Simple Summary
Hay is a major part of a horse’s diet, but if it contains too much dust or is contaminated with harmful microorganisms like mold or bacteria, it can cause serious breathing problems. Horse owners and caretakers often check hay quality using sight, touch and smell, but it is unclear whether this simple method can detect hay that might be harmful. In this study, researchers collected 50 hay samples from horse owners and examined them using both sensory examinations, as well as specific methods to measure dust levels and check for potentially harmful microorganisms. The results showed that hay with an abnormal smell was more likely to produce high levels of dust, and hay with visible dirt or debris was more likely to contain potentially harmful bacteria or fungi. This research highlights the value of basic sensory assessments as a rapid and low-cost means to monitor hay hygiene and protect horses’ respiratory health.
Abstract
Hay quality is a key factor in equine respiratory health, with microbiological contaminants in inhaled organic dust posing significant risks. Sensory assessment has been used to evaluate hay hygiene, but its value to identify deficiencies remains unclear. This study aimed to explore the potential of sensory assessment to predict both particulate matter (PM) dust concentrations and microbiological contamination. Fifty hay samples were collected from horse owners and evaluated using a structured sensory examination, microbiological analyses, and dust concentration measurements obtained with the Hay-Shaker device and a DustTrak DRX 8534. Sensory examination rated only 28% of samples as adequate, with 52% showing minor and 20% major deficiencies. Microbiological analysis found that 46% of samples met acceptable standards. Regression analysis showed that abnormal musty odor was the strongest predictor of increased dust concentrations, including the respirable fraction (PM4, <4 µm), while visible impurities were associated with microbial contamination. These findings suggest that sensory attributes such as odor and impurity are valuable indicators of hay hygiene. Structured protocols for sensory examination may offer a simple and cost-effective tool for assessing hay quality in equine environments.
1. Introduction
Hay is a major component of the equine diet, and its quality can significantly impact equine health, particularly of the respiratory system [1,2]. Poor-quality hay, particularly if contaminated with harmful microorganisms or debris, has been associated with airway inflammation and the development of equine asthma, a common respiratory condition among horses, affecting up to 50% in certain regions [3,4].
Hay quality is influenced by both its composition and potential contamination. It is important to differentiate between field flora—microorganisms naturally present on plants during growth—and spoilage flora, which proliferates under poor harvesting or storage conditions and may pose greater risks to respiratory health [5,6]. Contaminants can be classified into biological (toxic plants, dust, insects, bacteria, toxins), chemical (fertilizers, heavy metals, etc.), and physical (sand, glass, etc.) [7]. Among these, biological contaminants are of particular concern due to their presence in respirable organic dust. Inhalation of such organic dust—including mold spores, bacterial endotoxins present in fine particulate matter (PM)—has been widely recognized as a major contributor to lower airway inflammation and asthma exacerbation in horses [6,8,9]. The impact of these contaminants on respiratory health depends largely on the size of the airborne particles, which determines how deeply they can penetrate into the respiratory tract. These PM fractions describe the thoracic fraction (PM10, <10 μm) reaching the trachea and bronchi, the respirable fraction (PM4, <4 μm) reaching the bronchioles and the alveolar fractions (PM2.5 and PM1, <2.5 and <1 μm, respectively) that penetrate to the alveoli.
Experimental studies using dusty or moldy hay or nebulized hay dust suspensions consistently replicate the clinical and inflammatory features of equine asthma, especially in susceptible individuals [10,11,12]. Particles smaller than 4 μm (PM4) are especially hazardous, as they can reach the distal airways and trigger inflammatory responses [11,13,14,15,16]. A previous study found a positive association between visually apparent dust and elevated respirable particle levels, with dusty or moldy hay releasing significantly more PM compared to visually clean hay [17]. Mold spores and bacterial endotoxins in hay dust are widely recognized as key etiological components of this syndrome [18,19,20,21,22,23]. Among mold species, Aspergillus fumigatus is particularly relevant, as this mold produces spores capable of triggering severe asthma symptoms [14,24].
Two primary methodologies are employed to assess hay quality: sensory examination (also called organoleptic) and microbiological analysis. Sensory assessments, such as the protocol for sensory examination of roughage [25], offer practical advantages due to ease and speed, allowing early identification of potentially problematic forage. However, sensory examinations are inherently subjective and may fail to detect subtle microbiological contamination or quantify dust exposure accurately. Consequently, microbiological analyses, referencing established standards (Verband Deutscher Landwirtschaftlicher Untersuchungs und Forschungsanstalten, VDLUFA) [26], represent the gold standard to quantitatively evaluate bacterial, fungal, and yeast contents in hay samples.
The relationship between sensory evaluation and objective measures of hay quality remains poorly defined. Previous studies examining agreement between sensory and microbiological assessments yielded mixed results. Intemann et al. [14] found moderate correlations between sensory-detected abnormal odor and microbiological contamination levels, whereas Stickdorn et al. [27] reported weaker associations overall but noted trends relating sensory deficiencies to mold and yeast contamination. Importantly, no studies have directly examined whether sensory evaluation can predict dust release, despite its relevance for equine respiratory health. Thus, the practical value of sensory assessment as a predictive tool for hay safety remains uncertain.
This exploratory study aims to investigate the value of specific sensory attributes assessed using the adapted Kamphues protocol [25] to predict objective measures of hay quality, specifically dust concentrations and microbiological contamination. We hypothesized that certain sensory attributes would be positively associated with higher dust concentrations and greater microbiological contamination. By exploring sensory examination as a predictive tool, this research seeks to enhance the practical applicability of simple on-site evaluations for routine hay screening, thereby contributing to improved equine respiratory health management and prevention strategies.
2. Materials and Methods
2.1. Study Design and Sample Collection
This exploratory study employed a cross-sectional design to investigate the associations of sensory attributes with microbiological contamination and with dust concentrations in hay samples. Data on these hay samples, particularly regarding dust concentrations, were collected in a previous experimental study conducted at the Swiss Institute of Equine Medicine in Avenches, Switzerland, from April to October 2023, in which measurements obtained with a Hay-Shaker were compared to corresponding measurements in the horses’ breathing zone [28]. Fifty hay samples, each weighing approximately 5 kg, were collected by horse owners from recently opened hay bales. The hay samples consisted of untreated dry hay. Each hay sample was manually homogenized by gently mixing it in a large 110 L garbage bag. The homogenized material was then subdivided into two 1 kg sub-samples. During this process, portions were taken from the top, middle, and bottom of the bag to account for dust settling. Each sub-sample was placed in a separate 110 L garbage bag for subsequent sensory evaluation and dust analysis, with gentle mixing performed again in the smaller bags to ensure uniformity. Samples were weighed using a digital luggage scale (INTERTRONIC Digital Pèse-bagages, InterDiscount, Jegenstorf, Switzerland). Additionally, a 200 g sub-sample was prepared for microbiological analysis, weighted with a precision scale (SOEHNLE 65105 1 Style, Soehnle Industrial Solutions GmbH, Backnang, Germany) and sent to the Posieux Agroscope laboratory on the same day of collection.
2.2. Data Collection
2.2.1. Sensory Examination
The sensory examination was conducted according to the Kamphues protocol [25] (as described in Table 1), assessing four specific criteria: odor, texture, color, and visible impurities (Figure 1). Two trained examiners performed independent assessments blinded to the results of the other analyses. Scores were assigned for each attribute according to standardized criteria, with deductions ranging from 0 to −5 (texture and color), and from 0 to −10 (odor and impurities).

Table 1.
Sensory examination of hay hygienic status based on the criteria odor, texture, color and impurities. The sum of the deficiencies gives a total score (sum of points deducted).

Figure 1.
Illustration of sensory examination including odor, texture, color and impurities. (a) The examiner assessed the odor of the hay; (b) Hay color, and the presence of impurities were assessed visually, and haptic assessment (sense of touch) was used to evaluate texture.
2.2.2. Measurement of Dust Generated with the HS
A detailed description of this Hay-Shaker and its measurements are given in Bouverat et al. [28]. Dust concentrations were measured using the Hay-Shaker device in combination with the DustTrak DRX 8534 (TSI Incorporated, Shoreview, MN, USA) to generate dust from hay under controlled conditions independent of horse-related factors. The equipment provides real-time data on PM fractions, including PM1, PM2.5, PM4, PM10, representing particle size in μm, and total PM (PMT) in mg/m3. Each measurement was performed over a period of 10 min, with one data point recorded every second, resulting in a total of 600 values for each PM fraction per horse. Measurements were calibrated with ISO 12103-1 A1 [29] test dust and using airflow zero calibrations before and after each experiment, and the testing environment was controlled for consistent data collection, as described in detail by Bouverat et al. [28].
2.2.3. Microbiological Analyses
Sample preparation and platings.
The 50 hay samples were analyzed for the microbiological quality according to the VDLUFA method 28.1.1, 28.1.2, 28.1.3 and 28.1.4 [26]. Hay samples were manually cut down into approximatively <5 cm pieces with disinfected scissors. Twenty grams of the cut-down sample (including dust) were added to 380 mL of buffered peptone water (Millipore, Billerica, MA, USA, 1.07228) into a filtered stomacher bag, let soak for 5 min and homogenized with a stomacher for 5 min. The suspension was then decimally diluted. 0.1 mL of each dilution were pipetted onto two Petri dishes filled with appropriate culture media and evenly spread. The culture medium for aerobic mesophilic bacteria was Tryptose agar (Sigma-Aldrich, St. Louis, MO, USA, T2313) supplemented with 30 µM 2,3,5-Triphenyltetrazolium chloride (Sigma-Aldrich, St. Louis, MO, USA, 17770). The first culture media for yeasts and molds was Dichlorane Glycerol Agar (DG18 Agar, Oxoid, Basingstoke, UK, CM0729) supplemented with 120 µM oxytetracycline hydrochloride (Sigma-Aldrich, St. Louis, MO, USA, O5875). The second culture media for yeasts and molds was Rose-Bengal Agar (Oxoid, Basingstoke, UK, CM0549) with Tergitol (0.01% final) and 120 µM oxytetracycline hydrochloride (Sigma-Aldrich, St. Louis, MO, USA, O5875). The inoculated Perti dishes were then incubated upside-down according to the VDLUFA method (30 °C, 2 days for bacteria and 25 °C, 3 days for yeasts and molds, respectively).
Counting and classification of micro-organisms.
The colony-forming units (CFU) of aerobic mesophilic bacteria (AMB), molds and yeasts were each counted on their respective media (with regard to the dilutions they originated from). Petri dishes with CFU numbers ranging between 20 and 200 CFUs were considered. The obtained concentrations were given in CFU per gram. Then, each CFU was classified into 7 categories of micro-organisms according to their ecology and hazards: Group 1 represents the product-typical bacteria (such as members of the genera Pantoea, Enterobacter, Pseudomonas and Enterobacteriaceae to name a few). Group 2 includes the spoilage-indicating bacteria (including members of the genera Bacillus, Staphylococcus, Micrococcus). Group 3 concerns the spoilage-indicating Streptomycetes spp. Group 4 includes the product-typical fungi, living “on the field” and represented, among others, by the Dematicaeae and the genera Verticillium, Acremonium, Aureobasidium and Fusarium). Group 5 encompasses the spoilage-indicating molds, like the genera Penicillium, Aspergillus, Wallemia and others. Group 6 is formed by the spoilage-indicating Mucorales (like Mucor spp., Rhizopus spp. or Absidia spp.). Finally, Group 7 is made of all species of yeasts, which are also considered indicators of spoilage.
Assessment of the overall microbiological quality of hay.
The calculated concentrations of each of the 7 groups in a sample were compared to the orientation values for hay, as established by the VDLUFA method 28.1.4. The given orientations values for hay are shown in Table 2 (version 2024). If the concentrations of one (or more) of the 7 groups found in a sample were equal or below the concentrations given in the orientation values, the hay was considered “normal” (grade 1) in terms of microbiological quality. If the orientation values were exceeded up to 5 times by the concentrations found in the sample (in any of the 7 groups), the hay received a “slightly reduced” microbiological quality (grade 2). If the orientation values were exceeded up to 10 times by the concentrations found in the sample (in any of the 7 groups), the hay received a “distinctly reduced” microbiological quality (grade 3). If the orientations values were exceeded more than 10 times, then the microbiological quality was assessed as “significantly altered” (grade 4, i.e., not acceptable for feeding).

Table 2.
Microbiological assessment and the corresponding orientation values as colony forming units per gram (CFU/g), as established by the VDLUFA (Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten).
2.3. Data Processing
For statistical analyses we considered the mean PM levels (PM1, PM2.5, PM4, PM10, PMT described above in Section 2.2.2. and in [28] as the first continuous outcome variables. The other outcome variable, microbiological quality, entered the analyses as a categorical characteristic (grades 1–4) as outlined above in Section 2.2.3. Three types of summary ratings were generated from the sensory examination to assess hay quality. Rating 1 (Adapted from the protocol of Kamphues et al. 2024 [25]) reflected the overall hay quality based on the total score, Rating 2 considered the individual scores for odor, texture, color, and impurities, and Rating 3 applied a binary classification of the same four criteria. The specific scoring systems and categories for each rating are summarized in Table 3.

Table 3.
Overview of the three summary ratings generated from the sensory examination of hay samples. Rating 1 represents the total hay quality score, categorized into four quality levels. Rating 2 shows the individual scores assigned to each of the four criteria (odor, texture, color, impurities). Rating 3 applies a binary classification for the same four criteria, with 0 indicating acceptable and 1 indicating problematic. The table summarizes the scoring systems and categories for all three ratings.
2.4. Statistical Analyses
Statistical analyses were performed using R version 4.4.3 (R Core Team 2025; R Foundation for Statistical Computing, Vienna, Austria). For data import and export the package rio was used [30]. Descriptive statistics were computed for sensory scores (categorical) and microbiological scores (MBS, categorical), as presented in this paper. Descriptive statistics for all PM variables (continuous) are presented in Table 1 of Bouverat et al. [28].
Shapiro–Wilk tests were performed to test normality. PM measurements were square root-transformed to stabilize variance before regression analysis. Ordered logistic regressions were calculated using the package MASS [31]. To check the parallel line assumption, the package brant was used [32]. Models were compared using the package performance [33]. Parameters were extracted from the models either by hand or by using the package parameters [34]. For plotting the packages ggplot2 [35] and patchwork [36] were used. Model selection was based exclusively on the Bayesian Information Criterion (BIC). The significance level for all statistical tests was 0.05.
Agreement of sensory scores between the two examiners was assessed by Kendall’s tau-b correlation and Wilcoxon signed-rank test.
3. Results
3.1. Descriptive Statistics
3.1.1. Sensory Examination
Out of 50 evaluated hay samples, according to Rating 1, 28% (n = 14) were classified as adequate, 52% (n = 26) exhibited minor deficiencies, 16% (n = 8) had major deficiencies, and 4% (n = 2) demonstrated massive deficiencies.
For Rating 2, the vast majority of hay samples (98%, 49 out of 50) scored within the acceptable range for each sensory criterion: odor (0 to −5), texture (0 to −2), color (0 to −2), and impurities (0 to −5). Only 2% (1 sample) showed deficiencies with more negative scores in all categories: odor (−5.5 to −10), texture (−2.5 to −5), color (−2.5 to −5), and impurities (−5.5 to −20).
For Rating 3, which classifies sensory criteria as acceptable (0) or problematic (1), the proportion of hay samples rated problematic was odor 52% (26/50), texture 62% (31/50), color 62% (31/50), and impurities 80% (40/50). The remaining samples were classified as acceptable, corresponding to 48%, 38%, 38%, and 20%, respectively.
3.1.2. Microbiological Analysis
The microbiological analyses indicated substantial variability among samples. Overall, 46% (n = 23 grade 1) of hay samples were classified within normal microbiological quality limits. Slightly reduced quality was observed in 30% (n = 15 grade 2), distinctly reduced quality in 6% (n = 3 grade 3), and significantly altered quality in 18% (n = 9 grade 4) of the samples.
As shown in Table 4, hay samples with a score of 2 most frequently exceeded guideline values for field flora indicators, in particular for molds (80%) and AMB (20%), while yeasts were also commonly elevated (53%). As expected, samples with scores of 3 and 4 showed more frequent deviations in spoilage-related microorganisms. Among score 4 samples, 89% exceeded limits for spoilage-associated molds, 11% for spoilage-associated AMB, and 11% for Mucorales. These results indicate a shift from field flora exceedances at score 2 toward spoilage-related contamination at higher sensory scores.

Table 4.
Number of microbial groups exceeding VDLUFA (Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten) guideline values per microbiological score level. Grades were assigned based on the highest number of exceedances. Multiple exceedances per sample were possible.
3.2. Agreement of Sensory Examination Scores Between the Two Examiners
To evaluate consistency between the two examiners, Kendall’s tau-b was used, showing a strong correlation (τ = 0.842). An exact Wilcoxon signed-rank test revealed a small but significant difference between scores (p = 0.037), indicating a systematic divergence despite overall agreement, with one examiner tending to assign more severe scores than the other.
3.3. Regression Analyses: Sensory Predictors of Dust Concentrations
Rating 1, Rating 2 and Rating 3 were evaluated as predictors for dust concentration. A total of nine regression models (Supplementary Tables S1–S5) were constructed, varying in the combination of sensory predictors included. Across all PM fractions, models including binary sensory traits (Rating 3) consistently outperformed those based on the global score (Rating 1) or continuous sub-scores (Rating 2).
The best-performing model for PM1, PM2.5, PM4 and PMT, based on the BIC, included only “odor2”. From all the binary Rating 3 predictors, abnormal odor thus emerged as the most robust and consistent indicator of increased dust, explaining 30–40% of the variance (adjusted R2 values ranging from 0.31 to 0.40) across all PM fractions. Models including “Impurities2” yielded similar BIC values but did not offer significant improvement (p > 0.05) for these fractions, suggesting that impurity could play a role, though the limited number of data points may have influenced these results.
For PM10, however, impurity2 significantly improved model performance and was retained alongside odor, suggesting an additive effect. In contrast, texture and color provided limited predictive value across all PM fraction outcomes.
To illustrate, Table 5 presents both the initial full model (including all four binary variables) and the final model (odor2 only) for PM4.

Table 5.
Linear regression results for the outcome square root of particulate matter (PM) < 4 um in the Hay-Shaker and the binary predictors odor, texture, color and impurities as evaluated in the sensory examination (Rating 3). Intercept and coefficients with their corresponding p-values for the initial model are shown on the left, and for the final model after stepwise selection on the right.
Figure 2 illustrates this relationship for PM4 and PMT, with significantly higher dust levels observed in hay samples rated as having abnormal odor.
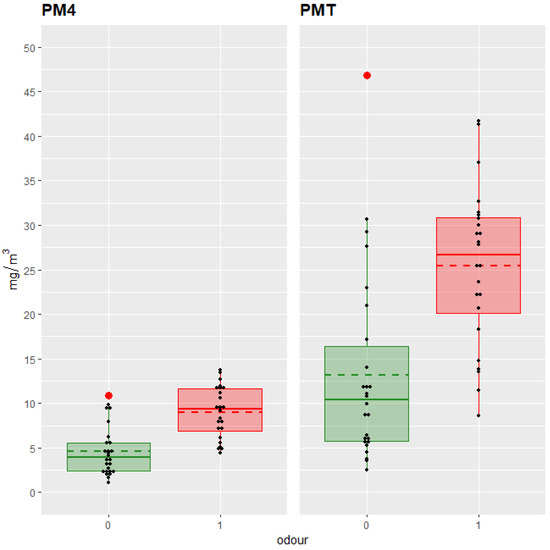
Figure 2.
Box-and-whiskers plots of mean dust concentration (mg/m3) of particulate matter < 4 μm (PM4) and total particulate matter (PMT) in the Hay-Shaker by Odor (0: adequate (green boxes and lines); 1; abnormal (red boxes and lines); n = 50). Individual data points are shown in black, outliers are highlighted in red, and dashed lines indicate the group means.
3.4. Regression Analyses: Sensory Predictors of Microbiological Quality
A total of ten regression models were evaluated to assess the predictive value of sensory assessment (Rating 1–3) for microbiological quality categories, as summarized in Supplementary Table S6. The models based on the continuous scoring of the sensory components: odor, texture, color and impurities (Rating 2) provided the best overall performance, both in terms of model fit (lower BIC) and assumption validity.
The best-fitting model (Table 6) included only impurity and showed that higher impurity levels tended to be associated with increased odds of microbiological contamination (OR = 0.541, p = 0.059). Despite the marginal significance, the model met the proportional odds assumption.

Table 6.
Final model: odds ratio for impurity score (Rating 2) and Brant test result.
In contrast, models incorporating binary sensory variables (odor2, texture2, color2, impurities2) exhibited higher BIC values, indicating poorer model fit and violated the parallel regression assumption, which assumes that the relationship between the predictor variables and the outcome is the same across all levels of the outcome variable. Moreover, these binary variables were not statistically significant, suggesting that they do not contribute effectively to the prediction of microbiological contamination.
4. Discussion
The increasing importance of equine asthma and its links to environmental organic dust exposures underlines the need for practical and reliable tools to assess forage quality. This study addressed whether simple sensory examination could predict dust concentration and microbiological contamination in hay samples. Our findings suggest that sensory examination, particularly assessment of abnormal odor, effectively predicts dust exposure across all PM fractions, while impurities may be useful for predicting microbiological contamination.
To our knowledge, this is the first systematic study to correlate structured sensory scoring with objective dust measures across a substantial number of hay samples. Among the sensory parameters assessed, abnormal odor consistently emerged as the most robust predictor of increased dust concentrations, across all PM fractions, explaining 30–40% of the variance. This specifically included the respirable particle fraction (PM4), which has been shown to be particularly relevant as a risk factor of equine asthma [15]. This observation aligns with previous reports indicating sensory detection of musty or moldy odors typically reflects deviating germ counts [14], mold contamination [14,24] and elevated respirable dust concentrations [17]. The clinical manifestation of respiratory signs, such as coughing, has been associated with elevated mold levels in the environment, particularly in hay and stable air, with Aspergillus spp. identified as a relevant respiratory allergen and potential trigger of equine asthma [14,37]. While odor assessment like all sensory examination is subjective, its consistent association with PM levels suggests it holds diagnostic value for identifying hay that may pose respiratory risks.
Similarly, visible impurities, including molds, beetles, and mites, were found to be associated with microbiological contamination and showed predictive potential for PM10 concentrations, with near-significant associations for other PM fractions. This dual role supports the utility of the impurity assessment not only for identifying microbial spoilage but also for estimating dust exposure. However, the presence of such impurities does not always reflect deficiencies likely to influence dust concentration or microbiological contamination. For instance, the presence of autumn crocus (Colchicum autumnalis) does not necessarily indicate such issues. Similarly, blister beetles (not found in temperate climates as in Switzerland) could be directly harmful to horses. Moreover, only a limited number of hay samples with marked impurity levels were included in the study; nevertheless, these samples showed a clear trend. While our results align with Intemann et al. [14], who reported similar associations between sensory examination and microbiological analysis, it is important to note that their study included a detailed analysis of individual microbial groups (e.g., aerobic bacteria, molds and yeasts). In contrast, we focused our statistical analyses on the overall microbiological classification (grades 1 to 4) according to the VDLUFA system [26]. This classification is itself derived from the quantified exceedance of threshold values in at least one microbial category (e.g., molds, yeasts or aerobic mesophilic bacteria), thereby integrating multiple contamination indicators into a single score. While we conducted descriptive analyses of these subcategories (Table 4), we did not formally model their individual associations with sensory scores. This approach allowed us to simplify the interpretation while maintaining a quantitative and standardized assessment of microbiological quality.
The microbiological profile of hay is influenced by harvest and storage conditions [25]. While the largest proportion of hay samples was graded as adequate (grade 1), in our study, the second largest group of hay samples with a grade 2 classification (slightly reduced) were most commonly dominated by field flora components, including molds and aerobic mesophilic bacteria (AMB) which reflect the normal environmental microbiota acquired during harvest. Their overrepresentation in these samples suggests suboptimal harvest conditions, such as high humidity or mechanical damage, rather than significant spoilage. Although not strictly part of the field flora, yeasts are commonly found in these samples and can proliferate under damp or anaerobic conditions, indicating suboptimal storage. In contrast, grades 3 and 4, which combined were assigned to almost a quarter of all hay samples, were primarily characterized by spoilage-indicating organisms, including principally molds, AMB and Mucorales, reflecting advanced microbial degradation during storage under poor conditions (elevated humidity, heat or physical damage, i.e., crushing, trampling, or fragmentation). This microbial shift is accompanied by a decrease in species diversity and an increase in spoilage-indicating bacteria and molds, along with sensory changes like musty or putrid odors and visible degradation [7]. These findings illustrate how the VDLUFA classification [26] link microbial contamination levels to hay quality, providing insight into harvest- and storage-related microbial risks.
While sensory assessment is based on seemingly simple traits such as odor and visible impurities, these attributes reflect complex biological processes—such as the production of volatile organic compounds by molds or bacteria during storage, or the presence of microbial and physical contaminants. Despite its simplicity and subjectivity, sensory evaluation offers an accessible, rapid, and cost-effective screening tool for daily use in stables, especially where laboratory diagnostics are not readily available. Standardized scoring systems such as the Kamphues protocol [25] can enhance its practicality and reproducibility. Although sensory assessment cannot replace precise dust measurements or comprehensive microbiological analyses, our findings suggest it holds value as a frontline screening method to help identify hay potentially unsuitable for horses at risk of equine asthma.
However, the limited predictive value of the sensory criteria texture and color highlights the need for careful interpretation. Possibly, abnormal “musty” odor may be an early sign of spoilage, preceding changes in color or texture. Furthermore, the subjective nature of these attributes potentially accounts for their weak correlations with microbiological contamination, echoing Stickdorn et al.’s [27] findings. Although overall inter-rater correlation was good, the tests revealed significant differences in severity perception between examiners, suggesting some level of rater-dependent scoring. Including a larger number of examiners in future studies could improve the assessment of inter-rater reliability and reduce individual bias. In this context, binary scoring systems, like Rating 3, which classify traits simply as normal or abnormal, may offer a more robust and reproducible alternative by reducing the influence of subjective gradation and simplifying decision-making in field conditions. Despite statistical model validation, several additional limitations must be considered. The sample size was modest, and severely contaminated hay samples were underrepresented, which may have restricted the variability observed. Moreover, no clinical respiratory health data were collected to directly link dust or microbiological levels with equine asthma. Finally, the explanatory power of our models was modest (R2 = 0.31–0.40), indicating that although odor and impurity assessments showed predictive potential, their ability to explain dust and microbiological variability is limited. Taken together, these factors underline that the present results should be regarded as exploratory. Nevertheless, sensory assessment offers a rapid and practical screening tool that can help avoid feeding dusty or microbiologically inadequate hay to horses at risk for equine asthma.
Future research involving larger, more diverse datasets, a greater number of examiners for sensory examination, and direct clinical correlations is necessary to further refine sensory protocols to enhance predictive accuracy. While sensory scoring offers a rapid low-cost approach, to assess dust levels it should ideally be complemented by objective tools, such as a recently described wearable real-time particulate monitor [38]. Furthermore, the present study concentrated on hay as a major dust source and contributor to lower airway inflammation. However, it would also be valuable to investigate the relationship of sensory examination with dust levels and microbiological evaluation of straw, which is commonly used as bedding material and can significantly contribute to organic dust exposure implicated in equine asthma [39]. Notably, a survey of 46 horse farms in Switzerland, found that the hygienic standard of straw was worse than that of hay [40]. Future research should build upon these findings to optimize sensory methods for comprehensive forage and potentially bedding quality assessment.
5. Conclusions
The study underscores the utility of sensory examination—particularly abnormal odor and visible impurities—as an effective initial screening tool for hay quality. Incorporating such simple and cost-effective assessments into routine stable management practices could significantly contribute to improved equine respiratory health outcomes by enabling timely interventions. The sensory analysis used was essential to identify hay samples unsuitable for horse consumption due to the high proportion of moldy and even spoiled material.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani15182688/s1, Table S1: Summary of linear regression models for PM1 fraction based on sensory examination ratings; Table S2: Summary of linear regression models for PM2.5 fraction based on sensory examination ratings; Table S3: Summary of linear regression models for PM4 fraction based on sensory examination ratings; Table S4: Summary of linear regression models for PM10 fraction based on sensory examination ratings; Table S5: Summary of linear regression models for PMT fraction based on sensory examination ratings; Table S6: Summary of linear regression models for microbiological scoring based on sensory examination ratings.
Author Contributions
Original draft, V.M.A.B.; Conceptualization, V.M.A.B. and V.G.; Sensory examination including training, V.M.A.B., E.G. and B.A.W.; microbiological analyses, N.P.; Statistical analyses, G.J.D. and V.M.A.B.; Writing—review and editing, V.M.A.B., N.P., B.A.W., E.G., G.J.D. and V.G.; supervision and funding acquisition, V.G. All authors have read and agreed to the published version of the manuscript.
Funding
Internal Research Fund of the Swiss Institute of Equine Medicine, Bern, Switzerland (ISMEquine Research No. 33-890). This study was also supported by a foundation based in Geneva, Switzerland wishing to remain anonymous.
Institutional Review Board Statement
This study did not require approval by an institutional animal care and use committee, as it did not involve any experimental procedures on animals.
Informed Consent Statement
The person in Figure 1 provided consent for publication of the figure in this research.
Data Availability Statement
The original data presented in the study are openly available in Zenodo at [10.5281/zenodo.16412564].
Acknowledgments
We acknowledge the support of the horse owners who provided hay samples and BEST OF HORSE HAY for support with recruitment. We thank Sophie Sage for her precious feedback on the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PM | Particulate Matter |
| VDLUFA | Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten |
| CFU | colony-forming units |
| BIC | Bayesian Information Criterion |
| AMB | Aerobic mesophilic bacteria |
References
- Couetil, L.; Cardwell, J.M.; Leguillette, R.; Mazan, M.; Richard, E.; Bienzle, D.; Bullone, M.; Gerber, V.; Ivester, K.; Lavoie, J.-P.; et al. Equine Asthma: Current Understanding and Future Directions. Front. Vet. Sci. 2020, 7, 450. [Google Scholar] [CrossRef]
- Art, T.; McGorum, B.C.; Lekeux, P. Environmental Control of Respiratory Disease. In Equine Respiratory Diseases; International Veterinary Information Service: Ithaca, NY, USA, 2002; Available online: https://www.ivis.org/library/equine-respiratory-diseases/environmental-control-of-respiratory-disease (accessed on 25 July 2025).
- Couëtil, L.L.; Cardwell, J.M.; Gerber, V.; Lavoie, J.-P.; Léguillette, R.; Richard, E.A. Inflammatory Airway Disease of Horses-Revised Consensus Statement. J. Vet. Intern. Med. 2016, 30, 503–515. [Google Scholar] [CrossRef]
- Diez de Castro, E.; Fernandez-Molina, J.M. Environmental Management of Equine Asthma. Animals 2024, 14, 446. [Google Scholar] [CrossRef] [PubMed]
- Ivester, K.M.; Couëtil, L.L. Management of chronic airway inflammation in the horse: A systematic review. Equine Vet. Educ. 2014, 26, 647–656. [Google Scholar] [CrossRef]
- Mańkowska, A.; Witkowska, D. The Most Common Environmental Risk Factors for Equine Asthma—A Narrative Review. Animals 2024, 14, 2062. [Google Scholar] [CrossRef] [PubMed]
- Kamphues, J. Feed hygiene and related disorders in horses. In Equine Applied and Clinical Nutrition; Geor, R.J., Harris, P.A., Coenen, M., Eds.; Elsevier Health Sciences: Maryland Heights, MO, USA, 2013; pp. 367–380. [Google Scholar]
- Robinson, N.E. International Workshop on Equine Chronic Airway Disease. Michigan State University 16–18 June 2000. Equine Vet. J. 2001, 33, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Pirie, R.S.; Dixon, P.M.; McGorum, B.C. Evaluation of nebulised hay dust suspensions (HDS) for the diagnosis and investigation of heaves. 3: Effect of fractionation of HDS. Equine Vet. J. 2002, 34, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Beeler-Marfisi, J.; Clark, M.E.; Wen, X.; Sears, W.; Huber, L.; Ackerley, C.; Viel, L.; Bienzle, D. Experimental induction of recurrent airway obstruction with inhaled fungal spores, lipopolysaccharide, and silica microspheres in horses. Am. J. Vet. Res. 2010, 71, 682–689. [Google Scholar] [CrossRef]
- Olave, C.J.; Ivester, K.M.; Couetil, L.L.; Kritchevsky, J.E.; Tinkler, S.H.; Mukhopadhyay, A. Dust exposure and pulmonary inflammation in Standardbred racehorses fed dry hay or haylage: A pilot study. Vet. J. 2021, 271, 105654. [Google Scholar] [CrossRef]
- McPherson, E.A.; Lawson, G.H.; Murphy, J.R.; Nicholson, J.M.; Breeze, R.G.; Pirie, H.M. Chronic obstructive pulmonary disease (COPD): Factors influencing the occurrence. Equine Vet. J. 1979, 11, 167–171. [Google Scholar] [CrossRef]
- Clarke, A.F.; Madelin, T. Technique for assessing respiratory health hazards from hay and other source materials. Equine Vet. J. 1987, 19, 442–447. [Google Scholar] [CrossRef]
- Intemann, S.; Reckels, B.; Schubert, D.; Wolf, P.; Kamphues, J.; Visscher, C. The Hygienic Status of Different Forage Types for Horses-A Retrospective Study on Influencing Factors and Associations with Anamnestic Reports. Vet. Sci. 2022, 9, 226. [Google Scholar] [CrossRef]
- Ivester, K.M.; Couëtil, L.L.; Zimmerman, N.J. Investigating the link between particulate exposure and airway inflammation in the horse. J. Vet. Intern. Med. 2014, 28, 1653–1665. [Google Scholar] [CrossRef]
- Pearson, C.C.; Sharples, T.J. Airborne Dust Concentrations in Livestock Buildings and the Effect of Feed. J. Agric. Eng. Res. 1995, 60, 145–154. [Google Scholar] [CrossRef]
- Vandenput, S.; Istasse, L.; Nicks, B.; Lekeux, P. Airborne dust and aeroallergen concentrations in different sources of feed and bedding for horses. Vet. Q. 1997, 19, 154–158. [Google Scholar] [CrossRef]
- Pirie, R.S.; Collie, D.D.S.; Dixon, P.M.; McGorum, B.C. Inhaled endotoxin and organic dust particulates have synergistic proinflammatory effects in equine heaves (organic dust-induced asthma). Clin. Exp. Allergy 2003, 33, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Moore-Colyer, M.J.; Taylor, J.L.; James, R. The Effect of Steaming and Soaking on the Respirable Particle, Bacteria, Mould, and Nutrient Content in Hay for Horses. J. Equine Vet. Sci. 2016, 39, 62–68. [Google Scholar] [CrossRef]
- Moore-Colyer, M.J.S.; Lumbis, K.; Longland, A.; Harris, P. The effect of five different wetting treatments on the nutrient content and microbial concentration in hay for horses. PLoS ONE 2014, 9, e114079. [Google Scholar] [CrossRef]
- Dauvillier, J.; ter Woort, F.; van Erck-Westergren, E. Fungi in respiratory samples of horses with inflammatory airway disease. J. Vet. Intern. Med. 2019, 33, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Berndt, A.; Derksen, F.J.; Robinson, N.E. Endotoxin concentrations within the breathing zone of horses are higher in stables than on pasture. Vet. J. 2010, 183, 54–57. [Google Scholar] [CrossRef]
- McGorum, B.C.; Dixon, P.M.; Halliwell, R.E. Responses of horses affected with chronic obstructive pulmonary disease to inhalation challenges with mould antigens. Equine Vet. J. 1993, 25, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.; Coenen, M.; Kamphues, J. A survey on the hygienic standard of feeds for horses associated with diseases. PHK 2005, 21, 24–25. [Google Scholar] [CrossRef][Green Version]
- Kamphues, J.; Wolf, P.; Coenen, M.; Eder, K.; Liesegang, A.; Paßlack, N.; Vervuert, I.; Visscher, C.; Zebeli, Q.; Zentek, J. Supplemente zur Tierernährung für Studium und Praxis; Schlütersche Verlagsgesellschaft mbH & Company KG: Hannover, Germany, 2024; ISBN 9783842600843. [Google Scholar]
- Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten (VDLUFA). Methodenbuch Band III—Die Untersuchung von Futtermitteln, Methods 28.1.1 to 28.1.4. ed.; VDLUFA-Verlag: Darmstadt, Germany, 2024. [Google Scholar]
- Stickdorn, T.; Ellis, A.; Kienzle, E. Horse feed hygiene evaluation with microbial and sensory examination. EAAP Sci. Ser. 2012, 132, 255–262. [Google Scholar] [CrossRef]
- Bouverat, V.M.A.; Naef, J.; Dolf, G.; Lamon, I.; Sage, S.E.; Gerber, V. Minimising feeding behaviour interference: A hay-shaker device to assess dust exposure in horses. Equine Vet. J. 2025. [Google Scholar] [CrossRef]
- ISO 12103-1; Road Vehicles—Test Contaminants for Filter Evaluation—Part 1: Arizona Test Dust. International Organization for Standardization: Geneva, Switzerland, 2024.
- Chan, C.; Leeper, T.; Becker, J.; Schoch, D. rio: A Swiss-Army Knife for Data File I/O. 2023. Available online: https://cran.r-project.org/web/packages/rio/index.html (accessed on 25 July 2025).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; corr. print; Springer: New York, NY, USA, 2007; ISBN 9780387954578. [Google Scholar]
- Schlegel, B.S.M. brant: Test for Parallel Regression Assumption. R Package Version 0.3-0. 2020. Available online: https://cran.r-project.org/web/packages/brant/index.html (accessed on 25 July 2025).
- Lüdecke, D.; Ben-Shachar, M.; Patil, I.; Waggoner, P.; Makowski, D. performance: An R Package for Assessment, Comparison and Testing of Statistical Models. JOSS 2021, 6, 3139. [Google Scholar] [CrossRef]
- Lüdecke, D.; Ben-Shachar, M.; Patil, I.; Makowski, D. Extracting, Computing and Exploring the Parameters of Statistical Models using R. JOSS 2020, 5, 2445. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Pedersen, T.L. patchwork: The Composer of Plots. R Package Version 1.3.0. 2024. Available online: https://CRAN.R-project.org/package=patchwork (accessed on 25 July 2025).
- Ward, M.P.; Couëtil, L.L. Climatic and aeroallergen risk factors for chronic obstructive pulmonary disease in horses. Am. J. Vet. Res. 2005, 66, 818–824. [Google Scholar] [CrossRef]
- Ivester, K.M.; Ni, J.-Q.; Couetil, L.L.; Peters, T.M.; Tatum, M.; Willems, L.; Park, J.H. A wearable real-time particulate monitor demonstrates that soaking hay reduces dust exposure. Equine Vet. J. 2024, 57, 1065–1073. [Google Scholar] [CrossRef]
- Siegers, E.W.; Anthonisse, M.; van Eerdenburg, F.J.C.M.; van den Broek, J.; Wouters, I.M.; Westermann, C.M. Effect of ionization, bedding, and feeding on air quality in a horse stable. J. Vet. Intern. Med. 2018, 32, 1234–1240. [Google Scholar] [CrossRef]
- Wichert, B.; Nater, S.; Wittenbrink, M.M.; Wolf, P.; Meyer, K.; Wanner, M. Judgement of hygienic quality of roughage in horse stables in Switzerland. J. Anim. Physiol. Anim. Nutr. 2008, 92, 432–437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).