Molecular Detection of Leishmania (V.) braziliensis and Leishmania (M.) martiniquensis Infecting Domestic Animals from Panama, Central America
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Serological Analysis
2.3. Molecular Analysis
2.3.1. DNA Extraction
2.3.2. PCR
2.4. Sequencing
2.5. Ethical Statement
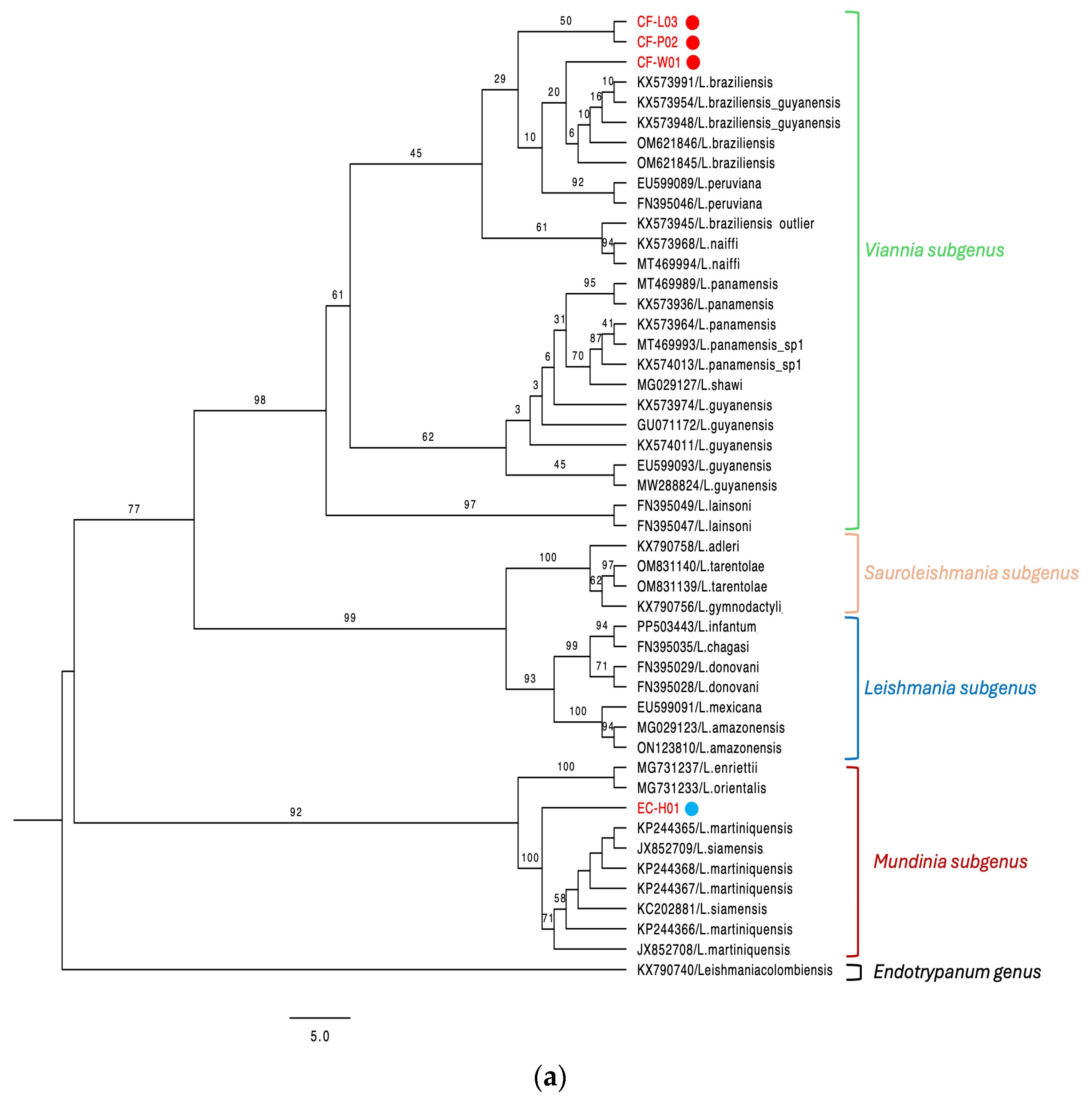
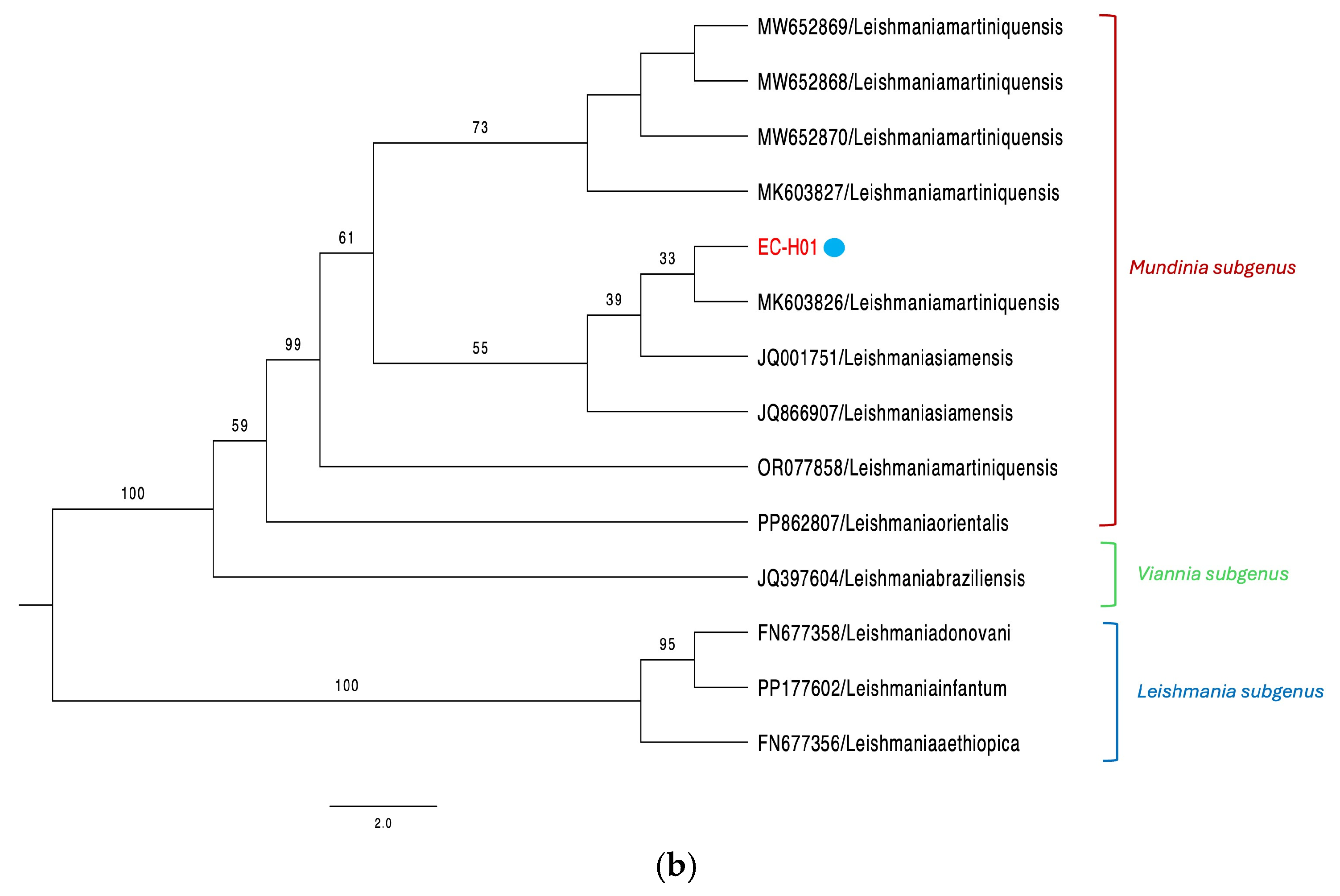
3. Results
3.1. Canine Cases
3.2. Equine Cases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACL | American cutaneous leishmaniasis |
| IFA | Indirect immunofluorescence assay |
| CL | Cutaneous leishmaniasis |
| CVL | Canine visceral leishmaniasis |
References
- Pan American Health Organization. Plan of Action to Strengthen the Surveillance and Control of Leishmaniasis in the Americas 2023–2030; Pan American Health Organization: Washington, DC, USA, 2024. [Google Scholar]
- Pan American Health Organization. Manual of Procedures for Leishmaniasis Surveillance and Control in the Region of the Americas; Pan American Health Organization: Washington, DC, USA, 2024. [Google Scholar]
- Pan American Health Organization. Leishmaniasis: Epidemiological Report for the Americas; Pan American Health Organization: Washington, DC, USA, 2024. [Google Scholar]
- Lainson, R. The Neotropical Leishmania Species: A Brief Historical Review of Their Discovery, Ecology and Taxonomy. Rev Pan-Amaz. Saude 2010, 1, 25–33. [Google Scholar] [CrossRef]
- Miranda, A.; Del, C.; González, K.A.; Samudio, F.; Pineda, V.J.; Calzada, J.E.; Capitan-Barrios, Z.; Jiménez, A.; Castillo, J.; Mendoza, Y.; et al. Molecular Identification of Parasites Causing Cutaneous Leishmaniasis in Panama. Am. J. Trop. Med. Hyg. 2021, 104, 1326. [Google Scholar] [CrossRef]
- Davila, M.; Pineda, V.; Calzada, J.E.; Saldaña, A.; Samudio, F. Evaluation of Cytochrome b Sequence to Identify Leishmania Species and Variants: The Case of Panama. Mem. Inst. Oswaldo Cruz. 2021, 116, e200572. [Google Scholar] [CrossRef]
- Vásquez, A.; Paz, H.; Alvar, J.; Perez, D.; Hernandez, C. Informe Final: Estudios Sobre La Epidemiología de la Leishmaniasis Panamá, en la Parte Occidental de la República de Panamá; Instituto Conmemorativo Gorgas de Estudios de la Salud-MINSA: Panama, Panama, 1998. [Google Scholar]
- de Vasquez, A.M.; Christensen, H.A.; Petersen, J.L. Short Report Epidemiologic Studies on Cutaneous Leishmaniasis in Eastern Panama. Am. J. Trop. Med. Hyg. 1999, 60, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Reina, A.M.; Mewa, J.C.; Calzada, J.E.; Saldaña, A. Characterization of Leishmania spp. Causing Cutaneous Lesions with a Negative Parasitological Diagnosis in Panama. Trop. Med. Infect. Dis. 2022, 7, 282. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Canine Leishmaniasis in the Americas: Etiology, Distribution, and Clinical and Zoonotic Importance. Parasit. Vectors 2024, 17, 198. [Google Scholar] [CrossRef]
- Barrera, C.; Le Pont, F.; Dereure, P.J.; Barrera, C.; Guerrini, F.; Martini, A.; Echeverria, R.; Guderianz, R.; Pont, F.L. Infestation Naturelle Du Chien Par Leishmania Panamensis. Ann. Soc. Belg. Med. Trop. 1994, 74, 29–33. [Google Scholar]
- Kent, A.; Ramkalup, P.; Mans, D.; Schallig, H. Is the Dog a Possible Reservoir for Cutaneous Leishmaniasis in Suriname? J. Trop. Med. 2013, 1, 324140. [Google Scholar] [CrossRef]
- Santaella, J.; Ocampo, C.B.; Saravia, N.G.; Méndez, F.; Góngora, R.; Gomez, M.A.; Munstermann, L.E.; Quinnell, R.J. Leishmania (Viannia) Infection in the Domestic Dog in Chaparral, Colombia. Am. J. Trop. Med. Hyg. 2011, 84, 674–680. [Google Scholar] [CrossRef]
- Medkour, H.; Davoust, B.; Dulieu, F.; Maurizi, L.; Lamour, T.; Marié, J.L.; Mediannikov, O. Potential Animal Reservoirs (Dogs and Bats) of Human Visceral Leishmaniasis Due to Leishmania infantum in French Guiana. PLoS Negl. Trop. Dis. 2019, 13, e0007456. [Google Scholar] [CrossRef]
- Ratzlaff, F.R.; Osmari, V.; da Silva, D.; de Paula Vasconcellos, J.S.; Pötter, L.; Fernandes, F.D.; de Mello Filho, J.A.; de Avila Botton, S.; Vogel, F.S.F.; Sangioni, L.A. Identification of Infection by Leishmania spp. in Wild and Domestic Animals in Brazil: A Systematic Review with Meta-Analysis (2001–2021). Parasitol. Res. 2023, 122, 1605–1619. [Google Scholar] [CrossRef]
- Belo, V.S.; Struchiner, C.J.; Werneck, G.L.; Barbosa, D.S.; de Oliveira, R.B.; Neto, R.G.T.; da Silva, E.S. A Systematic Review and Meta-Analysis of the Factors Associated with Leishmania infantum Infection in Dogs in Brazil. Vet. Parasitol. 2013, 195, 1–13. [Google Scholar] [CrossRef]
- Yamada, K.; Valderrama, A.; Gottdenker, N.; Cerezo, L.; Minakawa, N.; Saldaña, A.; Calzada, J.E.; Chaves, L.F. Macroecological Patterns of American Cutaneous Leishmaniasis Transmission across the Health Areas of Panamá (1980–2012). Parasite Epidemiol. Control 2016, 1, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Hage, R.; Dos, S.; Nunes e Silva, S.V.; Bohm, B.C.; Lima, J.V.; Bruhn, N.C.P.; Menezes, G.R.; Bruhn, F.R.P. Spatiotemporal Relationship between Agriculture, Livestock, Deforestation, and Visceral Leishmaniasis in Brazilian Legal Amazon. Sci. Rep. 2024, 14, 21542. [Google Scholar] [CrossRef] [PubMed]
- Calzada, J.E.; Saldaña, A.; González, K.; Rigg, C.; Pineda, V.; Santamaría, A.M.; Rodríguez, I.; Gottdenker, N.L.; Laurenti, M.D.; Chaves, L.F. Cutaneous Leishmaniasis in Dogs: Is High Seroprevalence Indicative of a Reservoir Role? Parasitology 2015, 142, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Vergel, C.; Walker, J.; Saravia, N.G. Amplification of human DNA by primers targeted to Leishmania kinetoplast DNA and post-genome considerations in the detection of parasites by a polumerase chain reaction. Am. J. Trop. Med. Hyg. 2005, 72, 423–429. [Google Scholar] [CrossRef]
- Montalvo, A.M.; Fraga, J.; Maes, I.; Dujardin, J.-C.; Van der Auwera, G. Three New Sensitive and Specific Heat-Shock Protein 70 PCRs for Global Leishmania Species Identification. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1453–1461. [Google Scholar] [CrossRef]
- Marques, M.J.; Volpini, Â.C.; Machado-Coelho, G.L.L.; Machado-Pinto, J.; da Costa, C.A.; Mayrink, W.; Genaro, O.; Romanha, A.J. Comparison of Polymerase Chain Reaction with Other Laboratory Methods for the Diagnosis of American Cutaneous Leishmaniasis. Diagn. Microbiol. Infect. Dis. 2006, 54, 37–43. [Google Scholar] [CrossRef]
- Songumpai, N.; Promrangsee, C.; Noopetch, P.; Siriyasatien, P.; Preativatanyou, K. First Evidence of Co-Circulation of Emerging Leishmania martiniquensis, Leishmania orientalis, and Crithidia sp. in Culicoides Biting Midges (Diptera: Ceratopogonidae), the Putative Vectors for Autochthonous Transmission in Southern Thailand. Trop. Med. Infect. Dis. 2022, 7, 379. [Google Scholar] [CrossRef]
- Srivarasat, S.; Brownell, N.; Siriyasatien, P.; Noppakun, N.; Asawanonda, P.; Rattanakorn, K.; Preativatanyou, K.; Kumtornrut, C. Case Report: Autochthonous Disseminated Cutaneous, Mucocutaneous, and Visceral Leishmaniasis Caused by Leishmania martiniquensis in a Patient with HIV/AIDS from Northern Thailand and Literature Review. Am. J. Trop. Med. Hyg. 2022, 107, 1196–1202. [Google Scholar] [CrossRef]
- Spanakos, G.; Piperaki, E.T.; Menounos, P.G.; Tegos, N.; Flemetakis, A.; Vakalis, N.C. Detection and Species Identification of Old World Leishmania in Clinical Samples Using a PCR-Based Method. Trans. R Soc. Trop. Med. Hyg. 2008, 102, 46–53. [Google Scholar] [CrossRef]
- Jariyapan, N.; Bates, M.D.; Bates, P.A. Molecular Identification of Two Newly Identified Human Pathogens Causing Leishmaniasis Using PCR-Based Methods on the 30 Untranslated Region of the Heat Shock Protein 70 (Type i) Gene. PLoS Negl. Trop. Dis. 2021, 15, e0009982. [Google Scholar] [CrossRef]
- Barlaam, A.; Traversa, D.; Papini, R.; Giangaspero, A. Habronematidosis in Equids: Current Status, Advances, Future Challenges. Front. Vet. Sci. 2020, 7, 358. [Google Scholar] [CrossRef]
- Traversa, D.; Otranto, D.; Iorio, R.; Carluccio, A.; Contri, A.; Paoletti, B.; Bartolini, R.; Giangaspero, A. Identification of the Intermediate Hosts of Habronema microstoma and Habronema muscae under Field Conditions. Med. Vet. Entomol. 2008, 22, 283–287. [Google Scholar] [CrossRef]
- Schwabl, P.; Boité, M.C.; Bussotti, G.; Jacobs, A.; Andersson, B.; Moreira, O.; Freitas-Mesquita, A.L.; Meyer-Fernandes, J.R.; Telleria, E.L.; Traub-Csekö, Y.; et al. Colonization and Genetic Diversification Processes of Leishmania infantum in the Americas. Commun. Biol. 2021, 4, 139. [Google Scholar] [CrossRef] [PubMed]
- Terrero, I.; Pineda, V.; Vásquez, V.; Miranda, A.; Saldaña, A.; Calzada, J.E.; González, K. First Report of Imported Canine Visceral Leishmaniasis Cases in Panama, Central America: Public Health Implications. Vet. Parasitol. Reg. Stud. Rep. 2022, 32, 100745. [Google Scholar] [CrossRef] [PubMed]
- Herrer, A.; Christensen, H.A. Natural Cutaneous Leishmaniasis among Dogs in Panama. Am. J. Trop. Med. Hyg. 1976, 25, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Lago, J.; Silva, J.A.; Borja, L.; Fraga, D.B.M.; Schriefer, A.; Arruda, S.; Lago, E.; Carvalho, E.M.; Bacellar, O. Clinical and Histopathologic Features of Canine Tegumentary Leishmaniasis and the Molecular Characterization of Leishmania braziliensis in Dogs. PLoS Negl. Trop. Dis. 2019, 13, e0007532. [Google Scholar] [CrossRef]
- Mhadhbi, M.; Sassi, A. Infection of the Equine Population by Leishmania Parasites. Equine Vet. J. 2020, 52, 28–33. [Google Scholar] [CrossRef]
- Ahmadi, S.; Hataminejad, M.; Rahimi Esboei, B.; Hosseini, S.A.; Fakhar, M. An Update on Leishmania martiniquensis Infections: Transmission, Clinical Characteristics, and Treatment. Parasite Epidemiol. Control 2024, 27, e00386. [Google Scholar] [CrossRef]
- Mendes, A.A.V.; Filgueira, C.P.B.; De Freitas Campos Miranda, L.; De Almeida, A.B.; Cantanhêde, L.M.; Fagundes, A.; Pereira, S.A.; Menezes, R.C.; Cupolillo, E. First Report of Leishmania (Mundinia) martiniquensis in South American Territory and Confirmation of Leishbunyavirus Infecting This Parasite in a Mare. Mem. Inst. Oswaldo Cruz 2023, 118, e220220. [Google Scholar] [CrossRef]
- Valderrama, A.; Tavares, M.G.; Andrade Filho, J.D. Anthropogenic Influence on the Distribution, Abundance and Diversity of Sandfly Species (Diptera: Phlebotominae: Psychodidae), Vectors of Cutaneous Leishmaniasis in Panama. Mem. Inst. Oswaldo Cruz 2011, 106, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.A.; Fairchild, G.B.; Herrer, A.; Johnson, C.M.; Young, D.G.; de Vásquez, A.M. The Ecology of Cutaneous Leishmaniasis in the Republic of Panama. J. Med. Entomol. 1983, 20, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Ortega-García, M.V.; Salguero, F.J.; García, N.; Domínguez, M.; Moreno, I.; Berrocal, A. Equine Infection with Leishmania spp. in Costa Rica: Study of Five Cases. Vet. Med. Sci. 2021, 7, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Fraga, J.; Montalvo, A.M.; De Doncker, S.; Dujardin, J.C.; Van der Auwera, G. Phylogeny of Leishmania Species Based on the Heat-Shock Protein 70 Gene. Infect. Genet. Evol. 2010, 10, 238–245. [Google Scholar] [CrossRef]
- Carbonara, M.; Mendoza-Roldan, J.A.; Bezerra-Santos, M.A.; de Abreu Teles, P.P.; Lia, R.P.; Locantore, F.; Iatta, R.; Volf, P.; Otranto, D. Leishmania spp. in Equids and Their Potential Vectors in Endemic Areas of Canine Leishmaniasis. PLoS Negl. Trop. Dis. 2024, 18, e0012290. [Google Scholar] [CrossRef]
| PCR | Primers | Primers Concentrations | DNA Used per Reaction | PCR Conditions | PCR Products Size |
|---|---|---|---|---|---|
| kDNA Viannia Vergel et al., 2005 [20] | B1: 5′-GGGGTTGGTGTAATATAGTGG-3′ LV: 5′-ATTTTTGAACGGGGTTTCTG-3′ | 0.6 μm/L each one | 5 μL | 5 cycles of 95 °C × 6 min 95 °C × 30 seg 64.5 °C × 2 min 72 °C × 1 min 35 cycles of 95 °C × 30 seg 64 °C × 1 min 72 °C × 1 min 72 °C × 10 min | 750 bp |
| Hsp-70 Montalvo et al., 2012 [21] | Hsp70-F25: 5′-GGACGCCGGCACGATTKCT-3′ Hsp70-R1310: 5′-CCTGGTTGTTGTTCAGCCACTC-3′ | 0.6 μm/L each one | 5 μL | 94 °C × 5 min 33 cycles of 94 °C × 30 seg 61 °C × 1 min 72 °C × 3 min 72 °C × 10 min | 1286 bp |
| kDNA L150/151 Marques et al., 2006 [22] | L-150: 5′-GGG(G/T)AGGGGCGTTCT(G/C)CGAA-3′ L-151: 5′-(G/C)(G/C)(G/C)A/C)CTAT(A/T)TTACACCAACCCC-3′ | 0.5 μm/L each one | 5 μL | 94 °C × 4 min 33 cycles of 94 °C × 30 seg 52.3 °C × 30 seg 72 °C × 30 seg 72 °C × 10 min | 120 bp |
| ITS1 Spanakos et al., 2008 [25] | LeR: 5′-CCAAGTCATCCATCGCGACACG-3′ LeF 5′-TCCGCCCGAAAGTTCACCGATA-3′ | 1.0 μm/L each one | 5 μL | 95 °C × 5 min 40 cycles of 95 °C × 1 min 65 °C × 1 min 72 °C × 1 min 72 °C × 10 min | 379 bp |
| ID | Animal Species | Sample Type | Region | DPP rk39 | IFI | PCR kDNA * | PCR Hsp-70 | Leishmania Species | GenBank Accession |
|---|---|---|---|---|---|---|---|---|---|
| CF-W01 | Dog | Biopsy | Colon | N/A | N/A | + | + | L. (V.) braziliensis | PV844902 |
| CF-P02 | Dog | Biopsy | Colon | N/A | N/A | + | + | L. (V.) braziliensis | PV844901 |
| CF-L03 | Dog | Biopsy | Panama Este | N/A | N/A | + | + | L. (V.) braziliensis | PV844903 |
| CF-C04 | Dog | Blood | Panama | - | - | - | - | N/A | N/A |
| CF-A05 | Dog | Serum/ Blood | Panama Oeste | - | - | - | - | N/A | N/A |
| CF-S06 | Dog | Serum/ Blood | Panama Oeste | - | - | - | - | N/A | N/A |
| CF-J07 | Dog | Serum/ Blood | Panama | + | + | + | + | N/A | N/A |
| CF-F08 | Dog | Serum/ Blood | Panama | - | - | - | - | N/A | N/A |
| CF-A09 | Dog | Serum/ Blood | Panama Norte | + | + | + | + | N/A | N/A |
| CF-S10 | Dog | Serum/ Blood | Panama Norte | - | - | - | - | N/A | N/A |
| EC-H01 | Horse | Biopsy | Panama Oeste | N/A | N/A | + | + | L. (M.) martiniquensis | PV658270 PV844900 |
| EC-H02 | Horse | Biopsy | Panama Este | N/A | N/A | - | - | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda, V.; Calzada, J.E.; Montilla, S.; Rodríguez, I.; Howard, E.; Torres, A.I.; Vasquez, V.; Reina, A.; Saldaña, A.; González, K. Molecular Detection of Leishmania (V.) braziliensis and Leishmania (M.) martiniquensis Infecting Domestic Animals from Panama, Central America. Animals 2025, 15, 2677. https://doi.org/10.3390/ani15182677
Pineda V, Calzada JE, Montilla S, Rodríguez I, Howard E, Torres AI, Vasquez V, Reina A, Saldaña A, González K. Molecular Detection of Leishmania (V.) braziliensis and Leishmania (M.) martiniquensis Infecting Domestic Animals from Panama, Central America. Animals. 2025; 15(18):2677. https://doi.org/10.3390/ani15182677
Chicago/Turabian StylePineda, Vanessa, Jose E. Calzada, Santiago Montilla, Indra Rodríguez, Erika Howard, Alicia I. Torres, Vanessa Vasquez, Adelys Reina, Azael Saldaña, and Kadir González. 2025. "Molecular Detection of Leishmania (V.) braziliensis and Leishmania (M.) martiniquensis Infecting Domestic Animals from Panama, Central America" Animals 15, no. 18: 2677. https://doi.org/10.3390/ani15182677
APA StylePineda, V., Calzada, J. E., Montilla, S., Rodríguez, I., Howard, E., Torres, A. I., Vasquez, V., Reina, A., Saldaña, A., & González, K. (2025). Molecular Detection of Leishmania (V.) braziliensis and Leishmania (M.) martiniquensis Infecting Domestic Animals from Panama, Central America. Animals, 15(18), 2677. https://doi.org/10.3390/ani15182677







