Neo-X-Linked Chromosome Polymorphism: Cytogenetic Insights from Passalites nemorivagus (Mammalia, Cervidae) †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
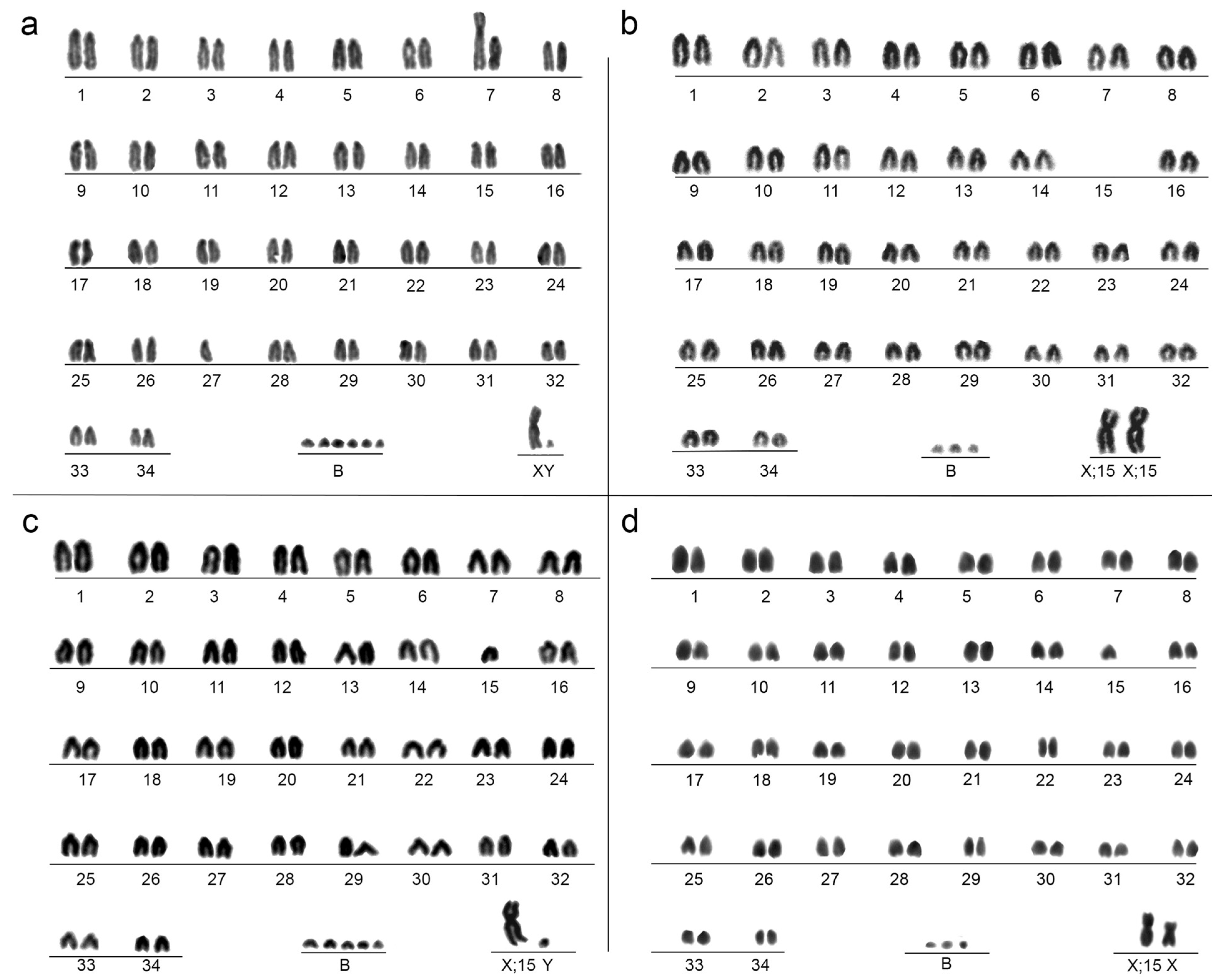
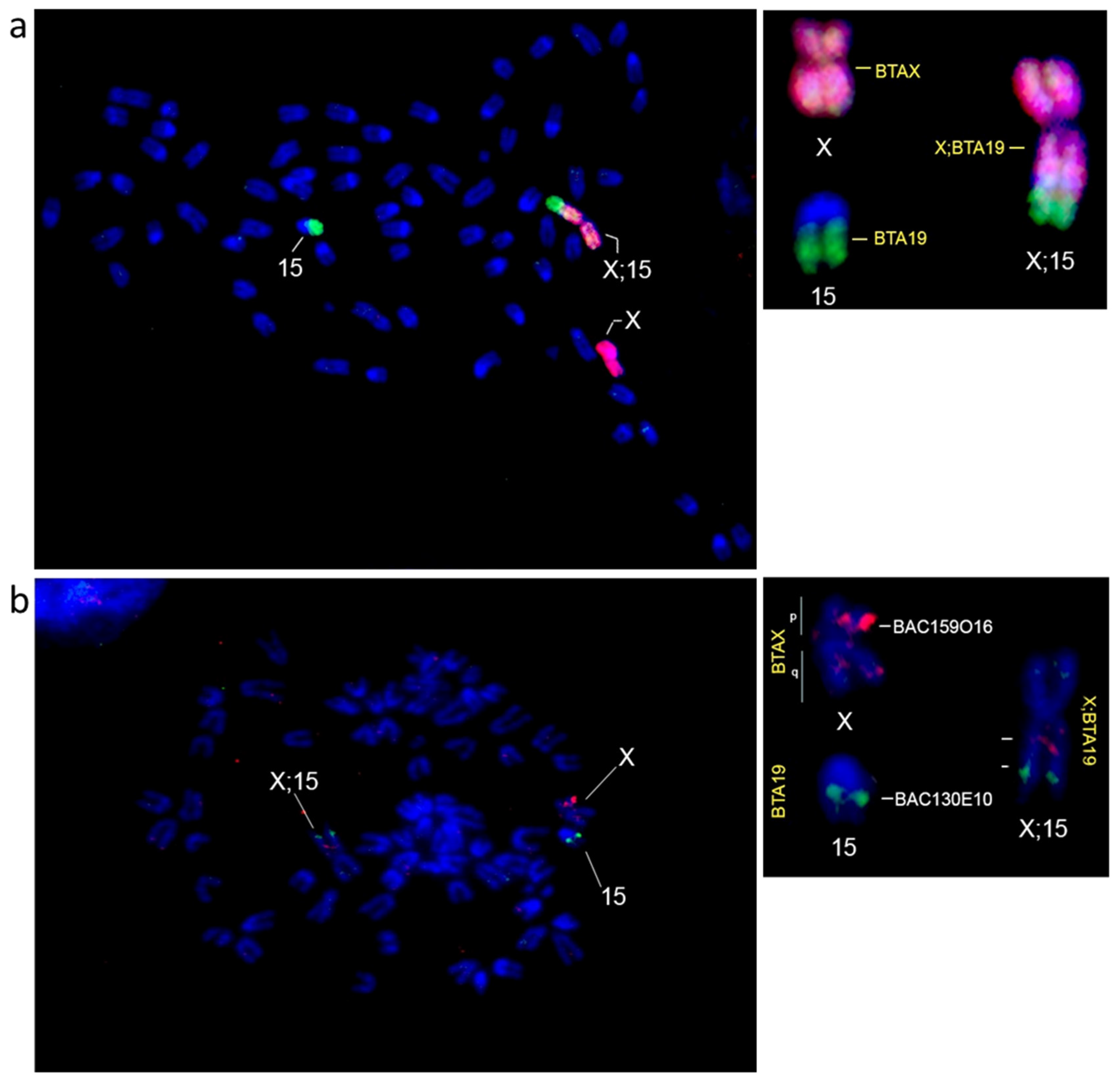
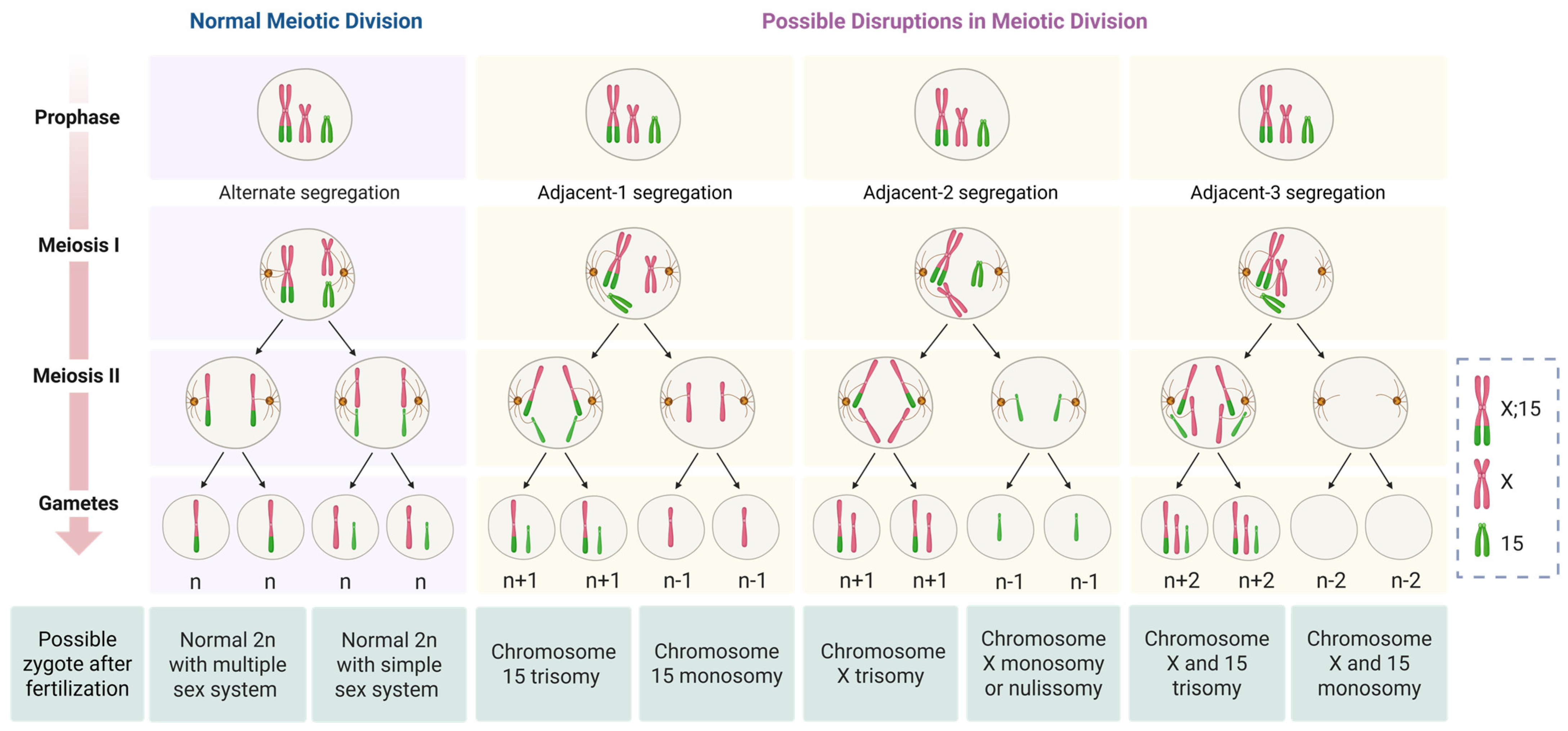
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rieseberg, L.H. Chromosomal Rearrangements and Speciation. Trends Ecol. Evol. 2001, 16, 351–358. [Google Scholar] [CrossRef]
- Faria, R.; Navarro, A. Chromosomal Speciation Revisited: Rearranging Theory with Pieces of Evidence. Trends Ecol. Evol. 2010, 25, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Graphodatsky, A.S.; Trifonov, V.A.; Stanyon, R. The Genome Diversity and Karyotype Evolution of Mammals. Mol. Cytogenet. 2011, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Ducos, A.; Berland, H.-M.; Bonnet, N.; Calgaro, A.; Billoux, S.; Mary, N.; Garnier-Bonnet, A.; Darré, R.; Pinton, A. Chromosomal Control of Pig Populations in France: 2002–2006 Survey. Genet. Sel. Evol. 2007, 39, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Dobigny, G.; Britton-Davidian, J.; Robinson, T.J. Chromosomal Polymorphism in Mammals: An Evolutionary Perspective. Biol. Rev. Camb. Philos. Soc. 2017, 92, 1–21. [Google Scholar] [CrossRef]
- Vargas-Munar, D.S.F.; Sarria-Perea, J.A.; Duarte, J.M.B. Different Responses to Doxorubicin-Induced Chromosome Aberrations in Brazilian Deer Species. Genet. Mol. Res. 2010, 9, 1545–1549. [Google Scholar] [CrossRef]
- Wurster, D.H.; Benirschke, K. Indian Muntjac, Muntiacus muntjak: A Deer with a Low Diploid Chromosome Number. Science 1970, 168, 1364–1366. [Google Scholar] [CrossRef]
- Fontana, F.; Rubini, M. Chromosomal Evolution in Cervidae. Biosystems 1990, 24, 157–174. [Google Scholar] [CrossRef]
- Bernegossi, A.M.; Vozdova, M.; Cernohorska, H.; Kubickova, S.; Galindo, D.J.; Kadlcikova, D.; Rubes, J.; Duarte, J.M.B. Cytogenetic Mapping of Cattle BAC Probes for the Hypothetical Ancestral Karyotype of the Family Cervidae. Cytogenet. Genome Res. 2022, 162, 140–147. [Google Scholar] [CrossRef]
- Proskuryakova, A.A.; Ivanova, E.S.; Makunin, A.I.; Larkin, D.M.; Ferguson-Smith, M.A.; Yang, F.; Uphyrkina, O.V.; Perelman, P.L.; Graphodatsky, A.S. Comparative Studies of X Chromosomes in Cervidae Family. Sci. Rep. 2023, 13, 11992. [Google Scholar] [CrossRef]
- Morales-Donoso, J.A.; Vacari, G.Q.; Bernegossi, A.M.; Sandoval, E.D.P.; Peres, P.H.F.; Galindo, D.J.; De Thoisy, B.; Vozdova, M.; Kubickova, S.; Barbanti Duarte, J.M. Revalidation of Passalites Gloger, 1841 for the Amazon Brown Brocket Deer P. nemorivagus (Cuvier, 1817) (Mammalia, Artiodactyla, Cervidae). ZooKeys 2023, 1167, 241–264. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.M.B.; González, S.; Maldonado, J.E. The Surprising Evolutionary History of South American Deer. Mol. Phylogenet. Evol. 2008, 49, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, B.F.; Sarria-Perea, J.A.; Abril, V.; Duarte, J.M.B. Cytogenetic Description of the Amazonian Brown Brocket Mazama nemorivaga (Artiodactyla, Cervidae). Comp. Cytogenet. 2013, 7, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Galindo, D.J.; Martins, G.S.; Vozdova, M.; Cernohorska, H.; Kubickova, S.; Bernegossi, A.M.; Kadlcikova, D.; Rubes, J.; Duarte, J.M. Chromosomal Polymorphism and Speciation: The Case of the Genus Mazama (Cetartiodactyla; Cervidae). Genes 2021, 12, 165. [Google Scholar] [CrossRef]
- Hughes, J.J.; Lagunas-Robles, G.; Campbell, P. The Role of Conflict in the Formation and Maintenance of Variant Sex Chromosome Systems in Mammals. J. Hered. 2024, 115, 601–624. [Google Scholar] [CrossRef]
- Kitano, J.; Peichel, C.L. Turnover of Sex Chromosomes and Speciation in Fishes. Environ. Biol. Fishes 2012, 94, 549–558. [Google Scholar] [CrossRef]
- Yoshida, K.; Kitano, J. The contribution of female meiotic drive to the evolution of neo-sex chromosomes. Evolution 2012, 66, 3198–3208. [Google Scholar] [CrossRef]
- Pathak, S.; Lin, C.C. Synaptonemal Complex of the Sex-Autosome Trivalent in a Male Indian Muntjac. Chromosoma 1981, 82, 367–376. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, H.; Zhang, X. Polymorphic Karyotypes and Sex Chromosomes in the Tufted Deer (Elaphodus cephalophus): Cytogenetic Studies and Analyses of Sex Chromosome-Linked Genes. Cytogenet. Genome Res. 2005, 109, 512–518. [Google Scholar] [CrossRef]
- Bernegossi, A.M.; Cartes, J.L.; Cernohorska, H.; Kubickova, S.; Vozdova, M.; Caparroz, R.; González, S.; Duarte, J.M.B. Resurrection of the Genus Subulo Smith, 1827 for the Gray Brocket Deer, with Designation of a Neotype. J. Mammal. 2023, 104, 619–633. [Google Scholar] [CrossRef]
- Aquino, C.I.; Abril, V.V.; Duarte, J.M.B. Meiotic Pairing of B Chromosomes, Multiple Sexual System, and Robertsonian Fusion in the Red Brocket Deer Mazama americana (Mammalia, Cervidae). Genet. Mol. Res. 2013, 12, 3566–3574. [Google Scholar] [CrossRef]
- Peres, P.H.F.; Luduvério, D.J.; Bernegossi, A.M.; Galindo, D.J.; Nascimento, G.B.; Oliveira, M.L.; Sandoval, E.D.P.; Vozdova, M.; Kubickova, S.; Cernohorska, H.; et al. Revalidation of Mazama rufa (Illiger 1815) (Artiodactyla: Cervidae) as a Distinct Species out of the Complex Mazama americana (Erxleben 1777). Front. Genet. 2021, 12, 742870. [Google Scholar] [CrossRef] [PubMed]
- Dobigny, G.; Ozouf-Costaz, C.; Bonillo, C.; Volobouev, V. Viability of X-Autosome Translocations in Mammals: An Epigenomic Hypothesis from a Rodent Case-Study. Chromosoma 2004, 113, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Deuve, J.L.; Bennett, N.C.; Ruiz-Herrera, A.; Waters, P.D.; Britton-Davidian, J.; Robinson, T.J. Dissection of a Y-Autosome Translocation in Cryptomys hottentotus (Rodentia, Bathyergidae) and Implications for the Evolution of a Meiotic Sex Chromosome Chain. Chromosoma 2008, 117, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Borodin, P.M.; Fedyk, S.; Chętnicki, W.; Torgasheva, A.A.; Pavlova, S.V.; Searle, J.B. Meiosis and Fertility Associated with Chromosomal Heterozygosity. In Shrews, Chromosomes and Speciation; Searle, J.B., Polly, P.D., Zima, J., Eds.; Cambridge Studies in Morphology and Molecules: New Paradigms in Evolutionary Bio; Cambridge University Press: Cambridge, UK, 2019; pp. 217–270. ISBN 978-1-107-01137-3. [Google Scholar]
- Rahn, M.I.; Noronha, R.C.; Nagamachi, C.Y.; Pieczarka, J.C.; Solari, A.J.; Sciurano, R.B. Protein Markers of Synaptic Behavior and Chromatin Remodeling of the Neo-XY Body in Phyllostomid Bats. Chromosoma 2016, 125, 701–708. [Google Scholar] [CrossRef]
- Vozdova, M.; Ruiz-Herrera, A.; Fernandez, J.; Cernohorska, H.; Frohlich, J.; Sebestova, H.; Kubickova, S.; Rubes, J. Meiotic Behaviour of Evolutionary Sex-Autosome Translocations in Bovidae. Chromosome Res. 2016, 24, 325–338. [Google Scholar] [CrossRef]
- De Oliveira, M.L.; De Faria Peres, P.H.; Gatti, A.; Morales-Donoso, J.A.; Mangini, P.R.; Duarte, J.M.B. Faecal DNA and Camera Traps Detect an Evolutionarily Significant Unit of the Amazonian Brocket Deer in the Brazilian Atlantic Forest. Eur. J. Wildl. Res. 2020, 66, 28. [Google Scholar] [CrossRef]
- Comizzoli, P.; Holt, W.V. Breakthroughs and New Horizons in Reproductive Biology of Rare and Endangered Animal Species. Biol. Reprod. 2019, 101, 514–525. [Google Scholar] [CrossRef]
- Oliveira, M.E.F.; Zanetti, E.D.S.; Cursino, M.S.; De Fátima Carvalho Peroni, E.; Rola, L.D.; Feliciano, M.A.R.; Canola, J.C.; Duarte, J.M.B. First Live Offspring of Amazonian Brown Brocket Deer (Mazama nemorivaga) Born by Artificial Insemination. Eur. J. Wildl. Res. 2016, 62, 767–770. [Google Scholar] [CrossRef][Green Version]
- Oliveira, C.C.C.; Queiroz Vacari, G.; Maurício Barbanti Duarte, J. A Method to Freeze Skin Samples for Cryobanks: A Test of Some Cryoprotectants for an Endangered Deer. Biopreserv. Biobank. 2024, 22, 211–216. [Google Scholar] [CrossRef]
- Verma, R.; Babu, A. Human Chromosomes: Principles & Techniques, 2nd ed.; McGraw-Hill, Inc.: New York, NY, USA, 1995. [Google Scholar]
- Telenius, H.; Carter, N.P.; Bebb, C.E.; Nordenskjöld, M.; Ponder, B.A.; Tunnacliffe, A. Degenerate Oligonucleotide-Primed PCR: General Amplification of Target DNA by a Single Degenerate Primer. Genomics 1992, 13, 718–725. [Google Scholar] [CrossRef]
- Kubickova, S.; Cernohorska, H.; Musilova, P.; Rubes, J. The Use of Laser Microdissection for the Preparation of Chromosome-Specific Painting Probes in Farm Animals. Chromosome Res. 2002, 10, 571–577. [Google Scholar] [CrossRef]
- Vozdova, M.; Kubickova, S.; Cernohorska, H.; Fröhlich, J.; Vodicka, R.; Rubes, J. Comparative Study of the Bush Dog (Speothos venaticus) Karyotype and Analysis of Satellite DNA Sequences and Their Chromosome Distribution in Six Species of Canidae. Cytogenet. Genome Res. 2019, 159, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.P.M.; Sharbel, T.F.; Beukeboom, L.W. B-Chromosome Evolution. Phil. Trans. R. Soc. Lond. B 2000, 355, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Abril, V.V.; Carnelossi, E.A.G.; González, S.; Duarte, J.M.B. Elucidating the Evolution of the Red Brocket Deer Mazama americana Complex (Artiodactyla; Cervidae). Cytogenet. Genome Res. 2010, 128, 177–187. [Google Scholar] [CrossRef]
- Huang, N.; Zhou, J.; Lu, W.; Luo, L.; Yuan, H.; Pan, L.; Ding, S.; Yang, B.; Liu, Y. Characteristics and Clinical Evaluation of X Chromosome Translocations. Mol. Cytogenet. 2023, 16, 36. [Google Scholar] [CrossRef]
- Bernegossi, A.M.; Galindo, D.J.; Peres, P.H.F.; Vozdova, M.; Cernohorska, H.; Kubickova, S.; Kadlcikova, D.; Rubes, J.; Duarte, J.M.B. Comparative Karyotype Analysis of the Red Brocket Deer (M. americana Sensu Lato and M. rufa) Complex: Evidence of Drastic Chromosomal Evolution and Implications on Speciation Process. J. Appl. Genet. 2024, 65, 601–614. [Google Scholar] [CrossRef]
- Sandoval, E.D.P.; Bernegossi, A.M.; Gallina, S.; Reyna-Hurtado, R.; Duarte, J.M.B. Cytogenetic, Molecular, and Morphological Characterization of Odocoileus pandora (Merriam, 1901) (Artiodactyla, Cervidae). Can. J. Zool. 2023, 101, 967–979. [Google Scholar] [CrossRef]
- Sandoval, E.D.P.; Jędrzejewski, W.; Molinari, J.; Vozdova, M.; Cernohorska, H.; Kubickova, S.; Bernegossi, A.M.; Caparroz, R.; Duarte, J.M.B. Description of Bisbalus, a New Genus for the Gray Brocket, Mazama cita Osgood, 1912 (Mammalia, Cervidae), as a Step to Solve the Neotropical Deer Puzzle. Taxonomy 2024, 4, 10–26. [Google Scholar] [CrossRef]
- Sandoval, E.D.P.; Bernegossi, A.M.; Gallina, S.; Reyna-Hurtado, R.; Duarte, J.M. Molecular Cytogenetics Markers Reveal the Existence of a Cryptic Complex of Mazama temama Species. Therya 2024, 15, 192–200. [Google Scholar] [CrossRef]
- Hayman, D. Marsupial Cytogenetics. Aust. J. Zool. 1989, 37, 331–349. [Google Scholar] [CrossRef]
- Toder, R.; O’Neill, R.J.W.; Wienberg, J.; O’Brien, P.C.M.; Voullaire, L.; Marshall-Graves, J.A. Comparative Chromosome Painting between Two Marsupials: Origins of an XX/XY1Y2 Sex Chromosome System. Mamm. Genome 1997, 8, 418–422. [Google Scholar] [CrossRef]
- Steinberg, E.R.; Bressa, M.J.; Mudry, M.D. Sex Chromosome Systems in Neotropical Primates: What Have We Learnt so Far from Cytogenetics and Genomics? J. Evol. Biol. 2022, 35, 1589–1600. [Google Scholar] [CrossRef]
- Di-Battista, A.; Favilla, B.P.; Zamariolli, M.; Nunes, N.; Defelicibus, A.; Armelin-Correa, L.; da Silva, I.T.; Reymond, A.; Moyses-Oliveira, M.; Melaragno, M.I. Premature Ovarian Insufficiency Is Associated with Global Alterations in the Regulatory Landscape and Gene Expression in Balanced X-Autosome Translocations. Epigenet. Chromatin 2023, 16, 19. [Google Scholar] [CrossRef]
- Povill, C.; de Oliveira, M.B.; de Abreu, F.V.S.; de Oliveira, R.L.; Perini, F.A.; Monticelli, C.; Bueno, C.; dos Santos, E.; Pissinatti, A.; Bonvicino, C.R. Genetic Diversity and Insights into the Distribution of Brown Howler Monkeys (Alouatta guariba Group) (Atelidae, Alouattinae). Int. J. Primatol. 2023, 44, 517–539. [Google Scholar] [CrossRef]
- Iannuzzi, A.; Parma, P.; Iannuzzi, L. Chromosome Abnormalities and Fertility in Domestic Bovids: A Review. Animals 2021, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, B.; Wall, J.D. Inbreeding, Heterozygote Advantage and the Evolution of Neo-X and Neo-Y Sex Chromosomes. Proc. R. Soc. B Biol. Sci. 1999, 266, 51. [Google Scholar] [CrossRef]
- Rice, W.R. Sex Chromosomes and the Evolution of Sexual Dimorphism. Evolution 1984, 38, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.J.; Harrison, W.R.; Ponce de León, F.A.; Davis, S.K.; Elder, F.F. A Molecular Cytogenetic Analysis of X Chromosome Repatterning in the Bovidae: Transpositions, Inversions, and Phylogenetic Inference. Cytogenet. Cell Genet. 1998, 80, 179–184. [Google Scholar] [CrossRef]
- Proskuryakova, A.A.; Kulemzina, A.I.; Perelman, P.L.; Makunin, A.I.; Larkin, D.M.; Farré, M.; Kukekova, A.V.; Lynn Johnson, J.; Lemskaya, N.A.; Beklemisheva, V.R.; et al. X Chromosome Evolution in Cetartiodactyla. Genes 2017, 8, 216. [Google Scholar] [CrossRef]
- Shi, L.; Yang, F.; Kumamoto, A. The Chromosomes of Tufted Deer (Elaphodus cephalophus). Cytogenet. Cell Genet. 1991, 56, 189–192. [Google Scholar] [CrossRef]
- Steiner, C.C.; Charter, S.J.; Goddard, N.; Davis, H.; Brandt, M.; Houck, M.L.; Ryder, O.A. Chromosomal Variation and Perinatal Mortality in San Diego Zoo Soemmerring’s Gazelles. Zoo. Biol. 2015, 34, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Solari, A.J.; Pigozzi, M.I. Fine Structure of the XY Body in the XY1Y2 Trivalent of the Bat Artibeus lituratus. Chromosome Res. 1994, 2, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Noronha, R.C.R.; Nagamachi, C.Y.; O’Brien, P.C.M.; Ferguson-Smith, M.A.; Pieczarka, J.C. Meiotic Analysis of XX/XY and Neo-XX/XY Sex Chromosomes in Phyllostomidae by Cross-Species Chromosome Painting Revealing a Common Chromosome 15-XY Rearrangement in Stenodermatinae. Chromosome Res. 2010, 18, 667–676. [Google Scholar] [CrossRef]
- Noronha, R.C.R.; Nagamachi, C.Y.; Pieczarka, J.C.; Marques-Aguiar, S.; Assis, M.F.L.; Barros, R.M.D.S. Meiotic Analyses of the Sex Chromosomes in Carolliinae-Phyllostomidae (Chiroptera): NOR Separates the XY1Y2 into Two Independent Parts. Caryologia 2004, 57, 1–9. [Google Scholar] [CrossRef]
- Ashley, T. X-Autosome Translocations, Meiotic Synapsis, Chromosome Evolution and Speciation. Cytogenet. Genome Res. 2002, 96, 33–39. [Google Scholar] [CrossRef]
- Cifuentes-Rincón, A.; Morales-Donoso, J.A.; Sandoval, E.D.P.; Tomazella, I.M.; Mantellatto, A.M.B.; De Thoisy, B.; Duarte, J.M.B. Designation of a Neotype for Mazama americana (Artiodactyla, Cervidae) Reveals a Cryptic New Complex of Brocket Deer Species. ZooKeys 2020, 958, 143–164. [Google Scholar] [CrossRef]
- Cursino, M.S.; Salviano, M.B.; Abril, V.V.; Zanetti, E.d.S.; Duarte, J.M.B. The Role of Chromosome Variation in the Speciation of the Red Brocket Deer Complex: The Study of Reproductive Isolation in Females. BMC Evol. Biol. 2014, 14, 40. [Google Scholar] [CrossRef]
- Salviano, M.B.; Cursino, M.S.; Zanetti, E.D.S.; Abril, V.V.; Duarte, J.M.B. Intraspecific Chromosome Polymorphisms Can Lead to Reproductive Isolation and Speciation: An Example in Red Brocket Deer (Mazama americana). Biol. Reprod. 2017, 96, 1279–1287. [Google Scholar] [CrossRef]
- Lyon, M.F. Gene Action in the X-Chromosome of the Mouse (Mus. musculus L.). Nature 1961, 190, 372–373. [Google Scholar] [CrossRef]
- Chatziparasidou, A.; Christoforidis, N.; Samolada, G.; Nijs, M. Sperm Aneuploidy in Infertile Male Patients: A Systematic Review of the Literature. Andrologia 2015, 47, 847–860. [Google Scholar] [CrossRef]
- Nadesapillai, S.; van der Velden, J.; Braat, D.; Fleischer, K.; Peek, R. Exploring X Chromosomal Aberrations in Ovarian Cells by Using Fluorescence In Situ Hybridization. J. Vis. Exp. 2023, 194, e64734. [Google Scholar] [CrossRef] [PubMed]
- Bonato, R.M.; Bernegossi, A.M.; Sandoval, E.D.P.; Cernohorska, H.; Vozdova, M.; Duarte, J.M.B. Genética da conservação: Polimorfismo sexual no Veado-roxo e implicações para o manejo reprodutivo em cativeiro. In Proceedings of the Anais do 48º Congresso da Associação de Zoológicos e Aquários do Brasil (AZAB), Brasília, Brazil, 10–13 June 2025. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonato, R.M.; Bernegossi, A.M.; Sandoval, E.D.P.; Cernohorska, H.; Vozdova, M.; Duarte, J.M.B. Neo-X-Linked Chromosome Polymorphism: Cytogenetic Insights from Passalites nemorivagus (Mammalia, Cervidae). Animals 2025, 15, 2557. https://doi.org/10.3390/ani15172557
Bonato RM, Bernegossi AM, Sandoval EDP, Cernohorska H, Vozdova M, Duarte JMB. Neo-X-Linked Chromosome Polymorphism: Cytogenetic Insights from Passalites nemorivagus (Mammalia, Cervidae). Animals. 2025; 15(17):2557. https://doi.org/10.3390/ani15172557
Chicago/Turabian StyleBonato, Raquel Muhlbeier, Agda Maria Bernegossi, Eluzai Dinai Pinto Sandoval, Halina Cernohorska, Miluse Vozdova, and José Maurício Barbanti Duarte. 2025. "Neo-X-Linked Chromosome Polymorphism: Cytogenetic Insights from Passalites nemorivagus (Mammalia, Cervidae)" Animals 15, no. 17: 2557. https://doi.org/10.3390/ani15172557
APA StyleBonato, R. M., Bernegossi, A. M., Sandoval, E. D. P., Cernohorska, H., Vozdova, M., & Duarte, J. M. B. (2025). Neo-X-Linked Chromosome Polymorphism: Cytogenetic Insights from Passalites nemorivagus (Mammalia, Cervidae). Animals, 15(17), 2557. https://doi.org/10.3390/ani15172557





