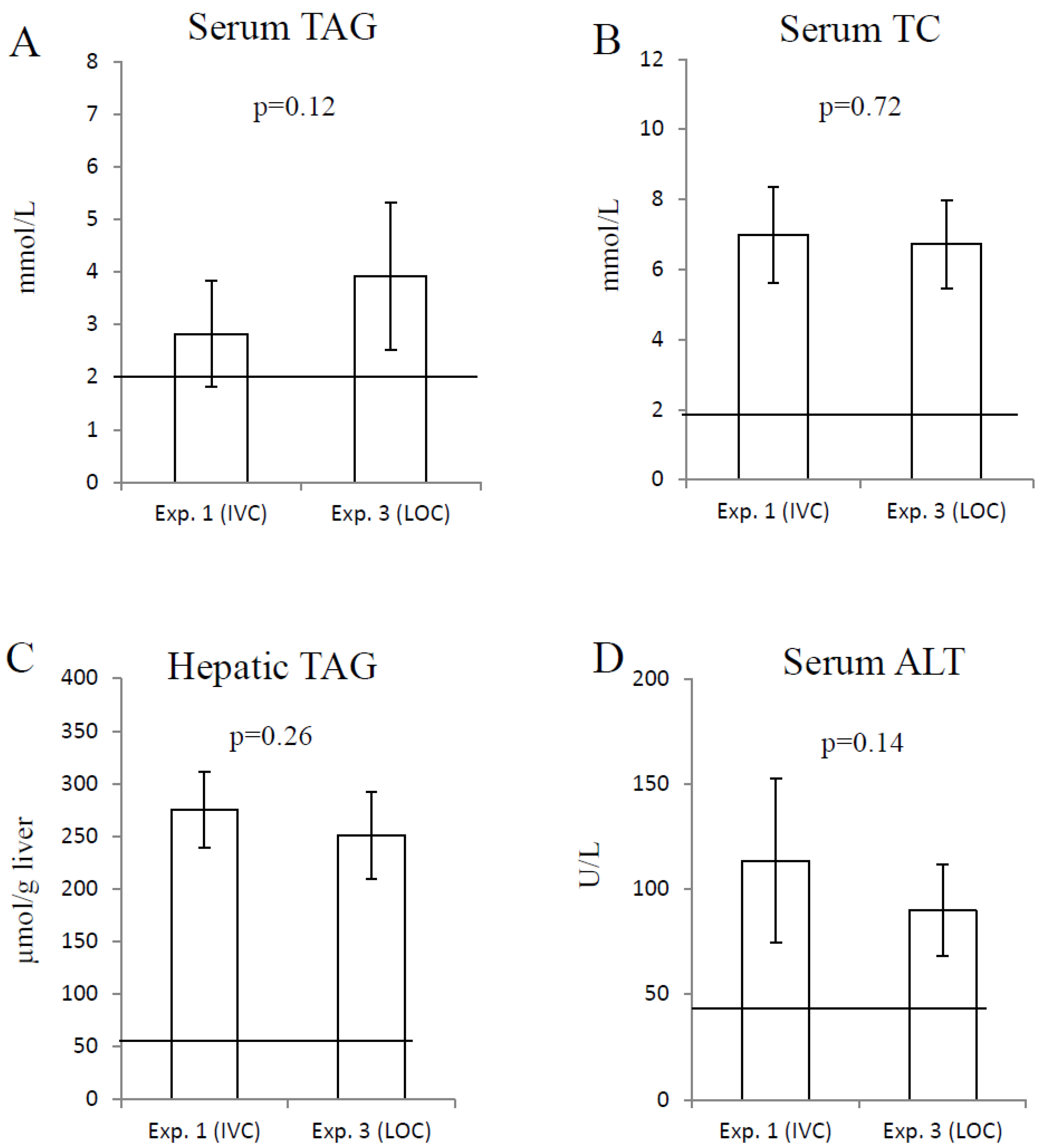
3. Results
3.1. Animal Welfare
3.2. Measurements
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine transaminase |
| IVC | Individually ventilated cages |
| LOC | Large open cage |
| MAP | Mean arterial blood pressure |
| WATepi | Epididymal white adipose tissue |
References
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. 2010. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32010L0063 (accessed on 31 July 2024).
- Regulations on the Use of Animals in Experiments. 2015. Available online: https://lovdata.no/dokument/SF/forskrift/2015-06-18-761/KAPITTEL_13#KAPITTEL_13 (accessed on 31 July 2024).
- Code of Practice for the Housing and Care of Animals Bred, Supplied or Used for Scientific Purposes. 2014. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/388535/CoPanimalsWeb.pdf (accessed on 31 July 2024).
- Guide-for the Care and Use of Laboratory Animals. 2011. Available online: https://olaw.nih.gov/sites/default/files/Guide-for-the-Care-and-Use-of-Laboratory-Animals.pdf (accessed on 31 July 2024).
- Baumans, V.; Van Loo, P.L.P. How to improve housing conditions of laboratory animals: The possibilities of environmental refinement. Vet. J. 2013, 195, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Ratuski, A.S.; Weary, D.M. Environmental Enrichment for Rats and Mice Housed in Laboratories: A Metareview. Animals 2022, 12, 414. [Google Scholar] [CrossRef]
- Bray, G.A. The Zucker-fatty rat: A review. Fed. Proc. 1977, 36, 148–153. [Google Scholar]
- Luo, H.; Wang, X.; Chen, C.; Wang, J.; Zou, X.; Li, C.; Xu, Z.; Yang, X.; Shi, W.; Zeng, C. Oxidative stress causes imbalance of renal renin angiotensin system (RAS) components and hypertension in obese Zucker rats. J. Am. Heart Assoc. 2015, 4, e001559. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.S.; Liu, Q.; Hammond, H.A.; Dugan, V.; Hey, P.J.; Caskey, C.J.; Hess, J.F. Leptin receptor missense mutation in the fatty Zucker rat. Nat. Genet. 1996, 13, 18–19. [Google Scholar] [CrossRef]
- Azain, M.J.; Martin, R.J. Effect of genetic obesity on the regulation of hepatic fatty acid metabolism. Am. J. Physiol. 1983, 244, R400–R406. [Google Scholar] [CrossRef] [PubMed]
- de Artinano, A.A.; Castro, M.M. Experimental rat models to study the metabolic syndrome. Br. J. Nutr. 2009, 102, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen & Co., Ltd.: London, UK, 1959. [Google Scholar]
- Makowska, I.J.; Weary, D.M. The importance of burrowing, climbing and standing upright for laboratory rats. R. Soc. Open Sci. 2016, 3, 160136. [Google Scholar] [CrossRef] [PubMed]
- Makowska, I.J.; Weary, D.M. A Good Life for Laboratory Rodents? ILAR J. 2019, 60, 373–388. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Vildmyren, I.; Oterhals, A.; Leh, S.; Samuelsen, T.A.; Halstensen, A.; Marti, H.P.; Gudbrandsen, O.A. Intake of residuals from Atlantic cod attenuated blood pressure increase but did not delay development of kidney damage in obese Zucker fa/fa rats. Food Nutr. Res. 2022, 66, 8708. [Google Scholar] [CrossRef] [PubMed]
- European Commission: Directorate-General for Environment. Caring for Animals Aiming for Better Science—Directive 2010/63/EU on Protection of Animals Used for Scientific Purposes—Severity Assessment Framework; Publications Office: 2018. Available online: https://data.europa.eu/doi/10.2779/068620 (accessed on 1 June 2025).
- ISO 9831:1998; Animal Feeding Stuffs, Animal Products, and Faeces or Urine—Determination of Gross Calorific Value—Bomb Calorimeter Method. International Organization for Standardization: Geneva, Switzerland, 1998.
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Wolford, S.T.; Schroer, R.A.; Gohs, F.X.; Gallo, P.P.; Brodeck, M.; Falk, H.B.; Ruhren, R. Reference range data base for serum chemistry and hematology values in laboratory animals. J. Toxicol. Environ. Health 1986, 18, 161–188. [Google Scholar] [CrossRef] [PubMed]
- Cait, J.; Cait, A.; Scott, R.W.; Winder, C.B.; Mason, G.J. Conventional laboratory housing increases morbidity and mortality in research rodents: Results of a meta-analysis. BMC Biol. 2022, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Murphy, M.L.M.; Prather, A.A. Ten Surprising Facts About Stressful Life Events and Disease Risk. Annu. Rev. Psychol. 2019, 70, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Wurbel, H. Ideal homes? Housing effects on rodent brain and behaviour. Trends Neurosci. 2001, 24, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Garner, J.P. Stereotypies and other abnormal repetitive behaviors: Potential impact on validity, reliability, and replicability of scientific outcomes. ILAR J. 2005, 46, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Baumans, V. Environmental enrichment for laboratory rodents and rabbits: Requirements of rodents, rabbits, and research. ILAR J. 2005, 46, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Poole, T. Happy animals make good science. Lab. Anim. 1997, 31, 116–124. [Google Scholar] [CrossRef]

| High-Level Categories | Areas to Focus on When Observing Rats | Specific Indicators to Monitor | Action/Clinical Scores 1 |
|---|---|---|---|
| Appearance | Body condition | Weight loss | 5–10% weight loss: 1–2 20% weight loss: 4 >20% weight loss: HEP |
| Coat and skin condition | Lack of grooming Fecal or urine staining | Feces in fur at the anus (wash with lukewarm water): 1 Recurring episodes with feces in fur >48 h: 3 Recurring episodes with feces in fur >72 h combined with lack of grooming: HEP | |
| Skin lesions Injury/wound | Palpation of rats at 3/week to check for bite wounds and swellings. Treat/wash wounds if necessary. Small bite wounds: 1 Small bite wounds that do not start to heal after 48 h: 4 Wounds that do not heal or are infected: HEP | ||
| Discharge | Porphyrin staining | Normal amount: 0–1 Increased amount: 2–3 | |
| Mouth | Malocclusion, Broken teeth | Weekly checks of teeth and feed intake; cut teeth before they become too long or cause problems for eating | |
| Body functions | Feed/water intake | Increased/decreased intake | Monitor feed and water intake, and monitor body weight. Change feed if necessary. |
| Environment | Enclosure environment, including any litter, nesting material, and enrichment items | Presence and consistency of feces | Loose stools or diarrhea: 2 Diarrhea >48 h: 4 |
| Whether rats are using enrichment items, e.g., nesting material, taking refuge, or using wooden chewing blocks | Nest building and chewing on blocks: 0 Slightly disorganized nesting: 1 Some attempts but unorganized and not chewing on blocks: 3 | ||
| Behaviors | Social interaction | Change from normal temperament; apprehensive or aggressive interactions with other rats; anxiety Isolated or withdrawn from other animals in social group | Normal behavior: 1 Aggressive: 3 Passive or withdrawn from cage-mates: 5 |
| Undesirable behaviors | Apathy | Slightly apathic: 1 No interest in greeting carer: 3 Not eating: HEP | |
| Increased aggression to humans or cage-mates | Tense and nervous on handling: 1 Markedly distressed/aggressive on handling: 3 Can no longer be handled: HEP | ||
| Procedure-specific indicators | Restitution after blood pressure measurement | Not recovered 1 h after measurements | Lethargic after 1–2 h: 1–2 Lethargic after >3 h: 4 Lethargic after >6 h and not drinking: HEP |
| Free observations | A severity assessment scheme was available for registration of any observations of unexpected negative welfare impacts. | ||
| High-Level Categories | Areas to Focus on When Observing Rats | Specific Indicators to Monitor | Clinical Scores for IVC Rats | Clinical Scores for LOC Rats |
|---|---|---|---|---|
| Appearance | Body condition | Weight loss | 0 | 0 |
| Coat and skin condition | Lack of grooming Fecal or urine staining | 0 | 0 | |
| Skin lesions | 0 | 0 | ||
| Discharge | Porphyrin staining | 0–1 | 0–1 | |
| Mouth | Malocclusion Broken teeth | 0 | 0 | |
| Body functions | Food/water intake | Increased/decreased intake | 0 | 0 |
| Environment | Enclosure environment | Presence and consistency of feces | 0 | 0 |
| Whether animal is using enrichment items | 1–3 | 0 | ||
| Behaviors | Social interaction | Change from normal temperament | 0 | 0 |
| Undesirable behaviors | Apathy | 3 | 0 | |
| Increased aggression to humans or cage-mates | 0 | 0 | ||
| Procedure-specific indicators | Restitution after blood pressure measurement | Not recovered 1 h after measurements | 0 | 0 |
| Free observations | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudbrandsen, O.A. Housing in a Large Open Cage Did Not Affect the Phenotypic Traits of Obese Male Zucker fa/fa Rats When Compared to IVC-Housed Rats, but Improved the Rats’ Well-Being. Animals 2025, 15, 2687. https://doi.org/10.3390/ani15182687
Gudbrandsen OA. Housing in a Large Open Cage Did Not Affect the Phenotypic Traits of Obese Male Zucker fa/fa Rats When Compared to IVC-Housed Rats, but Improved the Rats’ Well-Being. Animals. 2025; 15(18):2687. https://doi.org/10.3390/ani15182687
Chicago/Turabian StyleGudbrandsen, Oddrun Anita. 2025. "Housing in a Large Open Cage Did Not Affect the Phenotypic Traits of Obese Male Zucker fa/fa Rats When Compared to IVC-Housed Rats, but Improved the Rats’ Well-Being" Animals 15, no. 18: 2687. https://doi.org/10.3390/ani15182687
APA StyleGudbrandsen, O. A. (2025). Housing in a Large Open Cage Did Not Affect the Phenotypic Traits of Obese Male Zucker fa/fa Rats When Compared to IVC-Housed Rats, but Improved the Rats’ Well-Being. Animals, 15(18), 2687. https://doi.org/10.3390/ani15182687






