No Correlation Between Chronic Cough and Radiographic Signs of Bronchial Narrowing in Dogs with Cardiomegaly and Left Atrial Dilation Secondary to Primary Mitral Valve Regurgitation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Assessment of Radiographs
2.3. Statistical Analysis
2.3.1. Power Analysis
2.3.2. Descriptive Statistics and Contingency Table Analyses
2.3.3. Regression Models
2.3.4. Reliability Assessment
3. Results
3.1. Study Sample
3.2. Case Exclusions
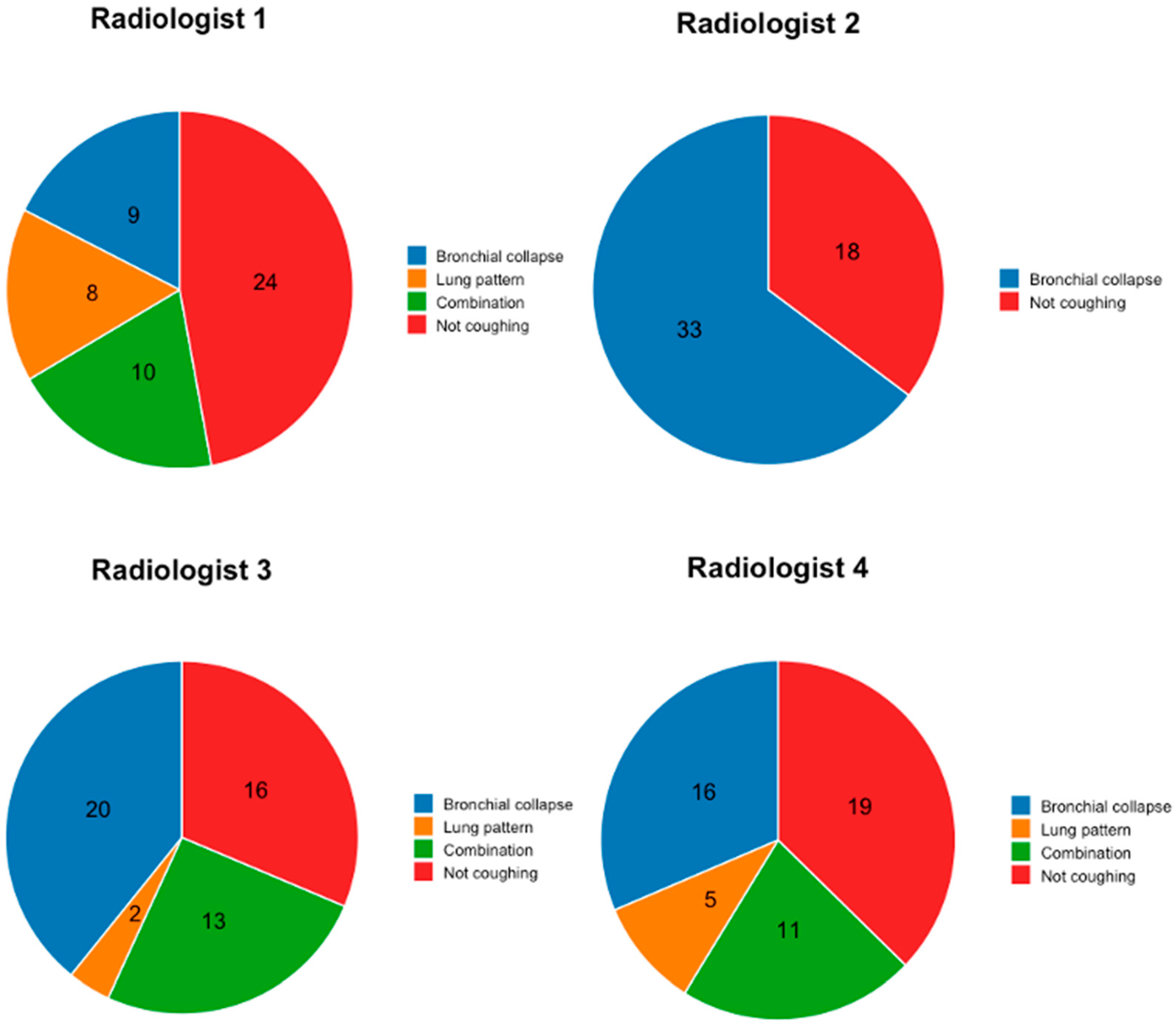
3.3. Image Review
3.4. Sensitivity and Specificity of Bronchial Compression/Collapse as a Predictor of Cough
3.5. Correlation of Radiographic Variables with Chronic Cough
3.6. Correlation Between Presumed Cough and Radiographic Variables
3.7. Interobserver Variability
3.8. Intraobserver Variability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef]
- Ljungvall, I.; Häggström, J. Valvular heart diseases of adult dogs and cats. Section XIV Cardiovascular diseases, Chapter 232. In Ettinger’s Textbook of Veterinary Internal Medicine, 9th ed.; Côté, E.E., Ettinger, S.J., Feldman, E.G., Eds.; Elsevier: Philadelphia, PA, USA, 2024; Volume 2, pp. 1348–1367. [Google Scholar]
- Borgarelli, M.; Buchanan, J.W. Historical review, epidemiology and natural history of degenerative mitral valve disease. J. Vet. Cardiol. 2012, 14, 93–101. [Google Scholar] [CrossRef]
- Buchanan, J.W. Chronic valvular disease (endocardiosis) in dogs. Adv. Vet. Sci. Comp. Med. 1977, 21, 75–106. [Google Scholar]
- Baisan, R.A.; Vulpe, V. Vertebral heart score and vertebral left atrial size as radiographic measurements for cardiac size in dogs—A literature review. Animals 2025, 15, 683. [Google Scholar] [CrossRef]
- Ferasin, L.; Crews, L.; Biller, D.S.; Lamb, K.E.; Borgarelli, M. Risk factors for coughing in dogs with naturally acquired myxomatous mitral valve disease. J. Vet. Intern. Med. 2013, 27, 286–292. [Google Scholar] [CrossRef]
- Johnson, L.R.; Pollard, R.E. Tracheal collapse and bronchomalacia in dogs: 58 cases (7/2001–1/2008). J. Vet. Intern. Med. 2010, 24, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Bottero, E.; Bellino, C.; De Lorenzi, D.; Ruggiero, P.; Tarducci, A.; D’Angelo, A.; Gianella, P. Clinical evaluation and endoscopic classification of bronchomalacia in dogs. J. Vet. Intern. Med. 2013, 27, 840–846. [Google Scholar] [CrossRef]
- Johnson, L.R.; Singh, M.K.; Pollard, R.E. Agreement among radiographs, fluoroscopy and bronchoscopy in documentation of airway collapse in dogs. J. Vet. Intern. Med. 2015, 29, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Adamama-Moraitou, K.K.; Pardali, D.; Day, M.J.; Prassinos, N.N.; Kritsepi-Konstantinou, M.; Patsikas, M.N.; Rallis, T.S. Canine bronchomalacia: A clinicopathological study of 18 cases diagnosed by endoscopy. J. Vet. Intern. Med. 2012, 191, 261–266. [Google Scholar] [CrossRef]
- Rozanski, E. Canine chronic bronchitis: An update. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Ferasin, L.; Linney, C. Coughing in dogs: What is the evidence for and against a cardiac cough? J. Small Anim. Pract. 2019, 60, 139–145. [Google Scholar] [CrossRef]
- Singh, M.K.; Johnson, L.R.; Kittleson, M.D.; Pollard, R.E. Bronchomalacia in dogs with myxomatous mitral valve degeneration. J. Vet. Intern. Med. 2012, 26, 312–319. [Google Scholar] [CrossRef]
- Lebastard, M.; Le Boedec, K.; Howes, M.; Joslyn, S.; Matheson, J.S.; O’Brien, R.T. Evaluation of bronchial narrowing in coughing dogs with heart murmurs using computed tomography. J. Vet. Intern. Med. 2021, 35, 1509–1518. [Google Scholar] [CrossRef]
- Gugliemini, C.; Diana, A.; Pietra, M.; Di Tommaso, M.; Cipone, M. Use of the vertebral heart score in coughing dogs with chronic degenerative mitral valve disease. J. Vet. Med. Sci. 2009, 71, 9–13. [Google Scholar] [CrossRef]
- Weisse, C. Insights in tracheobronchial stenting and a theory of bronchial compression. J. Small Anim. Pract. 2014, 55, 181–184. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzi, D.; Bertoncello, D.; Drigo, M. Bronchial abnormalities found in a consecutive series of 40 brachycephalic dogs. J. Am. Vet. Med. Assoc. 2009, 235, 835–840. [Google Scholar] [CrossRef]
- Buchanan, J.W.; Bücheler, J. Vertebral scale system to measure canine heart size in radiographs. J. Am. Vet. Med. Assoc. 1995, 206, 194–199. [Google Scholar] [CrossRef]
- Malcolm, E.L.; Visser, L.C.; Phillips, K.L.; Johnson, L.R. Diagnostic value of vertebral left atrial size as determined from thoracic radiographs for assessment of left atrial size in dogs with myxomatous mitral valve disease. J. Am. Vet. Med. Assoc. 2018, 253, 1038–1045. [Google Scholar] [CrossRef]
- Morice, A.H.; Millqvist, E.; Bieksiene, K.; Birring, S.S.; Dicpinigaitis, P.; Ribas, C.D.; Boon, M.H.; Kantar, A.; Lai, K.; McGarvey, L.; et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur. Respir. J. 2019, 55, 1901136. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropract Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Boswood, A.; Häggström, J.; Gordon, S.G.; Wess, G.; Stepien, R.L.; Oyama, M.A.; Keene, B.W.; Bonagura, J.; MacDonald, K.A.; Patteson, M.; et al. Effect of pimobendan in dogs with preclinical myxomatous mitral valve disease and cardiomegaly: The EPIC study—A randomized clinical trial. J. Vet. Intern. Med. 2016, 30, 1765–1779. [Google Scholar] [CrossRef] [PubMed]
- Côté, E.; Weisse, C.; Lamb, K.; Tozier, E. Computed tomographic assessment of principal bronchial anatomy in dogs of various thoracic conformations: 93 cases (2012–2017). J. Am. Vet. Med. Assoc. 2022, 260, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tudor, G.R.F.D.; Taub, N. An assessment of inter-observer agreement and accuracy when reporting plain radiographs. Clin. Radiol. 1977, 52, 235–238. [Google Scholar] [CrossRef]
- Albaum, M.N.; Hill, L.C.; Murphy, M.; Li, Y.-H.; Fuhrman, C.R.; Britton, C.A.; Kapoor, W.N.; Fine, M.J. Interobserver reliability of the chest radiograph in community-acquired pneumonia. Chest 1996, 110, 343–350. [Google Scholar] [CrossRef]
- Potchen, E.J. Measuring observer performance in chest radiology: Some experiences. J. Am. Coll. Radiol. 2006, 3, 423–432. [Google Scholar] [CrossRef]
- Bergamaschi, N.A.; Huber, L.; Ludewig, E.; Böhler, A.; Gumpenberger, M.; Hittmair, K.M.; Strohmayer, C.; Folkertsma, R.; Rowan, C. Association between clinical history in the radiographic request and diagnostic accuracy of thorax radiographs in dogs: A retrospective case-control study. J. Vet. Intern. Med. 2023, 37, 2453–2459. [Google Scholar] [CrossRef]
- Oh, D.; Lee, S.; Kim, S.; Choen, S.; Choi, M.; Yoon, J. Computed tomographic bronchial collapsibility values over 50% may be detected in healthy dogs. Vet. Radiol. Ultrasound 2019, 60, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Della Maggiore, A. An update on tracheal and airway collapse in dogs. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 419–430. [Google Scholar] [CrossRef]
- Reinero, C.R.; Masseau, I. Lower airway collapse: Revisiting the definition and clinicopathologic features of canine bronchomalacia. Vet. J. 2021, 273, 105682. [Google Scholar] [CrossRef]
- Blank, C.; Granger, L.A.; Gaschen, L.; Liu, C.-C.; Gaschen, F. Fluoroscopically measured bronchial collapse in healthy dogs during cough exceeds 25%, and a cutoff of 60% bronchial collapse can be used to distinguish healthy from chronically coughing dogs. Vet. Radiol. Ultrasound 2024, 65, 219–226. [Google Scholar] [CrossRef]
- Guillem, J.S.; Schiborra, F.; Rossanese, M.; Maddox, T.W.; Mortier, J.R. Prevalence of bronchial wall thickening and collapse in brachycephalic dogs with and without brachycephalic obstructive airway syndrome and in nonbrachycephalic dogs. J. Am. Vet. Med. Assoc. 2022, 261, 1–8. [Google Scholar] [CrossRef]
- Uehara, T.; Orito, K.; Fujii, Y. CT-based anatomical features of large airway and heart volume in dogs of different body size. Vet. J. 2019, 246, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Boissady, E.; de La Comble, A.; Zhu, X.; Hespel, A.-M. Artificial intelligence evaluating primary thoracic lesions has an overall lower error rate compared to veterinarians or veterinarians in conjunction with the artificial intelligence. Vet. Radiol. Ultrasound 2020, 61, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Fischetti, A.J.; Sreetharan, P.; Weltman, J.G.; Fox, P.R. Comparison of artificial intelligence to the veterinary radiologist’s diagnosis of canine cardiogenic pulmonary edema. Vet. Radiol. Ultrasound 2022, 63, 292–297. [Google Scholar] [CrossRef] [PubMed]
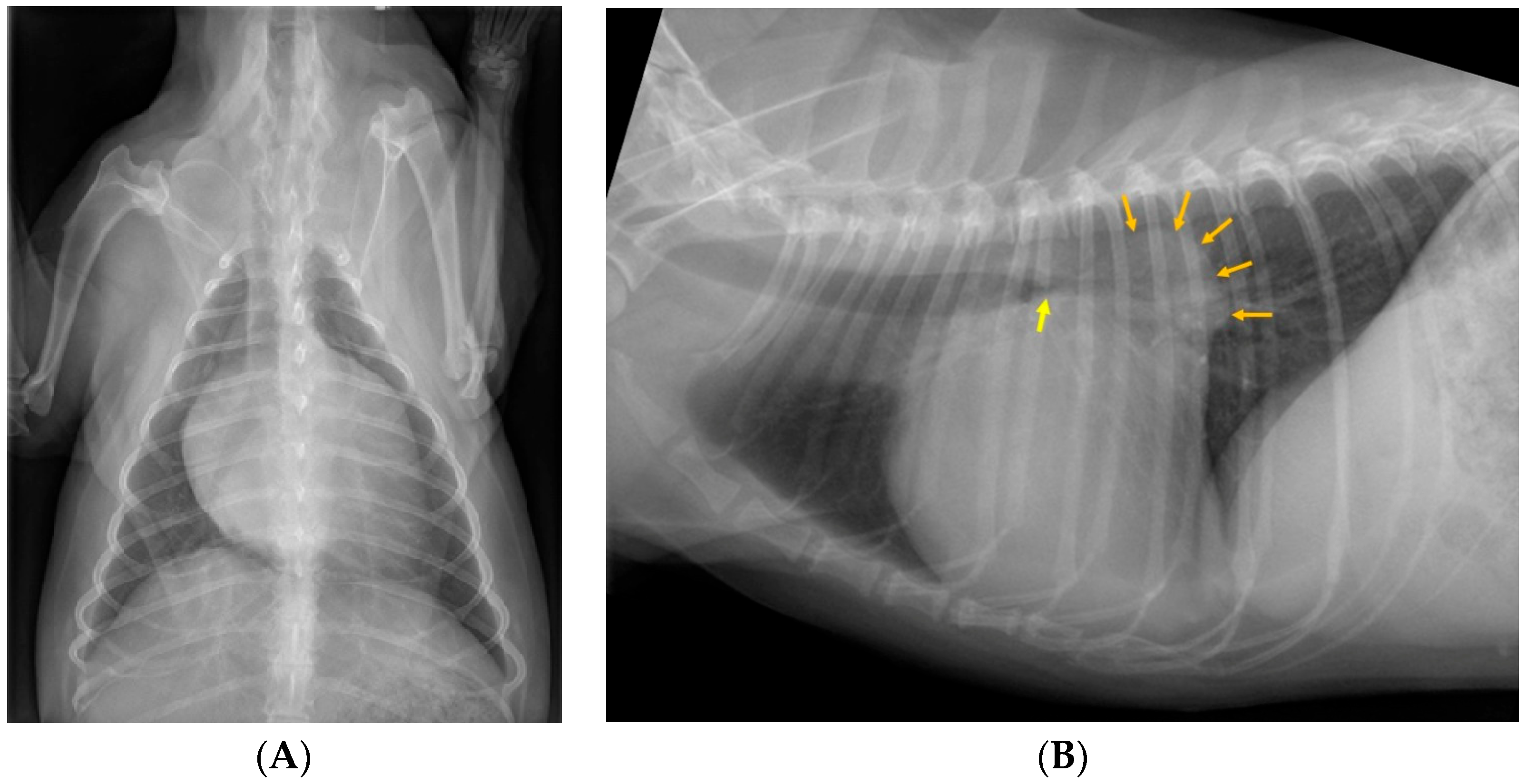
| Left Principal Bronchus | Left Caudal Lobar Bronchus | ||||
|---|---|---|---|---|---|
| Bronchial Narrowing | Non-Coughing (n = 26) | Coughing (n = 25) | Non-Coughing (n = 26) | Coughing (n = 25) | |
| Radiologist 1 | no | 7 | 3 | 23 | 19 |
| mild–moderate (<75%) | 8 | 10 | 1 | 0 | |
| severe (>75%) | 11 | 12 | 2 | 0 | |
| uninterpretable | 0 | 0 | 0 | 6 | |
| no + mild–moderate | 15 | 13 | 24 | 19 | |
| mild–moderate + severe | 19 | 22 | 3 | 0 | |
| Radiologist 2 | no | 9 | 4 | 20 | 23 |
| mild–moderate (<75%) | 8 | 6 | 4 | 2 | |
| severe (>75%) | 9 | 15 | 2 | 0 | |
| uninterpretable | 0 | 0 | 0 | 0 | |
| no + mild–moderate | 17 | 10 | 24 | 25 | |
| mild–moderate + severe | 17 | 21 | 6 | 2 | |
| Radiologist 3 | no | 12 | 3 | 19 | 14 |
| mild–moderate (<75%) | 4 | 5 | 3 | 5 | |
| severe (>75%) | 10 | 17 | 3 | 5 | |
| uninterpretable | 0 | 0 | 1 | 1 | |
| no + mild–moderate | 16 | 8 | 22 | 19 | |
| mild–moderate + severe | 14 | 22 | 6 | 10 | |
| Radiologist 4 | no | 22 | 17 | 13 | 4 |
| mild–moderate (<75%) | 4 | 7 | 9 | 17 | |
| severe (>75%) | 0 | 0 | 4 | 3 | |
| uninterpretable | 0 | 1 | 0 | 1 | |
| no + mild–moderate | 26 | 24 | 22 | 21 | |
| mild–moderate + severe | 4 | 7 | 13 | 20 | |
| Combined R 1–4 | no | 52 | 27 | 75 | 60 |
| no + mild–moderate | 74 | 55 | 92 | 84 | |
| mild–moderate + severe | 54 | 72 | 28 | 32 | |
| severe | 30 | 44 | 11 | 8 | |
| Combined R 1–3 | no | 30 | 10 | 62 | 56 |
| no + mild–moderate | 48 | 31 | 70 | 63 | |
| mild–moderate + severe | 50 | 65 | 15 | 12 | |
| severe | 30 | 44 | 7 | 5 | |
| Left Principal Bronchus | No and Mild–Moderate Versus Severe Narrowing | No Versus Mild–Moderate and Severe Narrowing | ||
|---|---|---|---|---|
| Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | |
| Radiologist 1 | 48.0 (27.8–68.7)% | 57.7 (36.9–76.7)% | 88.0 (68.8–97.5)% | 26.9 (11.6–47.8)% |
| Radiologist 2 | 60.0 (38.7–78.9)% | 65.4 (44.3–82.8)% | 84.0 (63.9–95.5)% | 34.6 (17.2–55.7)% |
| Radiologist 3 | 68.0 (46.5–85.0)% | 65.4 (44.3–82.8)% | 88.0 (68.8–97.5)% | 46.2 (26.6–66.6)% |
| Radiologist 4 | 0.0 (0.0–14.3)% | 100 (86.8–100)% | 29.2 (12.6–51.1)% | 84.6 (65.1–95.6)% |
| Combined R 1–4 | 59.5 (47.4–70.7)% | 57.4 (48.4–66.0)% | 57.1 (48.0–65.9)% | 65.8 (54.3–76.1)% |
| Combined R 1–3 | 59.5 (47.4–70.7)% | 60.8 (49.1–71.6)% | 56.5 (47.0–65.7)% | 75.0 (58.8–87.3)% |
| Left Caudal Lobar Bronchus | No and Mild–Moderate Versus Severe Narrowing | No Versus Mild–Moderate and Severe Narrowing | ||
|---|---|---|---|---|
| Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | |
| Radiologist 1 | 0.0 (0.0–17.7)% | 92.3 (74.9–99.1)% | 0.0 (0.0–17.7)% | 88.5 (69.9–97.6)% |
| Radiologist 2 | 0.0 (0.0–13.7)% | 92.3 (74.9–99.1)% | 8.0 (1.0–26.0)% | 76.9 (56.4–91.0)% |
| Radiologist 3 | 20.8 (7.1–42.2)% | 92.3 (74.9–99.1)% | 41.7 (22.1–63.4)% | 76.0 (54.9–90.7)% |
| Radiologist 4 | 12.5 (2.7–32.4)% | 88.5 (69.9–97.6)% | 83.3 (62.6–95.3)% | 50.0 (29.9–70.1)% |
| Combined R 1–4 | 42.1 (20.3–66.5)% | 52.3 (44.6–59.8)% | 53.3 (40.0–66.3)% | 55.6 (46.8–64.1)% |
| Combined R 1–3 | 41.7 (15.2–72.3)% | 52.6 (43.8–61.4)% | 44.4 (25.5–64.7)% | 52.5 (43.2–61.8)% |
| Accuracy | Variable | Odds Ratio | p-Value | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Radiologist 1 | 72% (18/25) correctly predicted as coughing 46% (12/26) correctly predicted as non-coughing | Left principal bronchus narrowing | 10.33 | <0.01 | 1.71–114.58 |
| Bronchial lung pattern | 5.16 | 0.02 | 1.15–33.06 | ||
| Radiologist 2 | 72% (18/25) correctly predicted as coughing 42% (11/26) correctly predicted as non-coughing | Left principal bronchus narrowing | 56.45 | <0.01 | 6.47–2761.87 |
| VHS | 3.99 | 0.01 | 1.54–13.57 | ||
| VLAS | 13.65 | 0.01 | 2.18–132.19 | ||
| Radiologist 3 | 84% (21/25) correctly predicted as coughing 46% (12/26) correctly predicted as non-coughing | Left principal bronchus narrowing | 28.50 | <0.01 | 5.10–232.24 |
| Right caudal bronchus narrowing | 12.13 | <0.01 | 1.54–562.64 | ||
| Bronchial lung pattern | Infinite | <0.01 | 1.83–infinite | ||
| VHS | 3.34 | 0.04 | 1.24–12.01 | ||
| VLAS | 21.75 | 0.01 | 2.56–341.50 | ||
| Radiologist 4 | 56% (14/25) correctly predicted as coughing 50% (13/26) correctly predicted as non-coughing | Left caudal bronchus narrowing | 4.08 | 0.04 | 1.03–18.63 |
| Interstitial lung pattern | 6.52 | <0.01 | 1.55–34.08 | ||
| Bronchial lung pattern | 11.25 | <0.01 | 2.47–73.88 |
| Radiologists | Variable | Kappa | p-Value |
|---|---|---|---|
| R1–R2 | Left principal bronchus compression/collapse | 0.24 | 0.02 |
| R1–R3 | Left principal bronchus compression/collapse | 0.21 | 0.03 |
| Left caudal lobar bronchus compression/collapse | 0.22 | <0.01 | |
| Interstitial lung pattern | 0.34 | <0.01 | |
| Bronchial lung pattern | 0.28 | 0.04 | |
| Prediction of cough | 0.35 | 0.01 | |
| Cause of cough | 0.36 | <0.01 | |
| R1–R4 | Right caudal lobar bronchus compression/collapse | 0.18 | 0.04 |
| Cause of cough | 0.34 | <0.01 | |
| R2–R3 | Left principal bronchus compression/collapse | 0.59 | <0.01 |
| Right caudal lobar bronchus compression/collapse | 0.20 | 0.04 | |
| Prediction of cough | 0.56 | <0.01 | |
| R2–R4 | Right caudal lobar bronchus compression/collapse | 0.42 | <0.01 |
| Prediction of cough | 0.28 | 0.04 | |
| R3–R4 | Right cranial lobar bronchus compression/collapse | 0.282 | 0.015 |
| Interstitial lung pattern | 0.20 | 0.036 | |
| Prediction of cough | 0.44 | <0.01 | |
| Cause of cough | 0.33 | <0.01 |
| ICC | p-Value | 95% Confidence Interval | |
|---|---|---|---|
| VHS | 0.76 | <0.01 | 0.65–0.84 |
| VLAS | 0.56 | <0.01 | 0.39–0.70 |
| Interstitial | Bronchial | |
|---|---|---|
| Radiologist 1 | 3 | 19 |
| Radiologist 2 | 1 | 0 |
| Radiologist 3 | 12 | 13 |
| Radiologist 4 | 34 | 20 |
| Dog 1a | Dog 1b | Dog 2a | Dog 2b | |
|---|---|---|---|---|
| Radiologist 1 | Left principal bronchus was not narrowed | Left principal bronchus collapse (<75%) | Left cranial (caudal) lobar bronchus was not narrowed VHS = 12.5 VLAS = 3.1 | Left cranial (caudal) lobar bronchus was uninterpretable VHS = 12.7 VLAS = 2.9 |
| Radiologist 2 | Reported as duplicate | Reported as duplicate | ||
| Radiologist 3 | VLAS = 3.0 | VLAS = 3.2 | Left caudal bronchus collapse (>75%) VLAS = 2.9 | Left caudal bronchus collapse (<75%) VLAS = 3.0 |
| Radiologist 4 | Right medial bronchus was uninterpretable Left cranial (cranial) lobar bronchus was uninterpretable Left cranial (caudal) lobar bronchus was uninterpretable Interstitial lung pattern was not present VHS = 12.2 VLAS = 2.8 | Right medial bronchus was not narrowed Left cranial (cranial) lobar bronchus not narrowed Left cranial (caudal) lobar bronchus not narrowed Interstitial lung pattern was present VHS = 12.0 VLAS = 2.5 | Left principial bronchus collapse (<75%) Interstitial lung pattern was not present VLAS = 2.9 | Left principial bronchus was not narrowed Interstitial lung pattern present VLAS = 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Opstal, K.Y.; Kittleson, M.D.; Teske, E.; Auriemma, E.; van den Broek, H.; Spattini, G.; Vilaplana Grosso, F.R.; Szatmári, V. No Correlation Between Chronic Cough and Radiographic Signs of Bronchial Narrowing in Dogs with Cardiomegaly and Left Atrial Dilation Secondary to Primary Mitral Valve Regurgitation. Animals 2025, 15, 2510. https://doi.org/10.3390/ani15172510
van Opstal KY, Kittleson MD, Teske E, Auriemma E, van den Broek H, Spattini G, Vilaplana Grosso FR, Szatmári V. No Correlation Between Chronic Cough and Radiographic Signs of Bronchial Narrowing in Dogs with Cardiomegaly and Left Atrial Dilation Secondary to Primary Mitral Valve Regurgitation. Animals. 2025; 15(17):2510. https://doi.org/10.3390/ani15172510
Chicago/Turabian Stylevan Opstal, Kira Y., Mark D. Kittleson, Erik Teske, Edoardo Auriemma, Henk van den Broek, Giliola Spattini, Federico R. Vilaplana Grosso, and Viktor Szatmári. 2025. "No Correlation Between Chronic Cough and Radiographic Signs of Bronchial Narrowing in Dogs with Cardiomegaly and Left Atrial Dilation Secondary to Primary Mitral Valve Regurgitation" Animals 15, no. 17: 2510. https://doi.org/10.3390/ani15172510
APA Stylevan Opstal, K. Y., Kittleson, M. D., Teske, E., Auriemma, E., van den Broek, H., Spattini, G., Vilaplana Grosso, F. R., & Szatmári, V. (2025). No Correlation Between Chronic Cough and Radiographic Signs of Bronchial Narrowing in Dogs with Cardiomegaly and Left Atrial Dilation Secondary to Primary Mitral Valve Regurgitation. Animals, 15(17), 2510. https://doi.org/10.3390/ani15172510








