Docosahexaenoic Acid (DHA) Decreases IL-6 and Prostaglandin-Endoperoxide Synthase 2 mRNA Expression and IL-6 Protein Release, While Increasing Resolvin D1 and CXCL8 mRNA Expression and Protein Release in BovineEndometrial Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture of Bovine Endometrial (BEND) Cells
2.2. Real Time-qPCR
2.3. IL-6, CXCL8, and RvD1 ELISA Assay
2.4. Immunoblot
2.5. Gas Chromatography–Mass Spectrometry (GC–MS) Metabolomics: Sample Preparation and Metabolomics Assay
2.6. Statistical Analysis
3. Results
3.1. DHA Reduces IL-6 and PTGS2 Expression and ERK1/2 and Akt Phosphorylation and Increases CXCL8 Induced by LPS in BEND Cells
3.2. DHA Induces RvD1 Production, a Metabolite Derived from DHA, in BEND Cells
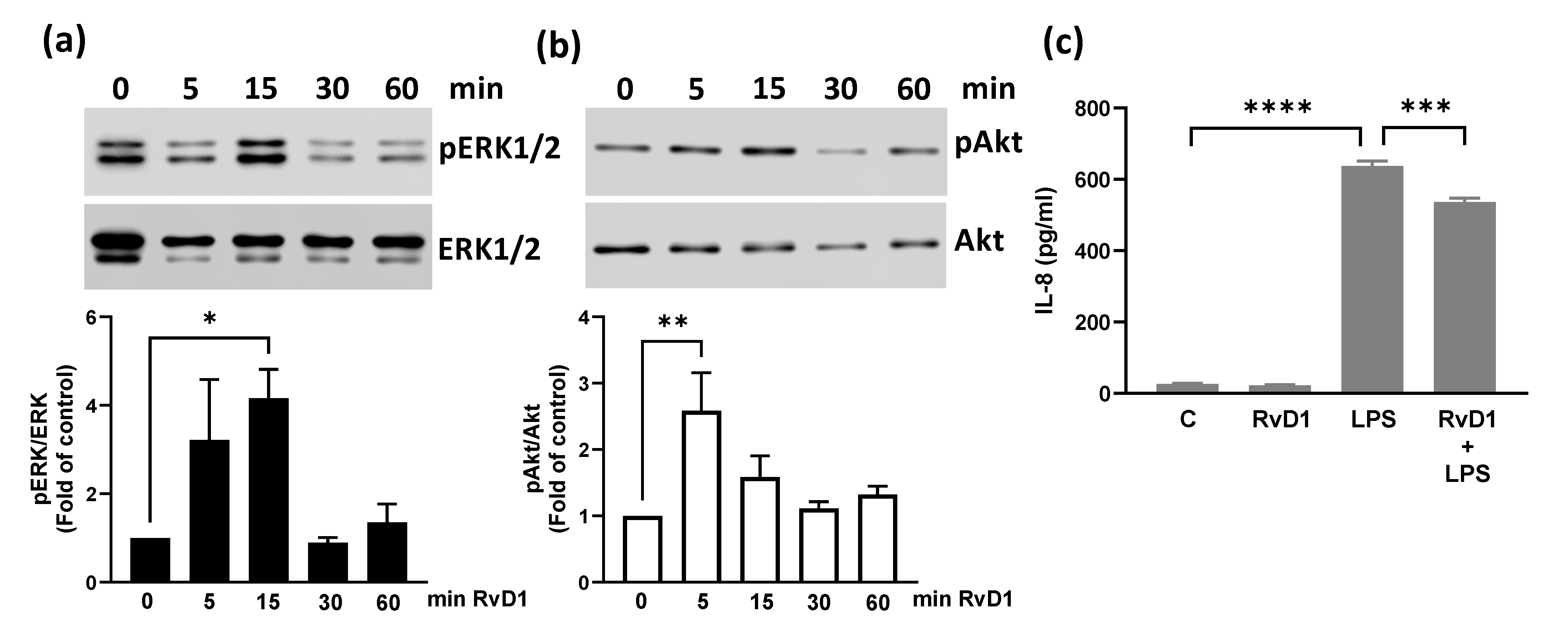
3.3. RvD1 Increases ERK1/2 and Akt Phosphorylation and Reduces LPS-Induced CXCL8 in BEND Cells
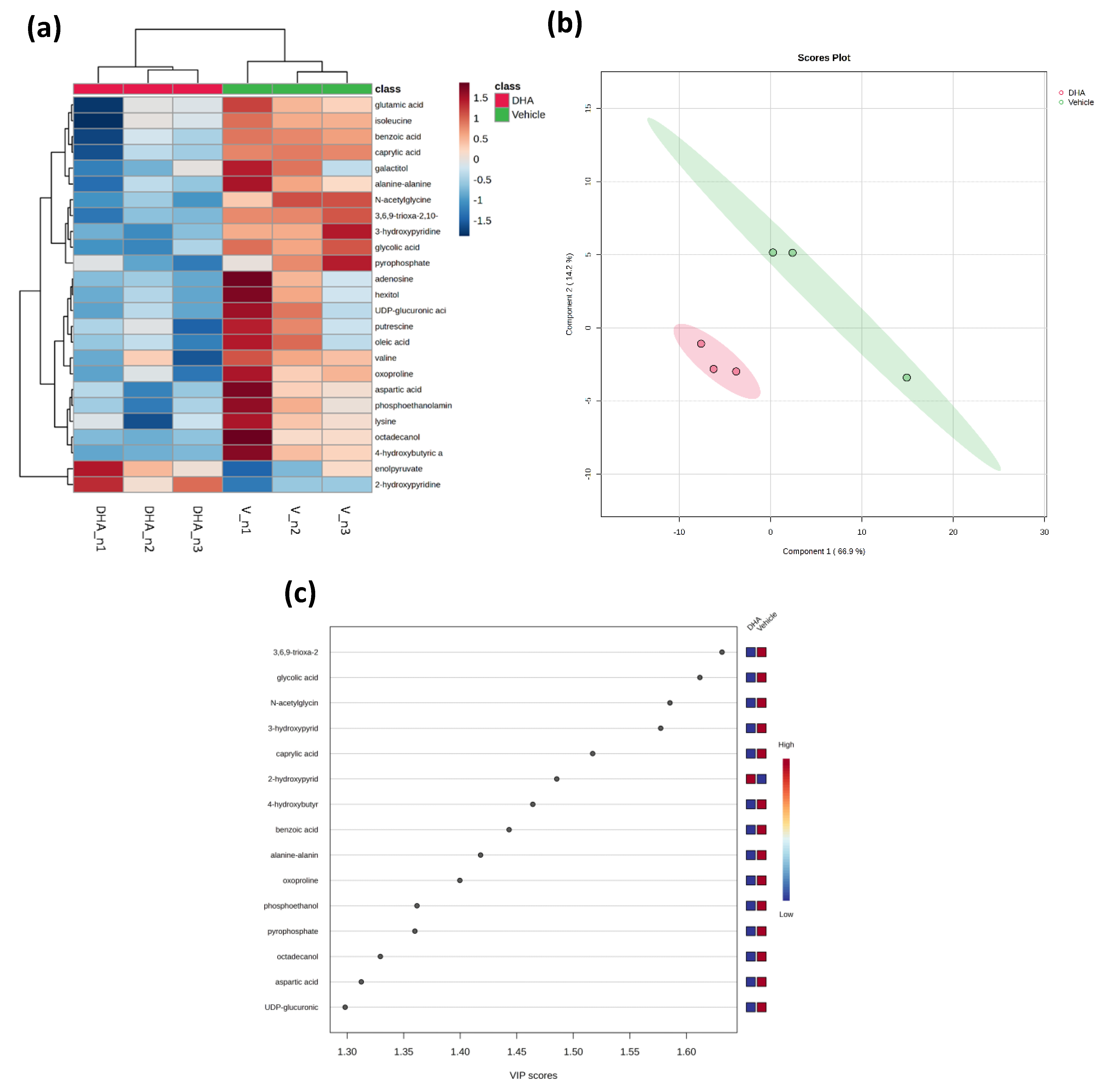
3.4. Metabolomics Changes Induced by DHA in BEND Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DHA | Docosahexaenoic acid |
| BEND | Bovine endometrial |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 |
| IL | Interleukin |
| RvD1 | Resolvin D1 |
| LPS | Lipopolysaccharide |
| ELISA | Enzyme-linked immunosorbent assay |
| RT-qPCR | Reverse transcription-quantitative polymerase chain reaction |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| NEFA | Non-esterified fatty acids |
| NEB | Negative energy balance |
| PUFA | Polyunsaturated fatty acid |
| TNF-α | Tumor necrosis factor-alpha |
| MMP-9 | Mmatrix metalloproteinase-9 |
| ROS | Reactive oxygen species |
| PGE2 | ProstaglandinE2 |
| SPM | Specialized pro-resolving mediators |
| FFA4 | Free Fatty Acid-4 |
| 15-LO | 15-lipoxygenase |
| GC-MS | Gas chromatography–mass spectrometry |
| HPLC | High performance liquid chromatography |
References
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef]
- LeBlanc, S.J. Postpartum uterine disease and dairy herd reproductive performance: A review. Vet. J. 2008, 176, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Ingvartsen, K.L.; Moyes, K. Nutrition, immune function and health of dairy cattle. Animals 2013, 7 (Suppl. S1), 112–122. [Google Scholar] [CrossRef] [PubMed]
- Bradford, B.J.; Yuan, K.; Farney, J.K.; Mamedova, L.K.; Carpenter, A.J. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J. Dairy Sci. 2015, 98, 6631–6650. [Google Scholar] [CrossRef] [PubMed]
- Pascottini, O.B.; LeBlanc, S.J. Modulation of immune function in the bovine uterus peripartum. Theriogenology 2020, 150, 193–200. [Google Scholar] [CrossRef]
- Carneiro, L.C.; Cronin, J.G.; Sheldon, I.M. Mechanisms linking bacterial infections of the bovine endometrium to disease and infertility. Reprod. Biol. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Cronin, J.G.; Turner, M.L.; Goetze, L.; Bryant, C.E.; Sheldon, I.M. Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biol. Reprod. 2012, 86, 51. [Google Scholar] [CrossRef]
- MacKintosh, S.B.; Schuberth, H.J.; Healy, L.L.; Sheldon, I.M. Polarised bovine endometrial epithelial cells vectorially secrete prostaglandins and chemotactic factors under physiological and pathological conditions. Reproduction 2013, 145, 57–72. [Google Scholar] [CrossRef]
- Waters, S.M.; Coyne, G.S.; Kenny, D.A.; MacHugh, D.E.; Morris, D.G. Dietary n-3 polyunsaturated fatty acid supplementation alters the expression of genes involved in the control of fertility in the bovine uterine endometrium. Physiol. Genom. 2012, 44, 878–888. [Google Scholar] [CrossRef]
- Dirandeh, E.; Towhidi, A.; Zeinoaldini, S.; Ganjkhanlou, M.; Ansari Pirsaraei, Z.; Fouladi-Nashta, A. Effects of different polyunsaturated fatty acid supplementations during the postpartum periods of early lactating dairy cows on milk yield, metabolic responses, and reproductive performances. J. Anim. Sci. 2013, 91, 713–721. [Google Scholar] [CrossRef]
- Greco, L.F.; Neves Neto, J.T.; Pedrico, A.; Lima, F.S.; Bisinotto, R.S.; Martinez, N.; Ribeiro, E.S.; Thatcher, W.W.; Staples, C.R.; Santos, J.E.P. Effects of altering the ratio of dietary n-6 to n-3 fatty acids on spontaneous luteolysis in lactating dairy cows. J. Dairy Sci. 2018, 101, 10536–10556. [Google Scholar] [CrossRef]
- Silvestre, F.T.; Carvalho, T.S.; Francisco, N.; Santos, J.E.; Staples, C.R.; Jenkins, T.C.; Thatcher, W.W. Effects of differential supplementation of fatty acids during the peripartum and breeding periods of Holstein cows: I. Uterine and metabolic responses, reproduction, and lactation. J. Dairy Sci. 2011, 94, 189–204. [Google Scholar] [CrossRef]
- Sinedino, L.D.; Honda, P.M.; Souza, L.R.; Lock, A.L.; Boland, M.P.; Staples, C.R.; Thatcher, W.W.; Santos, J.E. Effects of supplementation with docosahexaenoic acid on reproduction of dairy cows. Reproduction 2017, 153, 707–723. [Google Scholar] [CrossRef]
- Greco, L.F.; Neves Neto, J.T.; Pedrico, A.; Ferrazza, R.A.; Lima, F.S.; Bisinotto, R.S.; Martinez, N.; Garcia, M.; Ribeiro, E.S.; Gomes, G.C.; et al. Effects of altering the ratio of dietary n-6 to n-3 fatty acids on performance and inflammatory responses to a lipopolysaccharide challenge in lactating Holstein cows. J. Dairy Sci. 2015, 98, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; He, Q.; Sun, X.; Wang, W.; Li, S. Effects of increasing n3:n6 ratio by replacing extruded soybeans with extruded flaxseed on dry matter intake, rumen fluid bacteria, and liver lipid metabolism in transition cows. BMC Microbiol. 2025, 25, 138. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.; Simoes, P.; Bexiga, R.; Silva, E.; Mateus, L.; Fernandes, T.; Alves, S.P.; Bessa, R.J.B.; Lopes-da-Costa, L. Effects of feeding rumen-protected linseed fat to postpartum dairy cows on plasma n-3 polyunsaturated fatty acid concentrations and metabolic and reproductive parameters. J. Dairy Sci. 2022, 105, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Waters, S.M.; Coyne, G.S.; Kenny, D.A.; Morris, D.G. Effect of dietary n-3 polyunsaturated fatty acids on transcription factor regulation in the bovine endometrium. Mol. Biol. Rep. 2014, 41, 2745–2755. [Google Scholar] [CrossRef]
- Dos, S.S.P.; Butenko, Y.; Kra, G.; Malitsky, S.; Itkin, M.; Levin, Y.; Moallem, U.; Zachut, M. Omega-3 fatty acid supplementation from late pregnancy to early lactation attenuates the endocannabinoid system and immune proteome in preovulatory follicles and endometrium of Holstein dairy cows. J. Dairy Sci. 2025, 108, 4299–4317. [Google Scholar] [CrossRef]
- Bodur, M.; Yilmaz, B.; Agagunduz, D.; Ozogul, Y. Immunomodulatory Effects of Omega-3 Fatty Acids: Mechanistic Insights and Health Implications. Mol. Nutr. Food Res. 2025, 69, e202400752. [Google Scholar] [CrossRef]
- Khan, I.; Hussain, M.; Jiang, B.; Zheng, L.; Pan, Y.; Hu, J.; Khan, A.; Ashraf, A.; Zou, X. (Omega-3 long-chain polyunsaturated fatty acids: Metabolism and health implications). Prog. Lipid Res. 2023, 92, 101255. [Google Scholar] [CrossRef]
- Cucchi, D.; Camacho-Munoz, D.; Certo, M.; Niven, J.; Smith, J.; Nicolaou, A.; Mauro, C. Omega-3 polyunsaturated fatty acids impinge on CD4+ T cell motility and adipose tissue distribution via direct and lipid mediator-dependent effects. Cardiovasc. Res. 2020, 116, 1006–1020. [Google Scholar] [CrossRef]
- Hidalgo, M.A.; Carretta, M.D.; Burgos, R.A. Long Chain Fatty Acids as Modulators of Immune Cells Function: Contribution of FFA1 and FFA4 Receptors. Front. Physiol. 2021, 12, 668330. [Google Scholar] [CrossRef]
- Perez-Mojica, J.E.; Lillycrop, K.A.; Cooper, C.; Calder, P.C.; Burdge, G.C. Docosahexaenoic acid and oleic acid induce altered DNA methylation of individual CpG loci in Jurkat T cells. Prostaglandins Leukot. Essent. Fat. Acids 2020, 158, 102128. [Google Scholar] [CrossRef]
- Saidi, H.; Murtaza, B.; Khan, A.S.; Koceir, E.A.; Hichami, A.; Khan, N.A. DHA induces Jurkat T-cell arrest in G2/M phase of cell cycle and modulates the plasma membrane expression of TRPC3/6 channels. Biochimie 2021, 181, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Verlengia, R.; Gorjao, R.; Kanunfre, C.C.; Bordin, S.; Martins De Lima, T.; Martins, E.F.; Curi, R. Comparative effects of eicosapentaenoic acid and docosahexaenoic acid on proliferation, cytokine production, and pleiotropic gene expression in Jurkat cells. J. Nutr. Biochem. 2004, 15, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Wierenga, K.A.; Wee, J.; Gilley, K.N.; Rajasinghe, L.D.; Bates, M.A.; Gavrilin, M.A.; Holian, A.; Pestka, J.J. Docosahexaenoic Acid Suppresses Silica-Induced Inflammasome Activation and IL-1 Cytokine Release by Interfering with Priming Signal. Front. Immunol. 2019, 10, 2130. [Google Scholar] [CrossRef] [PubMed]
- Olmo, I.; Teuber, S.; Larrazabal, C.; Alarcon, P.; Raipane, F.; Burgos, R.A.; Hidalgo, M.A. Docosahexaenoic acid and TUG-891 activate free fatty acid-4 receptor in bovine neutrophils. Vet. Immunol. Immunopathol. 2019, 209, 53–60. [Google Scholar] [CrossRef]
- Valenzuela, P.; Teuber, S.; Manosalva, C.; Alarcon, P.; Figueroa, C.D.; Ratto, M.; Burgos, R.A.; Hidalgo, M.A. Functional expression of the free fatty acids receptor-1 and -4 (FFA1/GPR40 and FFA4/GPR120) in bovine endometrial cells. Vet. Res. Commun. 2019, 43, 179–186. [Google Scholar] [CrossRef]
- Harwood, J.L. Polyunsaturated Fatty Acids: Conversion to Lipid Mediators, Roles in Inflammatory Diseases and Dietary Sources. Int. J. Mol. Sci. 2023, 24, 8838. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Maillard, V.; Desmarchais, A.; Durcin, M.; Uzbekova, S.; Elis, S. Docosahexaenoic acid (DHA) effects on proliferation and steroidogenesis of bovine granulosa cells. Reprod. Biol. Endocrinol. 2018, 16, 40. [Google Scholar] [CrossRef]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Yacoubian, S.; Lee, C.H.; Yang, R.; Petasis, N.A.; Serhan, C.N. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 1660–1665. [Google Scholar] [CrossRef]
- Sousa, L.G.; Alves, P.; Teixeira, N.; Correia-da-Silva, G.; Fonseca, B.M. Alterations in the pro-resolving lipid mediator machinery within first trimester maternal tissue: Implications in decidualization and miscarriage risk. Prostaglandins Leukot. Essent. Fat. Acids 2024, 201, 102619. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.G.; Correia-da-Silva, G.; Teixeira, N.; Fonseca, B.M. Specialized pro-resolving mediators: Key regulators in placental function and pregnancy complications. J. Mol. Med. 2025, 103, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Guzeloglu, A.; Michel, F.; Thatcher, W.W. Differential effects of interferon-tau on the prostaglandin synthetic pathway in bovine endometrial cells treated with phorbol ester. J. Dairy Sci. 2004, 87, 2032–2041. [Google Scholar] [CrossRef]
- Turner, M.L.; Cronin, J.G.; Healey, G.D.; Sheldon, I.M. Epithelial and stromal cells of bovine endometrium have roles in innate immunity and initiate inflammatory responses to bacterial lipopeptides in vitro via Toll-like receptors TLR2, TLR1, and TLR6. Endocrinology 2014, 155, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Swangchan-Uthai, T.; Lavender, C.R.; Cheng, Z.; Fouladi-Nashta, A.A.; Wathes, D.C. Time course of defense mechanisms in bovine endometrium in response to lipopolysaccharide. Biol. Reprod. 2012, 87, 135. [Google Scholar] [CrossRef]
- Teuber, S.; Manosalva, C.; Alarcon, P.; Quiroga, J.; Pantoja, D.; Hidalgo, M.A.; Moran, G.; Burgos, R.A. Andrographolide Ameliorates Inflammatory Changes Induced by D-Lactate in Bovine Fibroblast-like Synoviocytes. Animals 2024, 14, 936. [Google Scholar] [CrossRef]
- Gutierrez, N.; Teuber, S.; Alarcon, P.; Burgos, R.A.; Hidalgo, M.A. ATP Induces Interleukin-8, Intracellular Calcium Release, and ERK1/2 Phosphorylation in Bovine Endometrial Cells, Partially through P2Y Receptors. Animals 2023, 13, 841. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Carretta, M.D.; Conejeros, I.; Hidalgo, M.A.; Burgos, R.A. Propionate induces the release of granules from bovine neutrophils. J. Dairy Sci. 2013, 96, 2507–2520. [Google Scholar] [CrossRef]
- Quiroga, J.; Alarcon, P.; Manosalva, C.; Teuber, S.; Taubert, A.; Hermosilla, C.; Hidalgo, M.A.; Carretta, M.D.; Burgos, R.A. Metabolic Reprogramming and Inflammatory Response Induced by D-Lactate in Bovine Fibroblast-Like Synoviocytes Depends on HIF-1 Activity. Front. Vet. Sci. 2021, 8, 625347. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Guo, S.; Guo, Y.F.; Zahoor, A.; Shaukat, A.; Chen, Y.; Umar, T.; Deng, P.G.; Guo, M. Upregulated-gene expression of pro-inflammatory cytokines (TNF-alpha, IL-1beta and IL-6) via TLRs following NF-kappaB and MAPKs in bovine mastitis. Acta Trop. 2020, 207, 105458. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.I.; Carretta, M.D.; Alarcon, P.; Manosalva, C.; Muller, A.; Navarro, M.; Hidalgo, M.A.; Kaehne, T.; Taubert, A.; Hermosilla, C.R.; et al. Pro-inflammatory mediators and neutrophils are increased in synovial fluid from heifers with acute ruminal acidosis. BMC Vet. Res. 2019, 15, 225. [Google Scholar] [CrossRef]
- Cui, L.; Wang, H.; Ding, Y.; Li, J.; Li, J. Changes in the blood routine, biochemical indexes and the pro-inflammatory cytokine expressions of peripheral leukocytes in postpartum dairy cows with metritis. BMC Vet. Res. 2019, 15, 157. [Google Scholar] [CrossRef]
- Ceciliani, F.; Ceron, J.J.; Eckersall, P.D.; Sauerwein, H. Acute phase proteins in ruminants. J Proteomics. 2012, 75, 4207–4231. [Google Scholar] [CrossRef]
- Byun, M.; Armon, R.; Souza, T.; Anderson, H.; Saleem, A.; Pauls, S.D. Omega-3 polyunsaturated fatty acids modify glucose metabolism in THP-1 monocytes. Biochem. Cell Biol. 2025, 103, 1–10. [Google Scholar] [CrossRef]
- Si, T.L.; Liu, Q.; Ren, Y.F.; Li, H.; Xu, X.Y.; Li, E.H.; Pan, S.Y.; Zhang, J.L.; Wang, K.X. Enhanced anti-inflammatory effects of DHA and quercetin in lipopolysaccharide-induced RAW264.7 macrophages by inhibiting NF-kappaB and MAPK activation. Mol. Med. Rep. 2016, 14, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Cheng, L.; Bi, X.; Zhang, X.; Liu, S.; Bai, X.; Li, F.; Zhao, A.Z. Elevation of omega-3 Polyunsaturated Fatty Acids Attenuates PTEN-deficiency Induced Endometrial Cancer Development through Regulation of COX-2 and PGE2 Production. Sci. Rep. 2015, 5, 14958. [Google Scholar] [CrossRef] [PubMed]
- Caswell, J.L.; Middleton, D.M.; Gordon, J.R. Production and functional characterization of recombinant bovine interleukin-8 as a specific neutrophil activator and chemoattractant. Vet. Immunol. Immunopathol. 1999, 67, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Goff, J.P.; Horst, R.L. Physiological changes at parturition and their relationship to metabolic disorders. J. Dairy Sci. 1997, 80, 1260–1268. [Google Scholar] [CrossRef]
- Kehrli, M.E., Jr.; Goff, J.P. Periparturient hypocalcemia in cows: Effects on peripheral blood neutrophil and lymphocyte function. J. Dairy Sci. 1989, 72, 1188–1196. [Google Scholar] [CrossRef]
- Hammon, D.S.; Evjen, I.M.; Dhiman, T.R.; Goff, J.P.; Walters, J.L. Neutrophil function and energy status in Holstein cows with uterine health disorders. Vet. Immunol. Immunopathol. 2006, 113, 21–29. [Google Scholar] [CrossRef]
- Paschoal, V.A.; Vinolo, M.A.; Crisma, A.R.; Magdalon, J.; Curi, R. Eicosapentaenoic (EPA) and docosahexaenoic (DHA) acid differentially modulate rat neutrophil function in vitro. Lipids 2013, 48, 93–103. [Google Scholar] [CrossRef]
- Pisani, L.F.; Lecchi, C.; Invernizzi, G.; Sartorelli, P.; Savoini, G.; Ceciliani, F. In vitro modulatory effect of omega-3 polyunsaturated fatty acid (EPA and DHA) on phagocytosis and ROS production of goat neutrophils. Vet. Immunol. Immunopathol. 2009, 131, 79–85. [Google Scholar] [CrossRef]
- Bicalho, M.L.S.; Zinicola, M.; Machado, V.S.; Lima, F.S.; Teixeira, A.G.V.; Narbus, C.; Xavier, M.R.; Higgins, H.; Bicalho, R.C. Effects of recombinant bovine interleukin-8 (rbIL-8) treatment on health, metabolism, and lactation performance in Holstein cattle I: Production and functional characterization of rbIL-8 in vitro and in vivo. J. Dairy Sci. 2019, 102, 10304–10315. [Google Scholar] [CrossRef]
- Zinicola, M.; Bicalho, M.L.S.; Santin, T.; Marques, E.C.; Bisinotto, R.S.; Bicalho, R.C. Effects of recombinant bovine interleukin-8 (rbIL-8) treatment on health, metabolism, and lactation performance in Holstein cattle II: Postpartum uterine health, ketosis, and milk production. J. Dairy Sci. 2019, 102, 10316–10328. [Google Scholar] [CrossRef]
- Dong, C.; Davis, R.J.; Flavell, R.A. MAP kinases in the immune response. Annu. Rev. Immunol. 2002, 20, 55–72. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef] [PubMed]
- Bora, G.; Yaba, A. The role of mitogen-activated protein kinase signaling pathway in endometriosis. J. Obstet. Gynaecol. Res. 2021, 47, 1610–1623. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, O.; Osuga, Y.; Hirota, Y.; Koga, K.; Hirata, T.; Harada, M.; Morimoto, C.; Yano, T.; Nishii, O.; Tsutsumi, O.; et al. Possible pathophysiological roles of mitogen-activated protein kinases (MAPKs) in endometriosis. Am. J. Reprod. Immunol. 2004, 52, 306–311. [Google Scholar] [CrossRef]
- Cui, L.; Wang, H.; Lin, J.; Wang, Y.; Dong, J.; Li, J.; Li, J. Progesterone inhibits inflammatory response in E. coli- or LPS-Stimulated bovine endometrial epithelial cells by NF-kappaB and MAPK pathways. Dev. Comp. Immunol. 2020, 105, 103568. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Fang, Z.; Qiao, L.; Jiang, Y.; Song, L.; Du, X.; Yao, H.; Xiao, L. Melatonin alleviates endometrial fibrosis in bovine endometritis by regulating TGF-beta/Smad and MAPK signaling pathways via MT2. J. Reprod. Immunol. 2025, 169, 104519. [Google Scholar] [CrossRef]
- Qin, C.X.; Norling, L.V.; Vecchio, E.A.; Brennan, E.P.; May, L.T.; Wootten, D.; Godson, C.; Perretti, M.; Ritchie, R.H. Formylpeptide receptor 2: Nomenclature, structure, signalling and translational perspectives: IUPHAR review 35. Br. J. Pharmacol. 2022, 179, 4617–4639. [Google Scholar] [CrossRef]
- Li, D.; Hodges, R.R.; Jiao, J.; Carozza, R.B.; Shatos, M.A.; Chiang, N.; Serhan, C.N.; Dartt, D.A. Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunol. 2013, 6, 1119–1130. [Google Scholar] [CrossRef]
- Kaye, R.; Botten, N.; Lippestad, M.; Li, D.; Hodges, R.R.; Utheim, T.P.; Serhan, C.N.; Dartt, D.A. Resolvin D1, but not resolvin E1, transactivates the epidermal growth factor receptor to increase intracellular calcium and glycoconjugate secretion in rat and human conjunctival goblet cells. Exp. Eye Res. 2019, 180, 53–62. [Google Scholar] [CrossRef]
- Odusanwo, O.; Chinthamani, S.; McCall, A.; Duffey, M.E.; Baker, O.J. Resolvin D1 prevents TNF-alpha-mediated disruption of salivary epithelial formation. Am. J. Physiol. Cell Physiol. 2012, 302, C1331–C1345. [Google Scholar] [CrossRef]
- Nelson, J.W.; Leigh, N.J.; Mellas, R.E.; McCall, A.D.; Aguirre, A.; Baker, O.J. ALX/FPR2 receptor for RvD1 is expressed and functional in salivary glands. Am. J. Physiol. Cell Physiol. 2014, 306, C178–C185. [Google Scholar] [CrossRef]
- Vomero, M.; Lamberti, L.; Corberi, E.; Currado, D.; Marino, A.; Berardicurti, O.; Fava, M.; Leuti, A.; Maccarrone, M.; Giacomelli, R.; et al. Specialized pro-resolving mediators and autoimmunity: Recent insights and future perspectives. Autoimmun. Rev. 2025, 24, 103896. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, N.; Suess, G.; Shirley, R. Resolvins RvD1 and 17(R)-RvD1 alleviate signs of inflammation in a rat model of endometriosis. Fertil. Steril. 2014, 102, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- de Faveri, C.; Fermino, P.M.P.; Piovezan, A.P.; Volpato, L.K. The Inflammatory Role of Pro-Resolving Mediators in Endometriosis: An Integrative Review. Int. J. Mol. Sci. 2021, 22, 4370. [Google Scholar] [CrossRef] [PubMed]
- Tomio, K.; Kawana, K.; Taguchi, A.; Isobe, Y.; Iwamoto, R.; Yamashita, A.; Kojima, S.; Mori, M.; Nagamatsu, T.; Arimoto, T.; et al. Omega-3 polyunsaturated Fatty acids suppress the cystic lesion formation of peritoneal endometriosis in transgenic mouse models. PLoS ONE 2013, 8, e73085. [Google Scholar] [CrossRef]
- Gemperle, C.; Tran, S.; Schmid, M.; Rimann, N.; Marti-Jaun, J.; Hartling, I.; Wawrzyniak, P.; Hersberger, M. Resolvin D1 reduces inflammation in co-cultures of primary human macrophages and adipocytes by triggering macrophages. Prostaglandins Leukot. Essent. Fatty. Acids 2021, 174, 102363. [Google Scholar] [CrossRef]
- Xiang, S.Y.; Ye, Y.; Yang, Q.; Xu, H.R.; Shen, C.X.; Ma, M.Q.; Jin, S.W.; Mei, H.X.; Zheng, S.X.; Smith, F.G.; et al. RvD1 accelerates the resolution of inflammation by promoting apoptosis of the recruited macrophages via the ALX/FasL-FasR/caspase-3 signaling pathway. Cell Death Discov. 2021, 7, 339. [Google Scholar] [CrossRef]
- Soliman, A.M.; Soliman, M.; Shah, S.S.H.; Baig, H.A.; Gouda, N.S.; Alenezi, B.T.; Alenezy, A.; Hegazy, A.M.S.; Jan, M.; Eltom, E.H. Molecular dynamics of inflammation resolution: Therapeutic implications. Front. Cell Dev. Biol. 2025, 13, 1600149. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H.; Liu, Q.; Li, Y.; Wang, J.; Yao, S.; Wu, Q. ResolvinD1 reduces apoptosis and inflammation in primary human alveolar epithelial type 2 cells. Lab. Investig. 2016, 96, 526–536. [Google Scholar] [CrossRef]
- Lee, H.N.; Kundu, J.K.; Cha, Y.N.; Surh, Y.J. Resolvin D1 stimulates efferocytosis through p50/p50-mediated suppression of tumor necrosis factor-alpha expression. J. Cell Sci. 2013, 126, 4037–4047. [Google Scholar] [CrossRef]
- Goncalves, D.S.; Melo, S.M.D.S.; Jacomini, A.P.; da Silva, M.J.; Pianoski, K.E.; Ames, F.Q.; Aguiar, R.P.; Oliveira, A.F.; Volpato, H.; Bidoia, D.L.; et al. Synthesis of novel 3,5,6-trisubstituted 2-pyridone derivatives and evaluation for their anti-inflammatory activity. Bioorg Med. Chem. 2020, 28, 115549. [Google Scholar] [CrossRef]
- Jung, F.; Liu, J.S.; Yang, S.H.; Tseng, H.Y.; Chou, S.P.; Lin, J.C.; Jow, G.M. FJU-C28 inhibits the endotoxin-induced pro-inflammatory cytokines expression via suppressing JNK, p38 MAPK and NF-kappaB signaling pathways. Pharmacol. Res. Perspect. 2021, 9, e00876. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemi, V.; Mojiri-Froshani, H.; Saghaei, L.; Fassihi, A. A study on the anti-inflammatory effects of new derivatives of 3-hydroxy pyridine-4-one. Adv. Biomed. Res. 2014, 3, 134. [Google Scholar] [CrossRef] [PubMed]
- Volchegorskii, I.A.; Pravdin, E.V.; Uzlova, T.V. Effects of 3-hydroxypyridine derivatives and succinic acid on endometrial leukocyte infiltration, cytokinemia and related affective symptoms during exacerbation of the chronic inflammation of the uterus and adnexa. Bull. Exp. Biol. Med. 2014, 156, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Chung, T.W.; Park, M.J.; Kim, H.S.; You, S.; Lee, M.S.; Joo, B.S.; Lee, K.S.; Kim, K.J.; Wee, G.; et al. Benzoic Acid Enhances Embryo Implantation through LIF-Dependent Expression of Integrin alphaVbeta3 and alphaVbeta5. J. Microbiol. Biotechnol. 2017, 27, 668–677. [Google Scholar] [CrossRef]
- Yamada, M.; Takanashi, K.; Hamatani, T.; Hirayama, A.; Akutsu, H.; Fukunaga, T.; Ogawa, S.; Sugawara, K.; Shinoda, K.; Soga, T.; et al. A medium-chain fatty acid as an alternative energy source in mouse preimplantation development. Sci. Rep. 2012, 2, 930. [Google Scholar] [CrossRef]
- Leonard, P.H.; Charlesworth, M.C.; Benson, L.; Walker, D.L.; Fredrickson, J.R.; Morbeck, D.E. Variability in protein quality used for embryo culture: Embryotoxicity of the stabilizer octanoic acid. Fertil. Steril. 2013, 100, 544–549. [Google Scholar] [CrossRef]
- Fredrickson, J.; Krisher, R.; Morbeck, D.E. The impact of the protein stabilizer octanoic acid on embryonic development and fetal growth in a murine model. J. Assist. Reprod. Genet. 2015, 32, 1517–1524. [Google Scholar] [CrossRef]
- Fluhr, L.; Mor, U.; Kolodziejczyk, A.A.; Dori-Bachash, M.; Leshem, A.; Itav, S.; Cohen, Y.; Suez, J.; Zmora, N.; Moresi, C.; et al. Gut microbiota modulates weight gain in mice after discontinued smoke exposure. Nature 2021, 600, 713–719. [Google Scholar] [CrossRef]
- Su, K.J.; Chen, X.Y.; Gong, R.; Zhao, Q.; Hu, S.D.; Feng, M.C.; Li, Y.; Lin, X.; Zhang, Y.H.; Greenbaum, J.; et al. Systematic metabolomic studies identified adult adiposity biomarkers with acetylglycine associated with fat loss in vivo. Front. Mol. Biosci. 2023, 10, 1166333. [Google Scholar] [CrossRef]
- Pederzolli, C.D.; Sgaravatti, A.M.; Braum, C.A.; Prestes, C.C.; Zorzi, G.K.; Sgarbi, M.B.; Wyse, A.T.; Wannmacher, C.M.; Wajner, M.; Dutra-Filho, C.S. 5-Oxoproline reduces non-enzymatic antioxidant defenses in vitro in rat brain. Metab. Brain Dis. 2007, 22, 51–65. [Google Scholar] [CrossRef]


| Metabolite | FC | log2(FC) | raw.pval | “=−LOG10(p)” |
|---|---|---|---|---|
| 3,6,9-trioxa-2,10-disilaundecane, 2,2,10,10-tetramethyl- | 0.02884 | −5.1158 | 0.0014157 | 2.849 |
| glycolic acid | 0.080281 | −3.6388 | 0.0026738 | 2.5729 |
| N-acetylglycine | 0.0054968 | −7.5072 | 0.0049903 | 2.3019 |
| 3-hydroxypyridine | 0.015087 | −6.0505 | 0.0058485 | 2.233 |
| caprylic acid | 0.021782 | −5.5207 | 0.014175 | 1.8485 |
| 2-hydroxypyridine | 2.7659 | 1.4677 | 0.019969 | 1.6996 |
| 4-hydroxybutyric acid | 0.088814 | −3.4931 | 0.024361 | 1.6133 |
| benzoic acid | 0.030144 | −5.052 | 0.029112 | 1.5359 |
| alanine-alanine | 0.032934 | −4.9243 | 0.035359 | 1.4515 |
| oxoproline | 0.016739 | −5.9007 | 0.040257 | 1.3952 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, G.; Gutierrez, N.; Moya, M.; Burgos, R.A.; Hidalgo, M.A. Docosahexaenoic Acid (DHA) Decreases IL-6 and Prostaglandin-Endoperoxide Synthase 2 mRNA Expression and IL-6 Protein Release, While Increasing Resolvin D1 and CXCL8 mRNA Expression and Protein Release in BovineEndometrial Cells. Animals 2025, 15, 2545. https://doi.org/10.3390/ani15172545
Sanchez G, Gutierrez N, Moya M, Burgos RA, Hidalgo MA. Docosahexaenoic Acid (DHA) Decreases IL-6 and Prostaglandin-Endoperoxide Synthase 2 mRNA Expression and IL-6 Protein Release, While Increasing Resolvin D1 and CXCL8 mRNA Expression and Protein Release in BovineEndometrial Cells. Animals. 2025; 15(17):2545. https://doi.org/10.3390/ani15172545
Chicago/Turabian StyleSanchez, Gisselle, Noemi Gutierrez, Mauricio Moya, Rafael A. Burgos, and Maria A. Hidalgo. 2025. "Docosahexaenoic Acid (DHA) Decreases IL-6 and Prostaglandin-Endoperoxide Synthase 2 mRNA Expression and IL-6 Protein Release, While Increasing Resolvin D1 and CXCL8 mRNA Expression and Protein Release in BovineEndometrial Cells" Animals 15, no. 17: 2545. https://doi.org/10.3390/ani15172545
APA StyleSanchez, G., Gutierrez, N., Moya, M., Burgos, R. A., & Hidalgo, M. A. (2025). Docosahexaenoic Acid (DHA) Decreases IL-6 and Prostaglandin-Endoperoxide Synthase 2 mRNA Expression and IL-6 Protein Release, While Increasing Resolvin D1 and CXCL8 mRNA Expression and Protein Release in BovineEndometrial Cells. Animals, 15(17), 2545. https://doi.org/10.3390/ani15172545






