Continuous Activity Monitoring Using a Wearable Sensor in Dogs with Osteoarthritis: An Exploratory Case Series
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Participants
2.2. Monitoring System Description
- Resting: prolonged periods of inactivity typically associated with sleep or deep rest.
- Quiet: awake but not in continuous motion, such as moving the head, chewing on a toy, licking, or standing still.
- Active: sustained, low-paced activity involving motion, such as walking or pacing.
- Excited: high-intensity activity, including trotting, running or jumping.
- Not resting: sum of Quiet, Active and Excited.
2.3. Procedures
2.4. AI-Vet Alert System and Clinical Access
2.5. Usability and Perception Assessment
2.6. Data Analysis Approach
3. Results
3.1. Study Population Overview
3.2. Clinical Characterization of Osteoarthritis in the Study Dogs
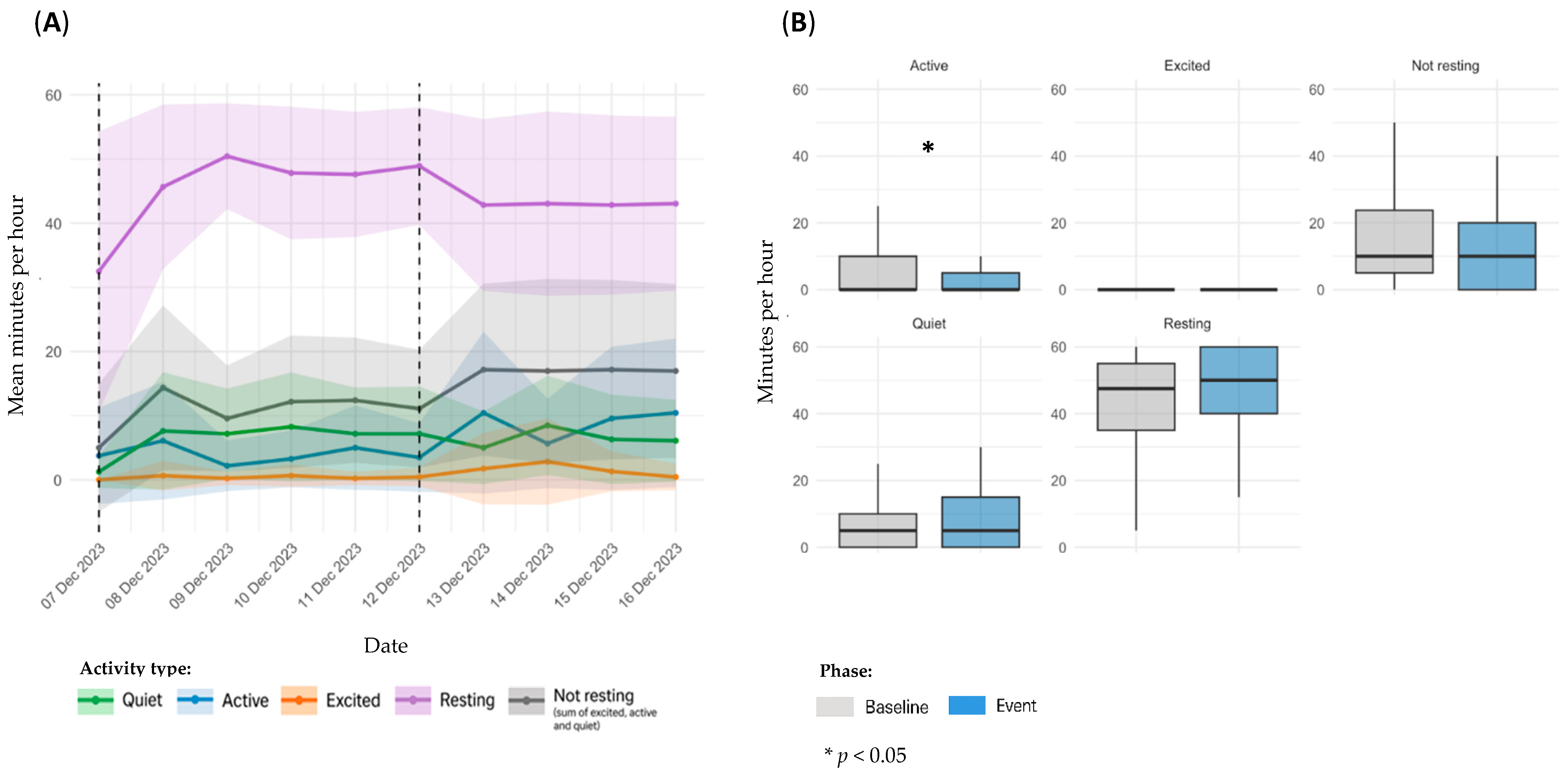
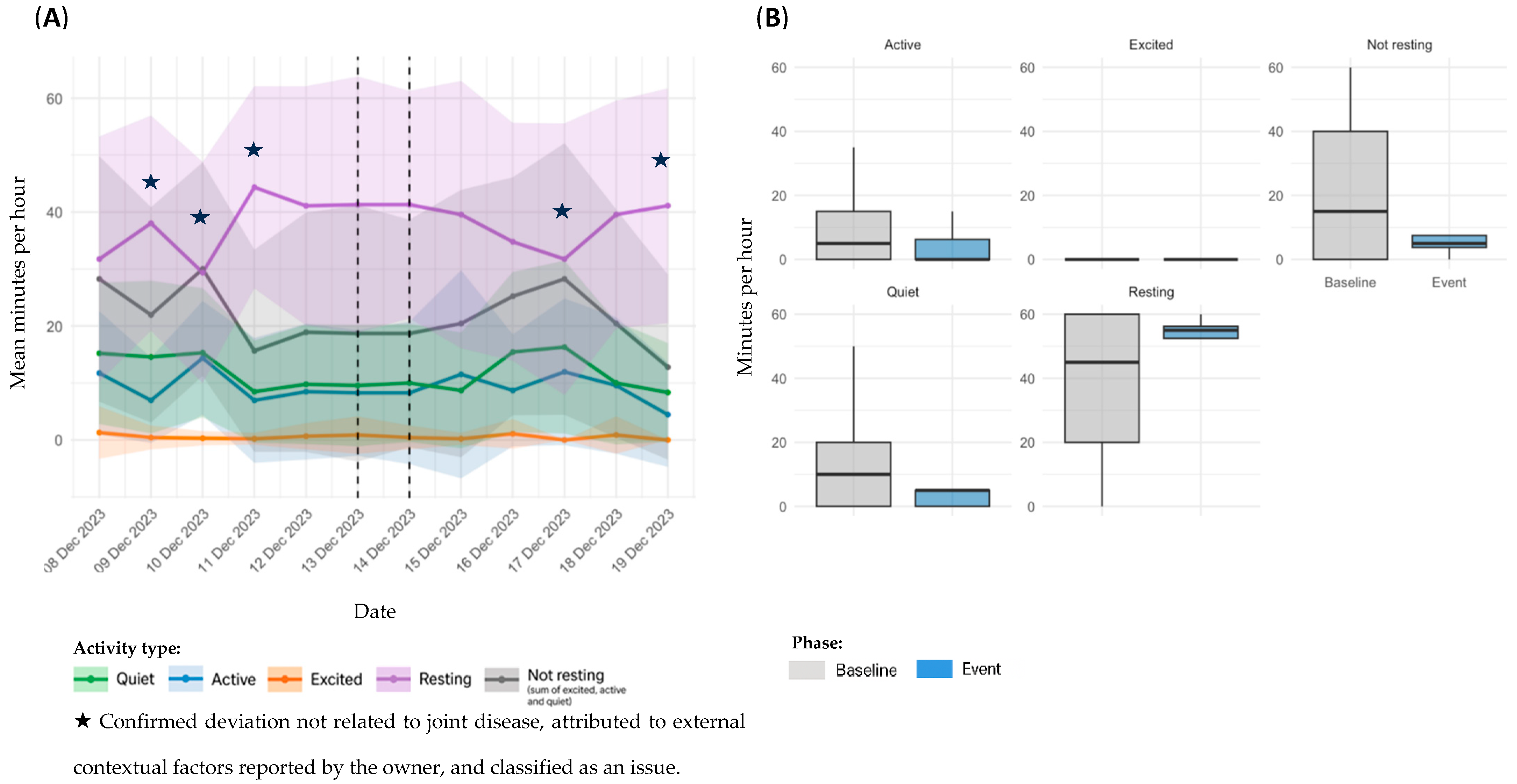
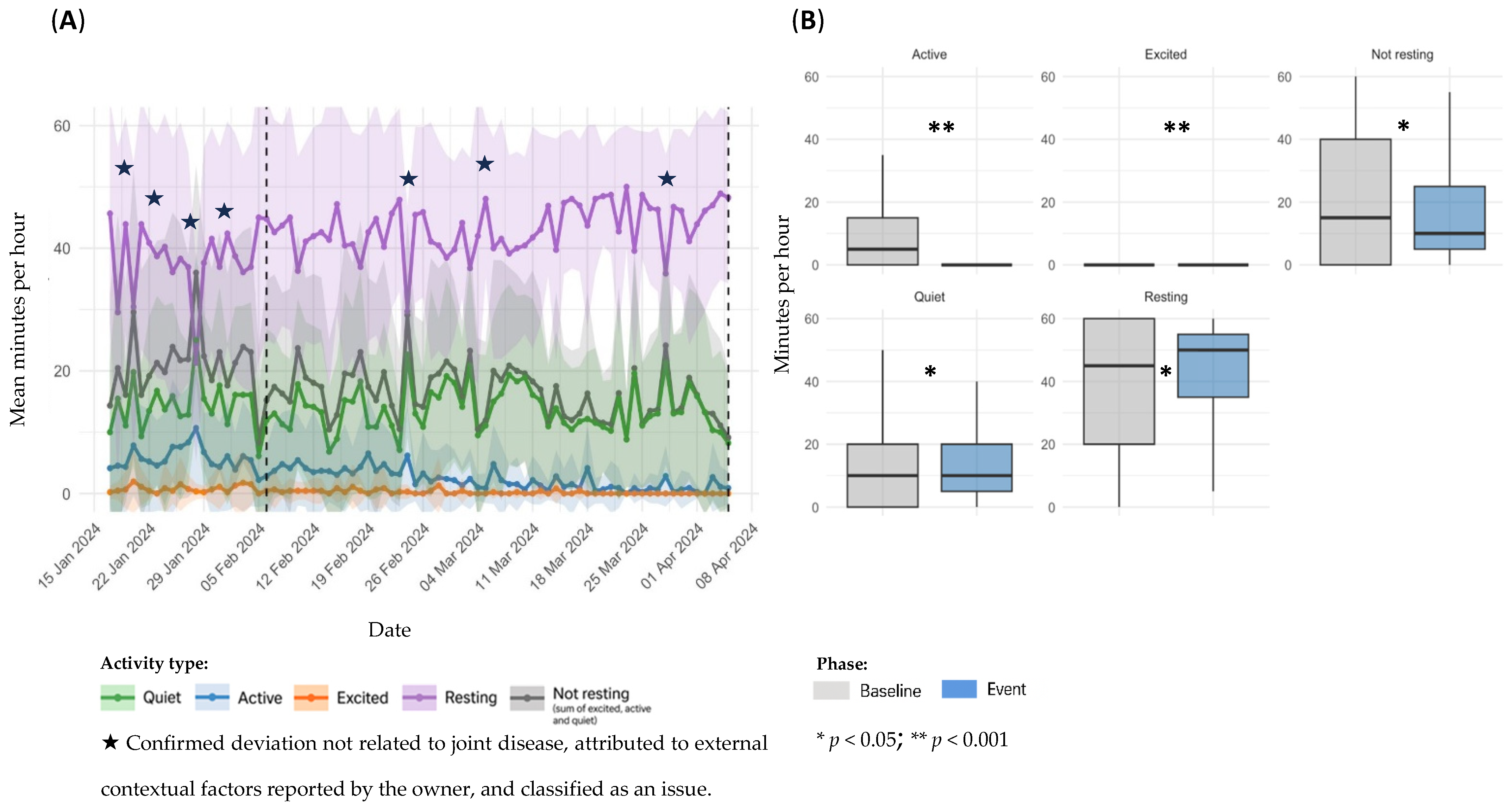
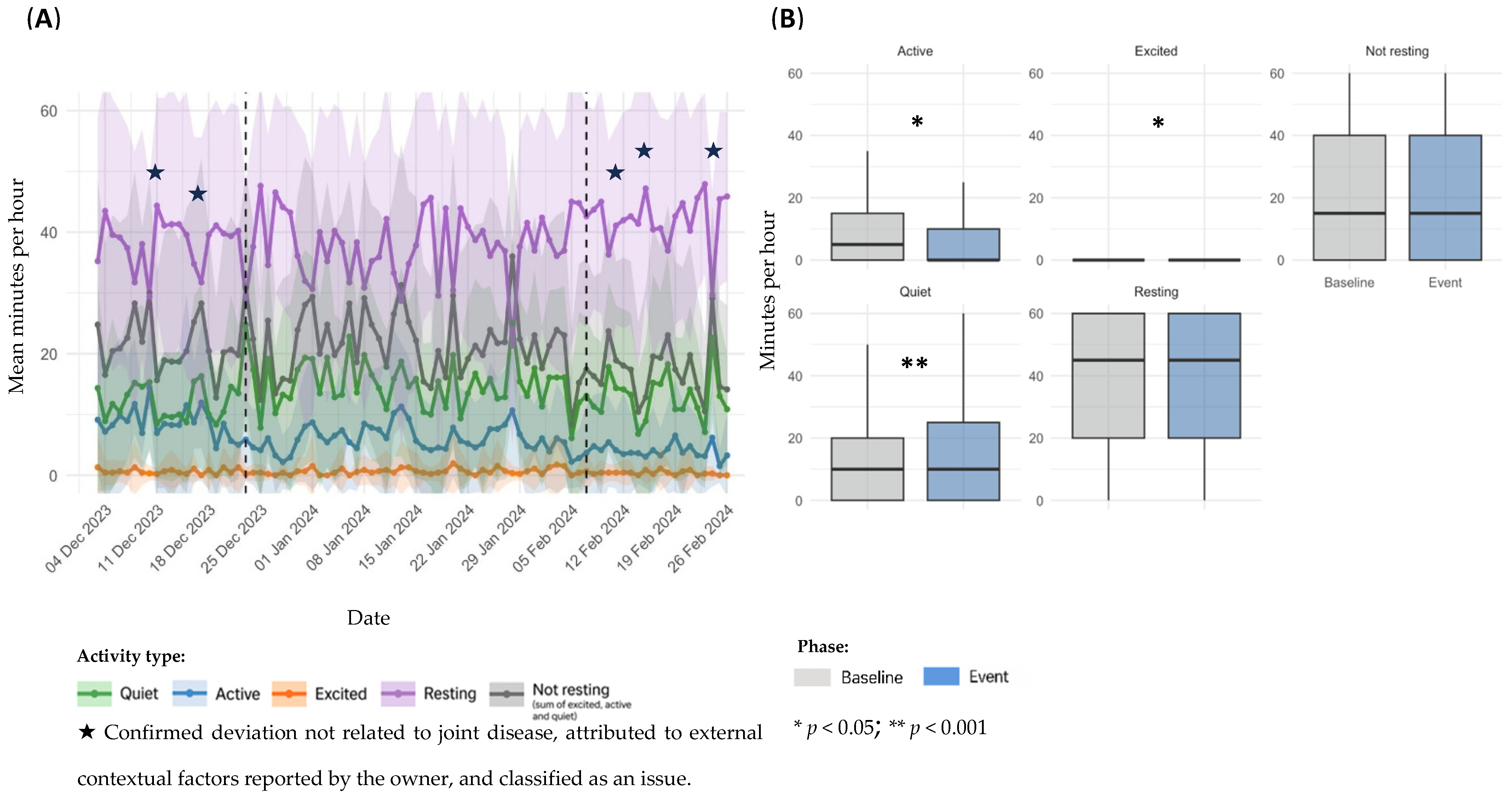
3.3. Event Classification and Distribution
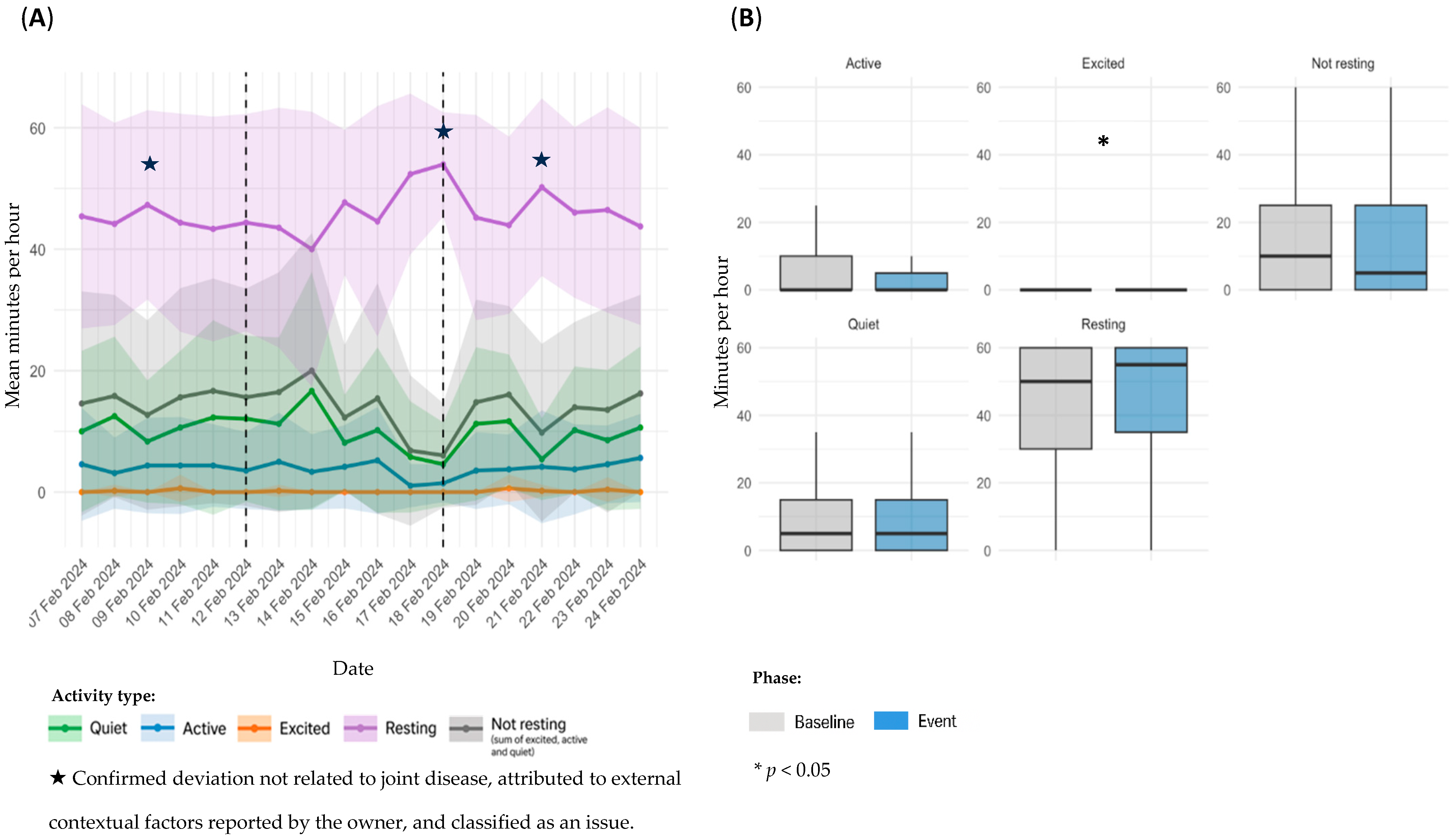
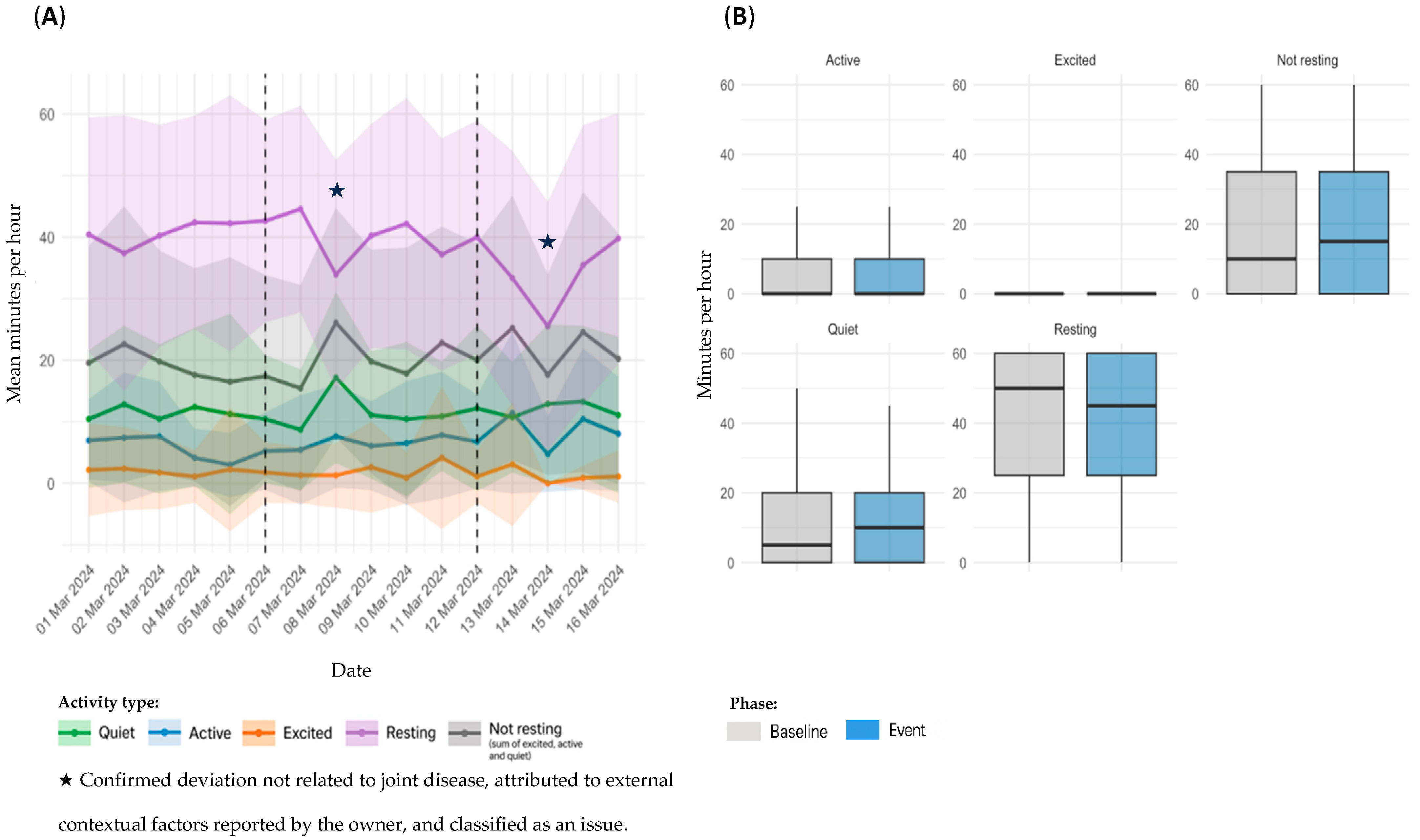
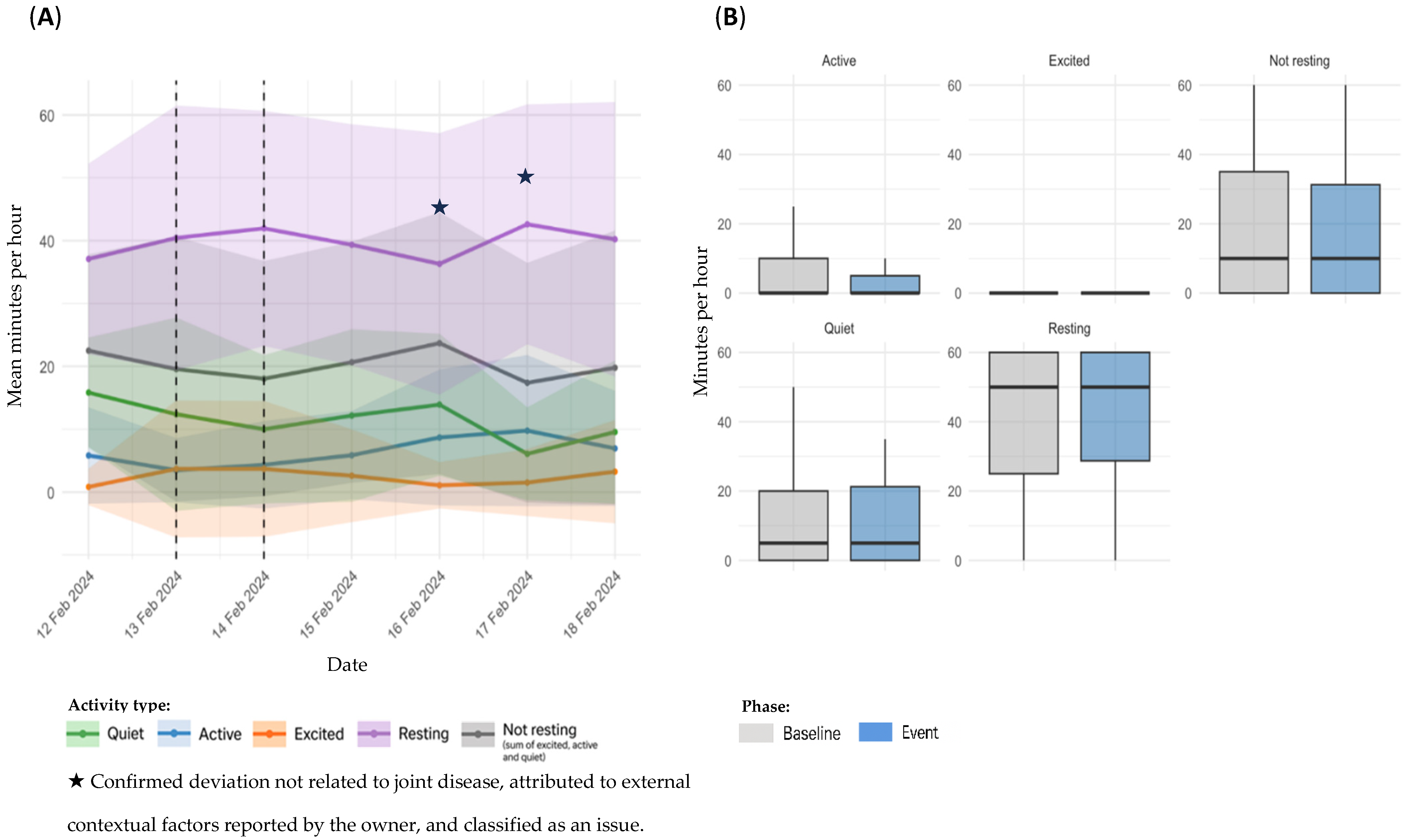
3.4. Sensor Based Activity Analysis by Event Type
3.4.1. OA Flare-Ups
3.4.2. Other Non-Orthopedic Health Events
3.5. Survey Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| NSAID(s) | Non-Steroidal Anti-Inflammatory Drug(s) |
| FLM | Functional Linear Modeling |
References
- Anderson, K.L.; O’Neill, D.G.; Brodbelt, D.C.; Church, D.B.; Meeson, R.L.; Sargan, D.; Summers, J.F.; Zulch, H.; Collins, L.M. Prevalence, Duration and Risk Factors for Appendicular Osteoarthritis in a UK Dog Population under Primary Veterinary Care. Sci. Rep. 2018, 8, 5641. [Google Scholar] [CrossRef]
- Bell, A.; Helm, J.; Reid, J. Veterinarians’ Attitudes to Chronic Pain in Dogs. Vet. Rec. 2014, 175, 428. [Google Scholar] [CrossRef]
- Malkani, R.; Paramasivam, S.; Wolfensohn, S. How Does Chronic Pain Impact the Lives of Dogs: An Investigation of Factors That Are Associated with Pain Using the Animal Welfare Assessment Grid. Front. Vet. Sci. 2024, 11, 1374858. [Google Scholar] [CrossRef]
- Pettitt, R.A.; German, A.J. Investigation and Management of Canine Osteoarthritis. Practice 2015, 37, 1–8. [Google Scholar] [CrossRef]
- Smith, M.; Mendl, M.; Murrell, J.C. Associations between Osteoarthritis and Duration and Quality of Night-Time Rest in Dogs. Appl. Anim. Behav. Sci. 2022, 253, 105661. [Google Scholar] [CrossRef]
- Belshaw, Z.; Dean, R.; Asher, L. Slower, Shorter, Sadder: A Qualitative Study Exploring How Dog Walks Change When the Canine Participant Develops Osteoarthritis. BMC Vet. Res. 2020, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Boston, R.C.; Coyne, J.C.; Farrar, J.T. Ability of the Canine Brief Pain Inventory to Detect Response to Treatment in Dogs with Osteoarthritis. J. Am. Vet. Med. Assoc. 2008, 233, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.B.; Cowderoy, E.; Lascelles, D.; Innes, J.F. Evaluation of Construct and Criterion Validity for the ‘Liverpool Osteoarthritis in Dogs’ (LOAD) Clinical Metrology Instrument and Comparison to Two Other Instruments. PLoS ONE 2013, 8, e58125. [Google Scholar] [CrossRef]
- Hielm-Björkman, A.K.; Kapatkin, A.S.; Rita, H.J. Reliability and Validity of a Visual Analogue Scale Used by Owners to Measure Chronic Pain Attributable to Osteoarthritis in Their Dogs. Am. J. Vet. Res. 2011, 72, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.C.; Santos, A.; Jorge, P.; Lavrador, C.; Carreira, L.M. Evaluation of Four Clinical Metrology Instruments for the Assessment of Osteoarthritis in Dogs. Animals 2022, 12, 2808. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.M.; Young, K.; Haskell, M.J.; Carter, A.J.; Clements, D.N. Mobility, Functionality and Functional Mobility: A Review and Application for Canine Veterinary Patients. Vet. J. 2024, 305, 106123. [Google Scholar] [CrossRef]
- Belshaw, Z.; Dean, R.; Asher, L. Could It Be Osteoarthritis? How Dog Owners and Veterinary Surgeons Describe Identifying Canine Osteoarthritis in a General Practice Setting. Prev. Vet. Med. 2020, 185, 105198. [Google Scholar] [CrossRef]
- Muller, C.; Gines, J.A.; Conzemius, M.; Meyers, R.; Lascelles, B.D.X. Evaluation of the Effect of Signalment and Owner-Reported Impairment Level on Accelerometer-Measured Changes in Activity in Osteoarthritic Dogs Receiving a Non-Steroidal Anti-Inflammatory. Vet. J. 2018, 242, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Detweiler, K.B.; Harper, T.A.; Knap, K.E.; de Godoy, M.R.C.; Swanson, K.S. Physical Activity Patterns of Free Living Dogs Diagnosed with Osteoarthritis. J. Anim. Sci. 2021, 99, skab204. [Google Scholar] [CrossRef]
- Rowlison de Ortiz, A.; Belda, B.; Hash, J.; Enomoto, M.; Robertson, J.; Lascelles, B.D.X. Frontiers | Initial Exploration of the Discriminatory Ability of the PetPace Collar to Detect Differences in Activity and Physiological Variables between Healthy and Osteoarthritic Dogs. Front. Pain Res. 2022, 3, 949877. [Google Scholar] [CrossRef]
- Marcato, M.; Tedesco, S.; O’Mahony, C.; O’Flynn, B.; Galvin, P. Machine Learning Based Canine Posture Estimation Using Inertial Data. PLoS ONE 2023, 18, e0286311. [Google Scholar] [CrossRef] [PubMed]
- Muminov, A.; Mukhiddinov, M.; Cho, J. Enhanced Classification of Dog Activities with Quaternion-Based Fusion Approach on High-Dimensional Raw Data from Wearable Sensors. Sensors 2022, 22, 9471. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R.D.; Yoder, N.C.; Carson, A.B.; Junge, C.; Allen, D.E.; Prescott, L.M.; Bradley, S.; Wymore, G.; Lloyd, K.; Lyle, S. Deep Learning Classification of Canine Behavior Using a Single Collar-Mounted Accelerometer: Real-World Validation. Animals 2021, 11, 1549. [Google Scholar] [CrossRef]
- Schork, I.G.; Manzo, I.A.; de Oliveira, M.R.B.; Costa, F.V.; Young, R.J.; De Azevedo, C.S. Testing the Accuracy of Wearable Technology to Assess Sleep Behaviour in Domestic Dogs: A Prospective Tool for Animal Welfare Assessment in Kennels. Animals 2023, 13, 1467. [Google Scholar] [CrossRef]
- Den Uijl, I.; Gómez Álvarez, C.B.; Bartram, D.; Dror, Y.; Holland, R.; Cook, A. External Validation of a Collar-Mounted Triaxial Accelerometer for Second-by-Second Monitoring of Eight Behavioural States in Dogs. PLoS ONE 2017, 12, e0188481. [Google Scholar] [CrossRef]
- Karimjee, K.; Harron, R.C.M.; Piercy, R.J.; Daley, M.A. A Standardised Approach to Quantifying Activity in Domestic Dogs. R. Soc. Open Sci. 2024, 11, 240119. [Google Scholar] [CrossRef] [PubMed]
- Belda, B.; Enomoto, M.; Case, B.C.; Lascelles, B.D.X. Initial Evaluation of PetPace Activity Monitor. Vet. J. 2018, 237, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, E.C.; Rudinsky, A.J.; Kieves, N.R. Commercially Available Wearable Health Monitors in Dogs Only Had a Very Strong Correlation during Longer Durations of Time: A Pilot Study. Am. J. Vet. Res. 2024, 85, ajvr.24.06.0162. [Google Scholar] [CrossRef]
- Brasier, N.; Wang, J.; Dincer, C.; Güder, F.; Schauwecker, I.; Schaffarczyk, D.; Ghaffari, R.; Goldhahn, J. Next-Generation Digital Biomarkers: Continuous Molecular Health Monitoring Using Wearable Devices. Trends Biotechnol. 2024, 42, 255–257. [Google Scholar] [CrossRef]
- Ates, H.C.; Nguyen, P.Q.; Gonzalez-Macia, L.; Morales-Narváez, E.; Güder, F.; Collins, J.J.; Dincer, C. End-to-End Design of Wearable Sensors. Nat. Rev. Mater. 2022, 7, 887–907. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.; Kawecki-Wright, E.; de Ortiz, A.R.; Thomson, A.; Aker, S.; Perry, E.; Haupt, E.; Mondino, A.; Enomoto, M.; Gruen, M.E.; et al. Frontiers | Factors Influencing, and Associated with, Physical Activity Patterns in Dogs with Osteoarthritis-Associated Pain. Front. Vet. Sci. 2025, 12, 1503009. [Google Scholar] [CrossRef] [PubMed]
- Gruen, M.E.; Samson, D.R.; Lascelles, B.D.X. Functional Linear Modeling of Activity Data Shows Analgesic-Mediated Improved Sleep in Dogs with Spontaneous Osteoarthritis Pain. Sci. Rep. 2019, 9, 14192. [Google Scholar] [CrossRef]
- Barale, L.; Monticelli, P.; Adami, C. Effects of Low-Level Laser Therapy on Impaired Mobility in Dogs with Naturally Occurring Osteoarthritis. Vet. Med. Sci. 2023, 9, 653–659. [Google Scholar] [CrossRef]
- Schuster, L.A.H.; de Carvalho, A.L.; dos Santos, E.A.R.; de Oliveira, M.P.; Camacho-Rozo, C.A.; Raposo Monteiro, E.; Ferreira, M.P.; Alievi, M.M. Physical Activity Measured with an Accelerometer in Dogs Following Extracapsular Stabilisation to Treat Cranial Cruciate Ligament Rupture. J. Small Anim. Pract. 2023, 64, 619–625. [Google Scholar] [CrossRef]
- Cachon, T.; Frykman, O.; Innes, J.F.; Lascelles, B.D.X.; Okumura, M.; Sousa, P.; Staffieri, F.; Steagall, P.V.; Van Ryssen, B. Face Validity of a Proposed Tool for Staging Canine Osteoarthritis: Canine OsteoArthritis Staging Tool (COAST). Vet. J. 2018, 235, 1–8. [Google Scholar] [CrossRef]
- Cachon, T.; Frykman, O.; Innes, J.F.; Lascelles, B.D.X.; Okumura, M.; Sousa, P.; Staffieri, F.; Steagall, P.V.; Van Ryssen, B. COAST Development Group’s International Consensus Guidelines for the Treatment of Canine Osteoarthritis. Front. Vet. Sci. 2023, 10, 1137888. [Google Scholar] [CrossRef] [PubMed]
- Millis, D.; Levine, D. Canine Rehabilitation and Physical Therapy, 2nd ed.; Saunders; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-1-4377-0309-2. [Google Scholar]
- Jones, G.M.C.; Pitsillides, A.A.; Meeson, R.L. Moving Beyond the Limits of Detection: The Past, the Present, and the Future of Diagnostic Imaging in Canine Osteoarthritis. Front. Vet. Sci. 2022, 9, 789898. [Google Scholar] [CrossRef]
- Mejia, S.; Duerr, F.M.; Salman, M. Comparison of Activity Levels Derived from Two Accelerometers in Dogs with Osteoarthritis: Implications for Clinical Trials. Vet. J. 2019, 252, 105355. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.M.; Scott, R.M.; Thomson, C.B.; Mesa, E.; Evans, R.; Conzemius, M.G. Evaluation of the Environmental Bias on Accelerometer-Measured Total Daily Activity Counts and Owner Survey Responses in Dogs with Osteoarthritis. Vet. Comp. Orthop. Traumatol. 2017, 30, 385–390. [Google Scholar] [CrossRef] [PubMed]
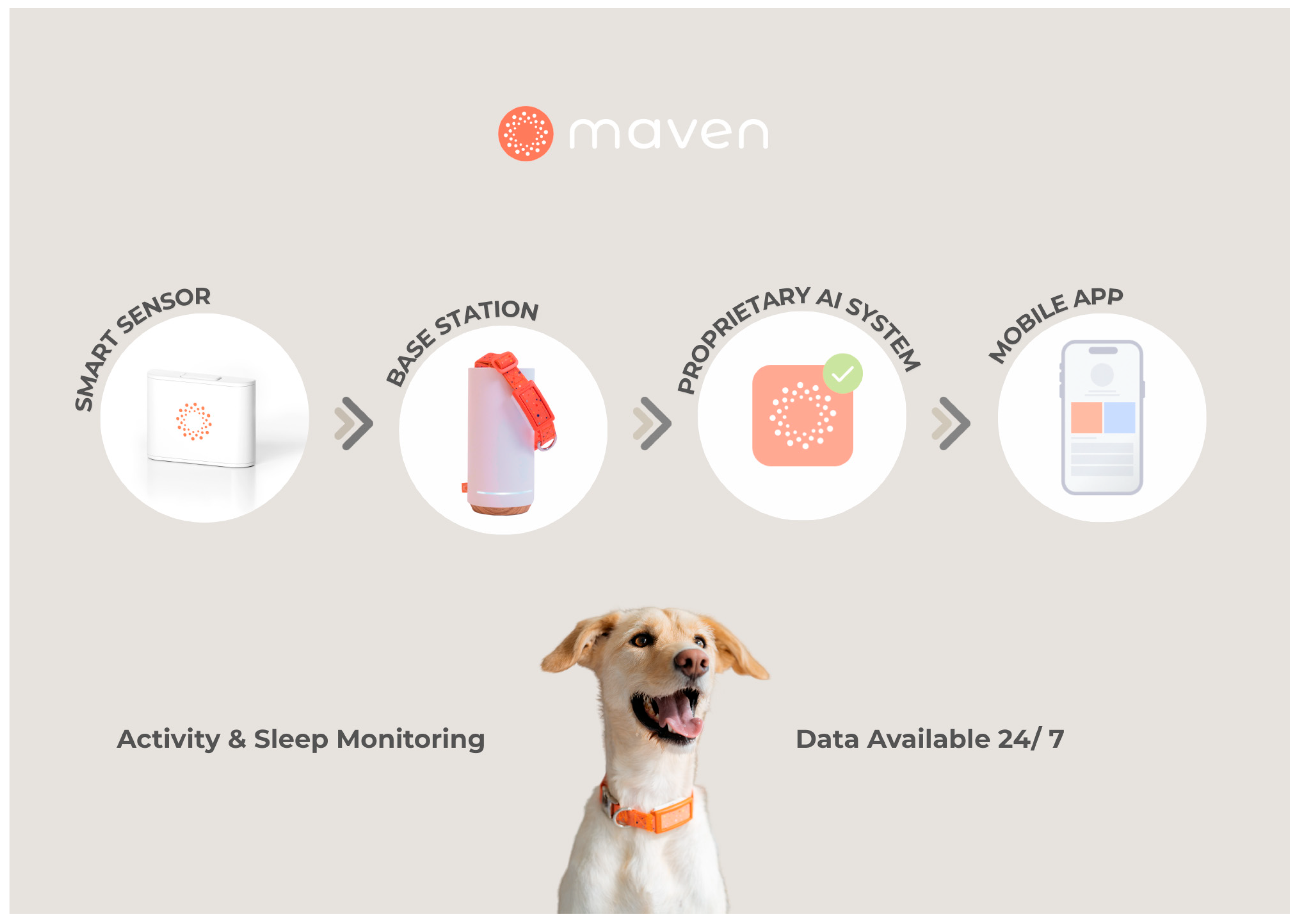


| Dog | Breed | Sex/Neuter Status | Age (Years) | Weight (kg) | Monitoring Duration (Days) | Valid Data (Hours) |
|---|---|---|---|---|---|---|
| D1 | Labrador Retriever | Female/Neutered | 10 | 26.9 | 84 | 1905 |
| D2 | Large Mixed Breed | Male/Neutered | 6 | 30 | 56 | 1248 |
| D3 | German Shepherd | Male/Intact | 7 | 43 | 119 | 2338 |
| D4 | Large Mixed Breed | Male/Neutered | 12 | 27.1 | 126 | 2873 |
| D5 | Jack Russell Terrier | Female/Neutered | 7 | 7 | 112 | 2357 |
| Dog | Diagnosis | Joints Affected | Severity at Baseline (by COAST/COASTer Stage Methods) | Lameness Grade * (Baseline) | Lameness Grade (Events/End) |
|---|---|---|---|---|---|
| D1 | OA secondary to hip dysplasia | Bilateral coxofemoral joints | Stage 3 COAST | 2 | 3 (event 1); 2 (end) |
| D2 | OA secondary to hip dysplasia | Bilateral coxofemoral joints | Stage 4 COAST | 3 | 5 (event 2 and 3); 3 (end) |
| D3 | OA secondary to hip dysplasia | Bilateral coxofemoral joints | Stage 3 COAST | 3 | 5 (event 4); 4 (event 5); 3 (end) |
| D4 | OA secondary to immune-mediated polyarthritis caused by ehrlichiosis | Bilateral tarsal joints | Stage 3 COASTer | 4 | 4 (events 6, 7 and 8); 4 (end) |
| D5 | OA | Unilateral right coxofemoral joint | Stage 3 COAST | 4 | 4 (event 9); 4 (end) |
| Event Classification | Dog | Event | Event Dates | Duration (Days) | Case Summary |
|---|---|---|---|---|---|
| OA flare-ups | D1 | 1 | 12–18 February 2024 | 7 | On 12 February, the owner reported abnormal gait, lameness, and neck rigidity via the Maven app. The dog was evaluated by a veterinarian on 14 February, and non-steroidal anti-inflammatory drugs (NSAIDs) and gabapentin were administered for five days. |
| D2 | 2 | 6–12 March 2024 | 7 | On 6 March, the owner reported lameness. Full recovery was observed by the owner after five days of robenacoxib treatment. | |
| 3 | 13 February 2024 | 1 | On 13 February, the owner reported limping and abnormal gait. An oral anti-inflammatory was administered around lunchtime, with resolution of clinical signs by the end of the day. | ||
| D3 | 4 | 24–29 March 2024 | 6 | On 24 March, the owner reported limping and difficulty rising, following a suspected episode of overexertion. Clinical signs improved after five days of rest and controlled activity. | |
| 5 | 7–12 December 2023 | 6 | On 7 December, the owner reported intermittent right pelvic limb lameness and mood changes. Same-day veterinary evaluation included laser therapy, acupuncture, and a rest recommendation. | ||
| D4 | 6 | 13 December 2023 | 1 | On 13 December, the owner reported limping in the morning and altered gait in the afternoon. Clinical signs resolved on the same day with rest | |
| 7 | 6 February–4 April 2024 | 58 | From 6 February onwards, the owner reported signs of lethargy. Subsequent veterinary assessment revealed findings consistent with OA of immune-mediated origin (ehrlichiosis), and a 28-day treatment with doxycycline was initiated on 17 March. Clinical signs persisted until the end of the study. | ||
| Other non-orthopedic health events | 8 | 23 December–7 February 2024 | 16 | On the morning of 23 December, the owner observed skin lesions on the nose and paws, and a corticosteroid injection was administered later that day. Oclacitinib was initiated on 7 February. The clinical signs were later confirmed as a dermatological flare-up. | |
| D5 | 9 | 6–9 March 2024 | 4 | On 7 March, the owner reported symptoms including elevated body temperature, soft stools, and dull coat. Veterinary evaluation the next day confirmed acute gastroenteritis, and treatment included probiotics and a gastrointestinal diet. |
| Owner Responses | ||
|---|---|---|
| 1. Frequency of system use | ||
| Parameter | Round 1 (%) | Round 2 (%) |
| Daily | 80 | 60 |
| Weekly | 20 | 40 |
| 2. Degree of agreement with statements about the system | ||
| Parameter | Round 1 (% Yes) | Round 2 (% Yes) |
| The system effectively records movement periods | 100 | 100 |
| Events on the dashboard match what I observe in reality. | 100 | 100 |
| The data correspond to my perception of sleep/activity. | 100 | 80 |
| The system is useful to understand my dog’s overall health. | 80 | 60 |
| The system helps identify moments of pain or discomfort. | 60 | 60 |
| Monitoring helps me understand my dog’s activity limits. | 80 | 80 |
| The system improved clinical follow-up by the veterinary team. | 40 | 40 |
| I see added value in long-term monitoring. | 80 | 80 |
| 3. System recommendation | ||
| Recommendation likelihood—mean score (0–10 scale) * | 7 | 8 |
| Veterinarian Responses | |
|---|---|
| Question | Response |
| Familiarity with the Maven application and its capabilities (0–10) * | 9 |
| Learned to use and explain the system to clients quickly and intuitively? (Yes/No) | Yes |
| Familiarity with how all data are collected and presented in the clinic portal (0–10) * | 8 |
| Overall ease of use of the portal (0–10) ** | 10 |
| Specific feature difficult to navigate or understand? (Yes/No) | No |
| Ease of integrating daily report reading into clinical workflow (Yes/No) | Yes |
| Who was responsible for reading and making decisions based on the system reports? | Myself |
| Time spent reading reports/emails and exploring patient data on portal | Some time weekly |
| Did monitoring become more efficient? If so, to what extent | In some cases, it helped maintain owner contact |
| Alerts received were clinically relevant? (Yes/No) | Yes |
| Practicality of information for diagnosis, treatment, and monitoring | Quite practical |
| Did report changes prompt calls or messages with owners? (Yes/No) | Yes, but only occasionally |
| Did alerts lead to scheduling new follow-up appointments? If yes, how many on average | No |
| Owners’ involvement affected relationship with clinical team (Positive/Negative/Neither) | Positively |
| Cases where system positively influenced diagnosis or treatment plan | In a few cases |
| Did use of Maven app increase veterinarians’ compliance with clinical recommendations? (Yes/No) | I did not notice |
| If previous answer positive, which feature is most relevant? | Not applicable |
| Did data provided help improve communication with owners? (Yes/No) | Yes |
| Suggestions to improve system’s usability and practical relevance | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacoor, C.; Leitão, S.; Domingues, C.; Babo, J.; Sá, C.M.; Cabeças, R.; Queiroga, F.L. Continuous Activity Monitoring Using a Wearable Sensor in Dogs with Osteoarthritis: An Exploratory Case Series. Animals 2025, 15, 2639. https://doi.org/10.3390/ani15182639
Sacoor C, Leitão S, Domingues C, Babo J, Sá CM, Cabeças R, Queiroga FL. Continuous Activity Monitoring Using a Wearable Sensor in Dogs with Osteoarthritis: An Exploratory Case Series. Animals. 2025; 15(18):2639. https://doi.org/10.3390/ani15182639
Chicago/Turabian StyleSacoor, Carina, Sara Leitão, Carolina Domingues, Joana Babo, Cátia M. Sá, Ricardo Cabeças, and Felisbina L. Queiroga. 2025. "Continuous Activity Monitoring Using a Wearable Sensor in Dogs with Osteoarthritis: An Exploratory Case Series" Animals 15, no. 18: 2639. https://doi.org/10.3390/ani15182639
APA StyleSacoor, C., Leitão, S., Domingues, C., Babo, J., Sá, C. M., Cabeças, R., & Queiroga, F. L. (2025). Continuous Activity Monitoring Using a Wearable Sensor in Dogs with Osteoarthritis: An Exploratory Case Series. Animals, 15(18), 2639. https://doi.org/10.3390/ani15182639





