Intrauterine Growth-Restricted Pig-Associated Testicular Transcriptome Analysis Reveals microRNA-mRNA Regulatory Networks
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Sample Collection
2.2. RNA Extraction and Sequencing
2.3. Transcriptomic Data Analysis
2.4. Enrichment Analysis, Target Gene Prediction, and miRNA-mRNA Regulatory Network Construction
2.5. RT-qPCR
2.6. Statistical Analysis
3. Results
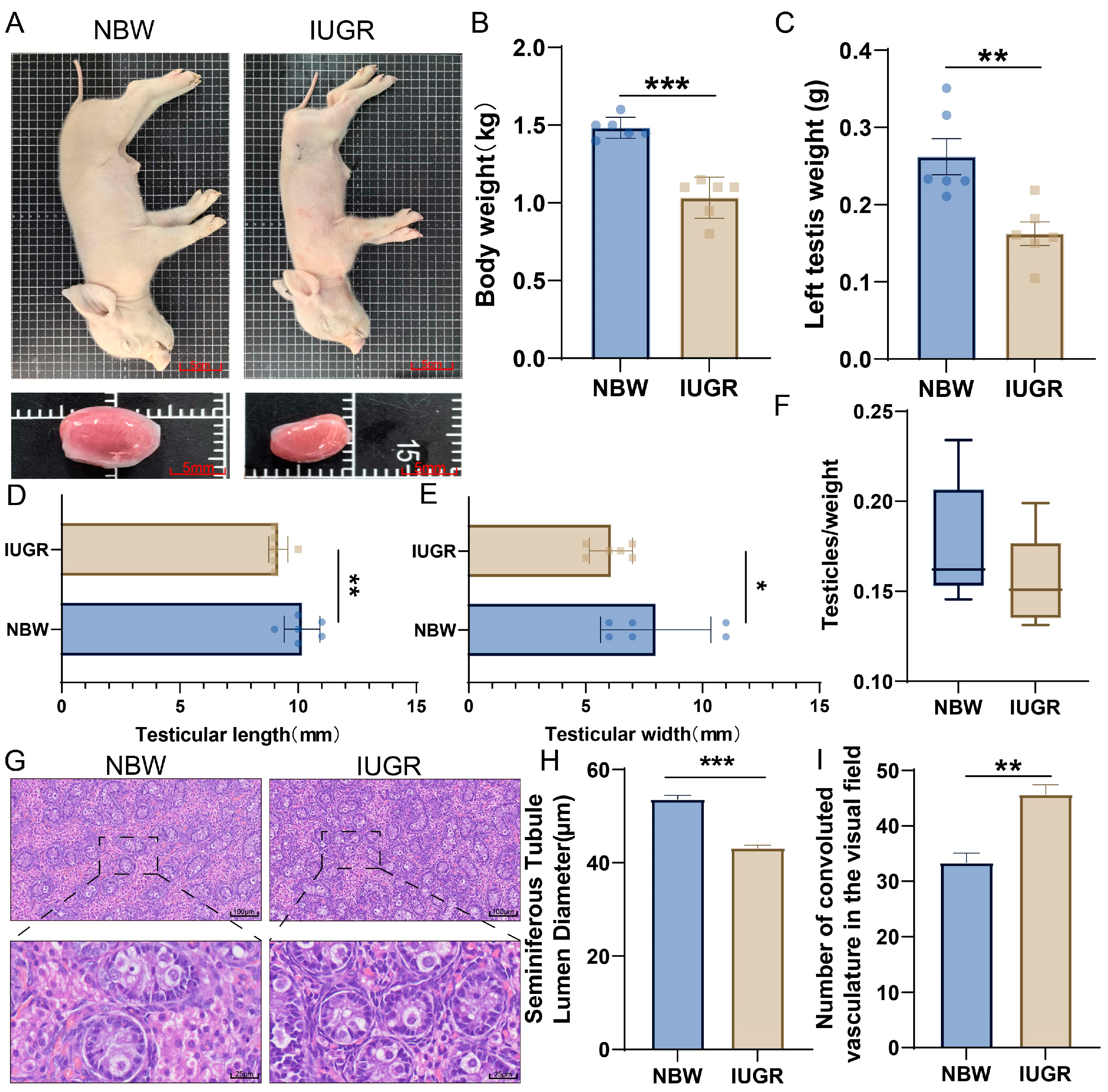
3.1. Phenotypic Differences in Testes Between NBW and IUGR Piglets
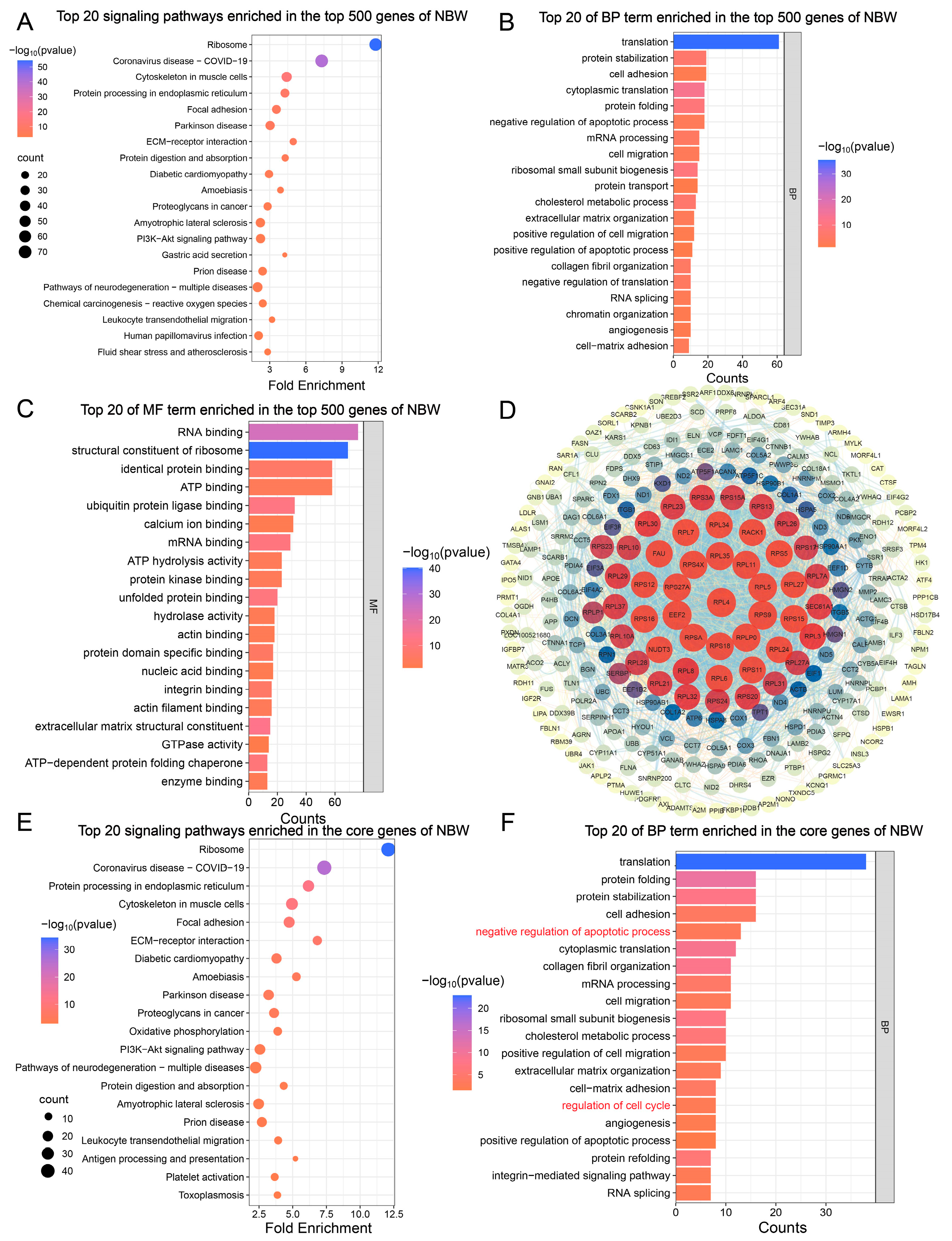
3.2. Enrichment Analysis of the Top 500 Genes in the Testicular Transcriptome of NBW Piglets
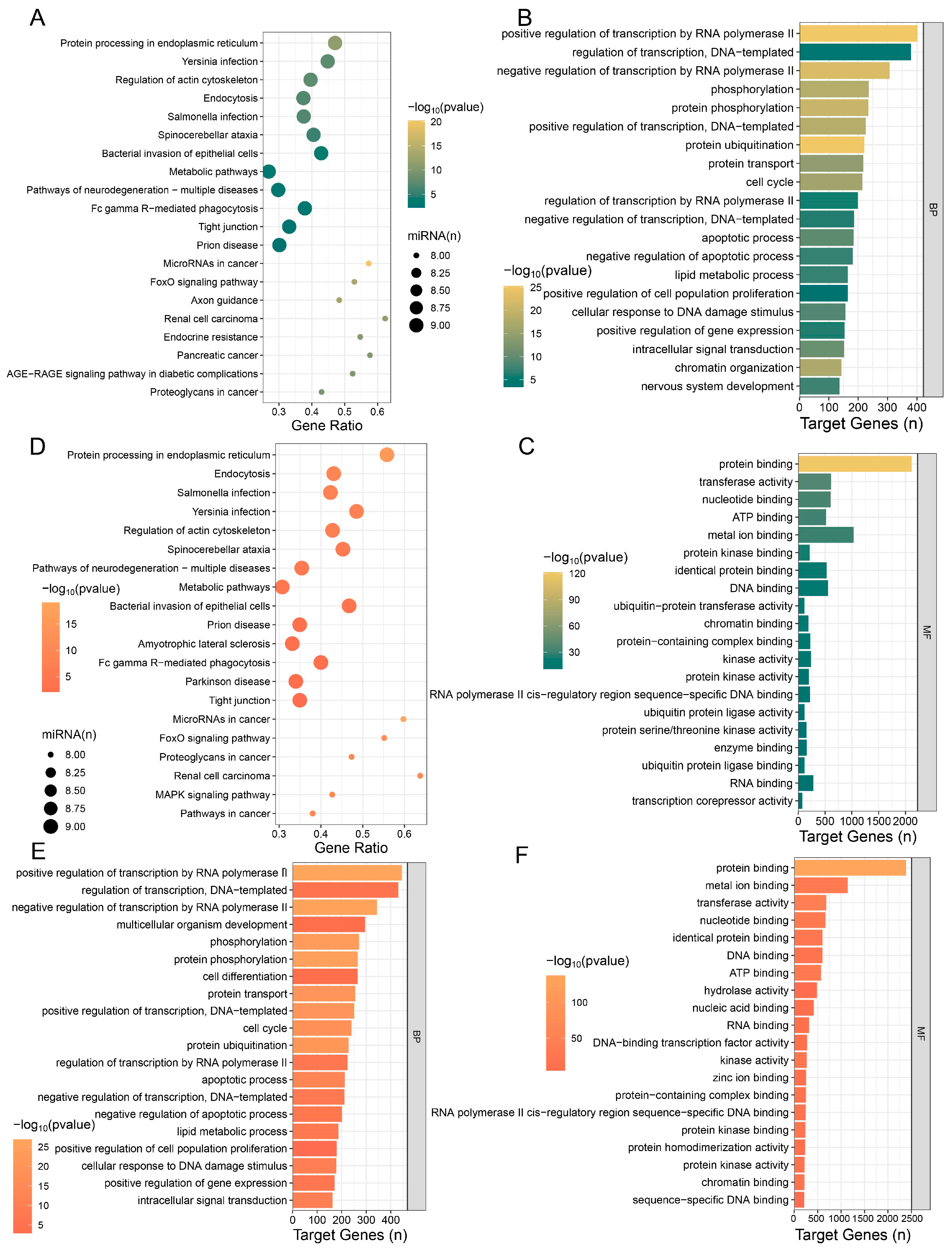
3.3. Enrichment Analysis of Top 500 Genes in the Testicular Transcriptome of IUGR Piglets
3.4. Analysis of mRNA Differences in Testes Between NBW and IUGR Piglets
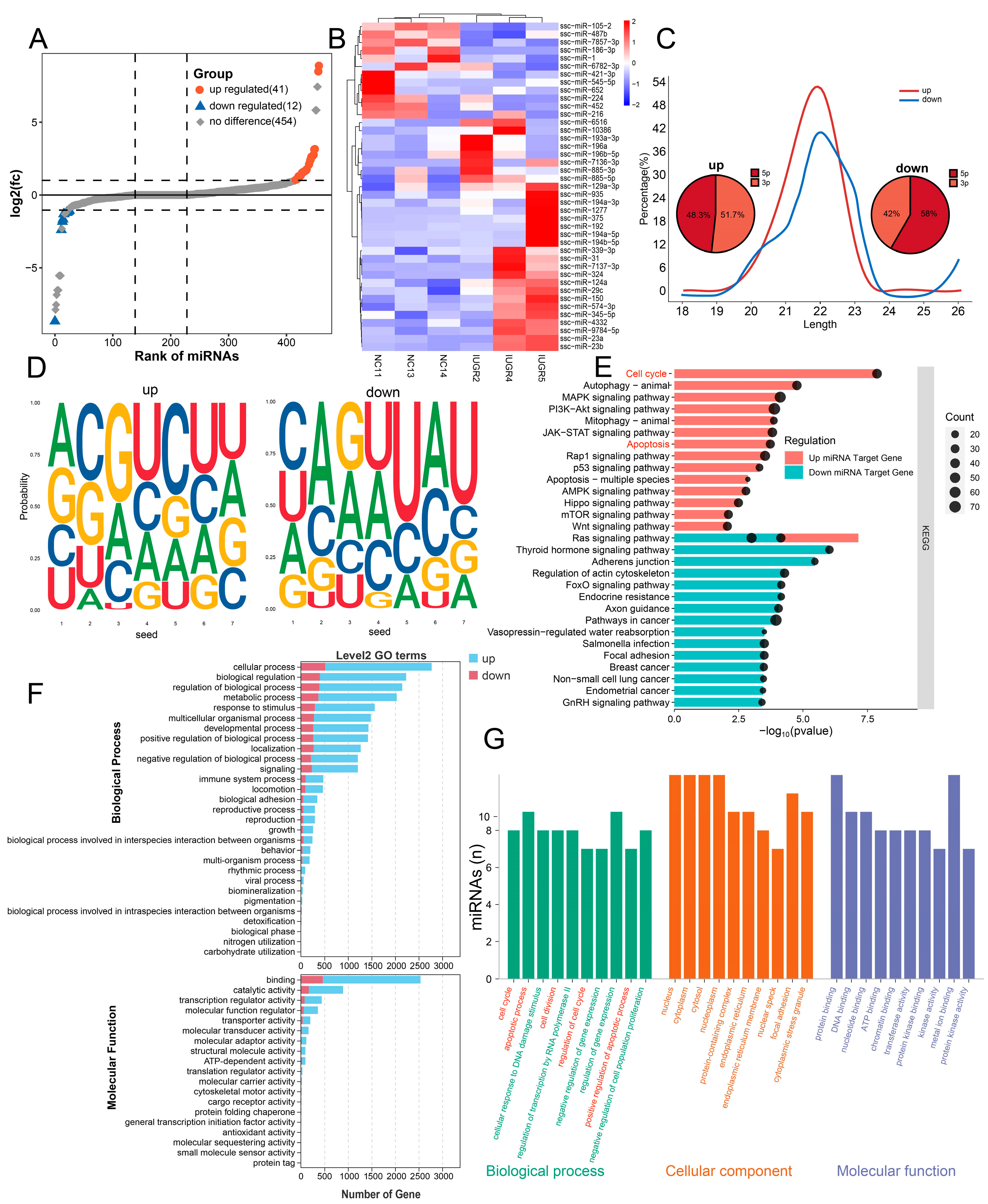
3.5. Enrichment Analysis of Top 10 miRNAs in Testes of NBW and IUGR Piglets
3.6. Analysis of miRNA Differences in Testes Between NBW and IUGR Piglets
3.7. Differential microRNA-mRNA Regulatory Network in Testicular Tissues Between NBW and IUGR Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IUGR | Intrauterine growth restriction |
| NBW | Normal birth weight |
| DEGs | Differentially expressed genes |
| PE | Preeclampsia |
Appendix A
Appendix A.1
| Top 25 BP Terms Enriched in the Top 500 Genes of NBW | |||
|---|---|---|---|
| Term | Count | p Value | Group |
| translation | 61 | 4.31 × 10−36 | BP |
| protein stabilization | 19 | 2.70 × 10−7 | BP |
| cell adhesion | 19 | 0.00521 | BP |
| cytoplasmic translation | 18 | 1.83 × 10−13 | BP |
| protein folding | 18 | 1.11 × 10−9 | BP |
| negative regulation of apoptotic process | 18 | 9.25 × 10−4 | BP |
| mRNA processing | 15 | 2.79 × 10−5 | BP |
| cell migration | 15 | 0.001047 | BP |
| ribosomal small subunit biogenesis | 14 | 3.77 × 10−10 | BP |
| protein transport | 14 | 0.04985 | BP |
| cholesterol metabolic process | 13 | 1.96 × 10−7 | BP |
| extracellular matrix organization | 12 | 9.22 × 10−4 | BP |
| positive regulation of cell migration | 12 | 0.009223 | BP |
| positive regulation of apoptotic process | 11 | 0.036481 | BP |
| collagen fibril organization | 10 | 7.55 × 10−6 | BP |
| negative regulation of translation | 10 | 1.59 × 10−5 | BP |
| RNA splicing | 10 | 0.001782 | BP |
| chromatin organization | 10 | 0.017809 | BP |
| angiogenesis | 10 | 0.019896 | BP |
| cell–matrix adhesion | 9 | 7.17 × 10−4 | BP |
| response to oxidative stress | 9 | 8.51 × 10−4 | BP |
| regulation of cell cycle | 9 | 0.03224 | BP |
| integrin-mediated signaling pathway | 8 | 0.012771 | BP |
| protein localization | 8 | 0.030255 | BP |
| actin filament organization | 8 | 0.03273 | BP |
Appendix A.2
| Top 30 MF Terms Enriched in the Top 500 Genes of NBW | |||
|---|---|---|---|
| Term | Count | p Value | Group |
| RNA binding | 76 | 1.12 × 10−24 | MF |
| structural constituent of ribosome | 69 | 5.88 × 10−41 | MF |
| identical protein binding | 58 | 9.80 × 10−7 | MF |
| ATP binding | 58 | 3.82 × 10−4 | MF |
| ubiquitin protein ligase binding | 32 | 1.57 × 10−12 | MF |
| calcium ion binding | 31 | 0.003062 | MF |
| mRNA binding | 29 | 7.93 × 10−12 | MF |
| ATP hydrolysis activity | 23 | 1.44 × 10−4 | MF |
| protein kinase binding | 23 | 3.31 × 10−4 | MF |
| unfolded protein binding | 20 | 3.83 × 10−12 | MF |
| hydrolase activity | 18 | 9.34 × 10−4 | MF |
| actin binding | 18 | 0.00163 | MF |
| protein domain-specific binding | 17 | 2.96 × 10−6 | MF |
| nucleic acid binding | 17 | 0.003561 | MF |
| integrin binding | 16 | 8.43 × 10−7 | MF |
| actin filament binding | 16 | 3.42 × 10−4 | MF |
| extracellular matrix structural constituent | 15 | 6.34 × 10−14 | MF |
| GTPase activity | 14 | 0.041937 | MF |
| ATP-dependent protein folding chaperone | 13 | 5.12 × 10−11 | MF |
| enzyme binding | 13 | 0.001505 | MF |
| protease binding | 12 | 1.53 × 10−5 | MF |
| transmembrane transporter binding | 12 | 1.23 × 10−4 | MF |
| heme binding | 12 | 0.007209 | MF |
| oxidoreductase activity | 11 | 0.02603 | MF |
| extracellular matrix structural constituent conferring tensile strength | 10 | 1.75 × 10−7 | MF |
| rRNA binding | 10 | 7.58 × 10−7 | MF |
| amyloid-beta binding | 10 | 7.30 × 10−6 | MF |
| mRNA 3′-UTR binding | 10 | 9.11 × 10−5 | MF |
| translation initiation factor activity | 10 | 9.11 × 10−5 | MF |
| lipid binding | 10 | 0.01612 | MF |
Appendix A.3
| Top 25 MFs of IUGR Piglet Top 500 Genes Sorted by p-Value | |||
|---|---|---|---|
| Term | Count | p Value | Group |
| structural constituent of ribosome | 78 | 2.65 × 10−50 | MF |
| RNA binding | 97 | 3.07 × 10−40 | MF |
| mRNA binding | 35 | 1.36 × 10−16 | MF |
| extracellular matrix structural constituent | 13 | 3.94 × 10−11 | MF |
| unfolded protein binding | 19 | 4.38 × 10−11 | MF |
| ubiquitin protein ligase binding | 27 | 5.79 × 10−9 | MF |
| rRNA binding | 11 | 6.86 × 10−8 | MF |
| pre-mRNA binding | 7 | 1.22 × 10−7 | MF |
| platelet-derived growth factor binding | 7 | 1.22 × 10−7 | MF |
| extracellular matrix structural constituent conferring tensile strength | 10 | 1.91 × 10−7 | MF |
| mRNA 3′-UTR binding | 12 | 2.32 × 10−6 | MF |
| NADH dehydrogenase (ubiquinone) activity | 7 | 2.87 × 10−6 | MF |
| ATP-dependent protein folding chaperone | 9 | 3.09 × 10−6 | MF |
| mRNA 5′-UTR binding | 7 | 1.55 × 10−5 | MF |
| translation initiation factor activity | 11 | 1.60 × 10−5 | MF |
| nucleic acid binding | 22 | 2.44 × 10−5 | MF |
| disordered domain-specific binding | 7 | 2.67 × 10−5 | MF |
| translation regulator activity | 6 | 2.79 × 10−5 | MF |
| ribosome binding | 10 | 4.35 × 10−5 | MF |
| amyloid-beta binding | 9 | 6.15 × 10−5 | MF |
| miRNA binding | 7 | 6.85 × 10−5 | MF |
| laminin binding | 6 | 7.46 × 10−5 | MF |
| integrin binding | 13 | 1.09 × 10−4 | MF |
| ATP hydrolysis activity | 23 | 1.66 × 10−4 | MF |
| protein domain-specific binding | 14 | 2.34 × 10−4 | MF |
Appendix A.4
| Top 30 BP Terms Enriched in the Core Genes of IUGR | |||
|---|---|---|---|
| Term | Count | p Value | Group |
| translation | 45 | 4.83 × 10−33 | BP |
| cytoplasmic translation | 18 | 3.19 × 10−18 | BP |
| protein stabilization | 13 | 2.25 × 10−6 | BP |
| ribosomal small subunit biogenesis | 12 | 5.80 × 10−11 | BP |
| protein folding | 12 | 1.30 × 10−7 | BP |
| mRNA processing | 12 | 4.64 × 10−6 | BP |
| cell adhesion | 11 | 0.021248 | BP |
| collagen fibril organization | 10 | 3.10 × 10−8 | BP |
| mRNA splicing, via spliceosome | 10 | 8.14 × 10−5 | BP |
| RNA splicing | 9 | 9.35 × 10−5 | BP |
| negative regulation of apoptotic process | 9 | 0.033283 | BP |
| protein ubiquitination | 9 | 0.047604 | BP |
| angiogenesis | 8 | 0.005006 | BP |
| skeletal system development | 7 | 2.23 × 10−4 | BP |
| extracellular matrix organization | 7 | 0.0107 | BP |
| positive regulation of cell migration | 7 | 0.039137 | BP |
| DNA repair | 7 | 0.041843 | BP |
| negative regulation of mRNA splicing, via spliceosome | 6 | 2.00 × 10−6 | BP |
| mitochondrial electron transport, NADH to ubiquinone | 6 | 1.58 × 10−5 | BP |
| protein refolding | 6 | 1.94 × 10−5 | BP |
| regulation of RNA splicing | 6 | 2.94 × 10−4 | BP |
| regulation of alternative mRNA splicing, via spliceosome | 6 | 7.52 × 10−4 | BP |
| cell–matrix adhesion | 6 | 0.003386 | BP |
| regulation of apoptotic process | 6 | 0.033365 | BP |
| chromatin remodeling | 6 | 0.041067 | BP |
| regulation of cell cycle | 6 | 0.043147 | BP |
| positive regulation of telomere maintenance via telomerase | 5 | 1.18 × 10−4 | BP |
| alternative mRNA splicing, via spliceosome | 5 | 1.18 × 10−4 | BP |
| ribosomal small subunit assembly | 5 | 1.74 × 10−4 | BP |
| ribosomal large subunit assembly | 5 | 2.47 × 10−4 | BP |
References
- Romo, A.; Carceller, R.; Tobajas, J. Intrauterine Growth Retardation (IUGR): Epidemiology and Etiology. Pediatr. Endocrinol. Rev. 2009, 6 (Suppl. S3), 332–336. [Google Scholar]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-Invited Review: Intrauterine Growth Retardation: Implications for the Animal Sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef]
- Hales, J.; Moustsen, V.A.; Nielsen, M.B.F.; Hansen, C.F. Individual Physical Characteristics of Neonatal Piglets Affect Preweaning Survival of Piglets Born in a Noncrated System. J. Anim. Sci. 2013, 91, 4991–5003. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.; Bee, G.; Ollagnier, C. Review: Intrauterine Growth Restriction, Diagnosis and Physiological Characterization in Pigs. Animal 2025, 19, 101590. [Google Scholar] [CrossRef] [PubMed]
- Jianfeng, M.; Mailin, G.; Yiting, Y.; Lei, C.; Ye, Z.; Lili, N.; Yan, W.; Shunhua, Z.; Jingyong, W.; Li, Z.; et al. tRNA-Derived Small RNA Dataset in Multiple Organs of Intrauterine Growth-Restricted Pig. Sci. Data 2023, 10, 793. [Google Scholar] [CrossRef] [PubMed]
- Cromi, A.; Ghezzi, F.; Raffaelli, R.; Bergamini, V.; Siesto, G.; Bolis, P. Ultrasonographic Measurement of Thymus Size in IUGR Fetuses: A Marker of the Fetal Immunoendocrine Response to Malnutrition. Ultrasound Obstet. Gynecol. 2009, 33, 421–426. [Google Scholar] [CrossRef]
- Ma, J.; Gan, M.; Chen, J.; Chen, L.; Zhao, Y.; Zhu, Y.; Niu, L.; Zhang, S.; Jiang, Y.; Guo, Z.; et al. Characteristics of tRNA-Derived Small RNAs and microRNAs Associated with Immunocompromise in an Intrauterine Growth-Restricted Pig Model. Animals 2022, 12, 2102. [Google Scholar] [CrossRef]
- Tao, S.; Bai, Y.; Li, T.; Li, N.; Wang, J. Original Low Birth Weight Deteriorates the Hindgut Epithelial Barrier Function in Pigs at the Growing Stage. FASEB J. 2019, 33, 9897–9912. [Google Scholar] [CrossRef]
- Santos, T.G.; Fernandes, S.D.; de Araújo, S.B.O.; Felicioni, F.; de Paula, T.M.D.E.; Caldeira-Brant, A.L.; Ferreira, S.V.; de Naves, L.P.; de Souza, S.P.; Campos, P.H.R.F.; et al. Intrauterine Growth Restriction and Its Impact on Intestinal Morphophysiology Throughout Postnatal Development in Pigs. Sci. Rep. 2022, 12, 11810. [Google Scholar] [CrossRef]
- Ma, J.; Gan, M.; Chen, S.; Shi, Y.; Yang, Y.; Liu, C.; Zhang, S.; Chen, L.; Zhu, K.; Zhang, T.; et al. Metabolome and Transcriptome Profiling Reveal tRNA-Derived Small RNAs Regulated Glutathione Metabolism in Intrauterine Growth-Restricted Pigs. Int. J. Biol. Macromol. 2025, 293, 139167. [Google Scholar] [CrossRef]
- Gao, H.; Chen, X.; Zhao, J.; Xue, Z.; Zhang, L.; Zhao, F.; Wang, B.; Wang, L. Integrative Analysis of Liver Metabolomics and Transcriptomics Reveals Oxidative Stress in Piglets with Intrauterine Growth Restriction. Biology 2022, 11, 1430. [Google Scholar] [CrossRef]
- Lin, Y.; Cheng, X.; Sutovsky, P.; Wu, D.; Che, L.-Q.; Fang, Z.-F.; Xu, S.-Y.; Ren, B.; Dong, H.-J. Effect of Intra-Uterine Growth Restriction on Long-Term Fertility in Boars. Reprod. Fertil. Dev. 2017, 29, 374–382. [Google Scholar] [CrossRef]
- Meng, F.; Yao, M.; Li, S.; Tian, A.; Zhang, C.; Luo, X. The Impact of Impaired Intrauterine Growth on Male Fertility: A Systematic Review and Meta-Analysis. Andrology 2024, 12, 1651–1660. [Google Scholar] [CrossRef]
- Schreurs, N.M.; Garcia, F.; Jurie, C.; Agabriel, J.; Micol, D.; Bauchart, D.; Listrat, A.; Picard, B. Meta-Analysis of the Effect of Animal Maturity on Muscle Characteristics in Different Muscles, Breeds, and Sexes of Cattle. J. Anim. Sci. 2008, 86, 2872–2887. [Google Scholar] [CrossRef] [PubMed]
- Stenhouse, C.; Cortes-Araya, Y.; Donadeu, F.X.; Ashworth, C.J. Associations Between Testicular Development and Fetal Size in the Pig. J. Anim. Sci. Biotechnol. 2022, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, J.-A.; Koskenniemi, J.J.; Virtanen, H.E.; Toppari, J. Testis Development. Endocr. Rev. 2019, 40, 857–905. [Google Scholar] [CrossRef]
- Ding, H.; Wang, Y.; Zhao, H.; Wang, J.; Huang, D. Negative Effect of Seasonal Heat Stress on Testis Morphology and Transcriptomes in Angora Rabbit. BMC Genom. 2025, 26, 478. [Google Scholar] [CrossRef]
- Smit, M.N.; Spencer, J.D.; Almeida, F.R.C.L.; Patterson, J.L.; Chiarini-Garcia, H.; Dyck, M.K.; Foxcroft, G.R. Consequences of a Low Litter Birth Weight Phenotype for Postnatal Lean Growth Performance and Neonatal Testicular Morphology in the Pig. Animal 2013, 7, 1681–1689. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Ali, A.; Murani, E.; Hadlich, F.; Liu, X.; Wimmers, K.; Ponsuksili, S. Prenatal Skeletal Muscle Transcriptome Analysis Reveals Novel MicroRNA-mRNA Networks Associated with Intrauterine Growth Restriction in Pigs. Cells 2021, 10, 1007. [Google Scholar] [CrossRef]
- Ali, A.; Hadlich, F.; Abbas, M.W.; Iqbal, M.A.; Tesfaye, D.; Bouma, G.J.; Winger, Q.A.; Ponsuksili, S. MicroRNA–mRNA Networks in Pregnancy Complications: A Comprehensive Downstream Analysis of Potential Biomarkers. Int. J. Mol. Sci. 2021, 22, 2313. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Gan, M.; Xie, Z.; Ma, J.; Chen, L.; Zhang, S.; Zhao, Y.; Niu, L.; Wang, Y.; Zhu, L.; et al. Characteristics of microRNAs in Skeletal Muscle of Intrauterine Growth-Restricted Pigs. Genes 2023, 14, 1372. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wang, J.; Ji, X.; Chai, J.; Chen, L.; Zhang, T.; Long, X.; Tu, Z.; Chen, S.; Zhang, L.; et al. Maternal Hypermethylated Genes Contribute to Intrauterine Growth Retardation of Piglets in Rongchang Pigs. Int. J. Mol. Sci. 2024, 25, 6462. [Google Scholar] [CrossRef] [PubMed]
- Mailin, G.; Yang, Y.; Liu, C.; Jing, Y.; Wang, Y.; Ma, J.; Liao, T.; Shen, L.; Zhu, L. The RNA-Seq Mapping of Testicular Development After Heat Stress in Sexually Mature Mice. Sci. Data 2024, 11, 913. [Google Scholar] [CrossRef]
- Luo, J.; Fan, Y.; Shen, L.; Niu, L.; Zhao, Y.; Jiang, D.; Zhu, L.; Jiang, A.; Tang, Q.; Ma, J.; et al. The Pro-Angiogenesis of Exosomes Derived from Umbilical Cord Blood Of Intrauterine Growth Restriction Pigs Was Repressed Associated with MiRNAs. Int. J. Biol. Sci. 2018, 14, 1426–1436. [Google Scholar] [CrossRef]
- Gan, M.; Liu, L.; Zhang, S.; Guo, Z.; Tan, Y.; Luo, J.; Yang, Q.; Pan, H.; Li, X.; Wang, J.; et al. Expression Characteristics of microRNA in Pig Umbilical Venous Blood and Umbilical Arterial Blood. Animals 2021, 11, 1563. [Google Scholar] [CrossRef]
- Wang, L.; Gu, H.; Liao, T.; Lei, Y.; Qiu, Y.; Chen, Q.; Chen, L.; Zhang, S.; Wang, J.; Hao, X.; et al. tsRNA Landscape and Potential Function Network in Subcutaneous and Visceral Pig Adipose Tissue. Genes 2023, 14, 782. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, J.; Pan, H.; Gan, M.; Shen, L. MiR-29a Family as a Key Regulator of Skeletal Muscle Dysplasia in a Porcine Model of Intrauterine Growth Retardation. Biomolecules 2022, 12, 1193. [Google Scholar] [CrossRef]
- Lin, G.; Wang, X.; Wu, G.; Feng, C.; Zhou, H.; Li, D.; Wang, J. Improving Amino Acid Nutrition to Prevent Intrauterine Growth Restriction in Mammals. Amino Acids 2014, 46, 1605–1623. [Google Scholar] [CrossRef]
- Auler, P.A.; Moreira, G.H.F.A.; Hogg, C.O.; Ashworth, C.J.; Bortolozzo, F.P.; Chiarini-Garcia, H.; Almeida, F.R.C.L. Testicular Parameters and Spermatogenesis in Different Birthweight Boars. Reprod. Fertil. Dev. 2017, 29, 1720–1728. [Google Scholar] [CrossRef]
- Boeri, L.; Ventimiglia, E.; Capogrosso, P.; Ippolito, S.; Pecoraro, A.; Paciotti, M.; Scano, R.; Galdini, A.; Valsecchi, L.; Papaleo, E.; et al. Low Birth Weight Is Associated with a Decreased Overall Adult Health Status and Reproductive Capability—Results of a Cross-Sectional Study in Primary Infertile Patients. PLoS ONE 2016, 11, e0166728. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhang, X.; Liu, Z.; Huo, H.; Chang, Y.; Zhao, J.; Gong, S.; Zhao, G.; Huo, J. Isoform-Resolution Single-Cell RNA Sequencing Reveals the Transcriptional Panorama of Adult Baoshan Pig Testis Cells. BMC Genom. 2025, 26, 459. [Google Scholar] [CrossRef] [PubMed]
- Svingen, T.; Koopman, P. Building the Mammalian Testis: Origins, Differentiation, and Assembly of the Component Cell Populations. Genes Dev. 2013, 27, 2409–2426. [Google Scholar] [CrossRef] [PubMed]
- Mojadadi, A.; Au, A.; Cerda, T.O.; Shao, J.-Y.; O’Neil, T.; Bell-Anderson, K.; Andersen, J.W.; Webb, J.; Salah, W.; Ahmad, G.; et al. Dietary Supplementation of Male Mice with Inorganic, Organic or Nanoparticle Selenium Preparations: Evidence Supporting a Putative Gut-Thyroid-Male Fertility Axis. Redox Rep. 2025, 30, 2495367. [Google Scholar] [CrossRef]
- Pontelo, T.P.; Miranda, J.R.; Felix, M.A.R.; Pereira, B.A.; da Silva, W.E.; Avelar, G.F.; Mariano, F.C.M.Q.; Guimarães, G.C.; Zangeronimo, M.G. Histological Characteristics of the Gonads of Pig Fetuses and Their Relationship with Fetal Anatomical Measurements. Res. Vet. Sci. 2018, 117, 28–36. [Google Scholar] [CrossRef]
- Sudhakaran, G.; Kesavan, D.; Kandaswamy, K.; Guru, A.; Arockiaraj, J. Unravelling the Epigenetic Impact: Oxidative Stress and Its Role in Male Infertility-Associated Sperm Dysfunction. Reprod. Toxicol. 2024, 124, 108531. [Google Scholar] [CrossRef]
- Ao, Z.; Wu, Z.; Hu, G.; Gong, T.; Zhang, C.; Yang, Z.; Zhang, Y. Implications for miR-339-5p Regulation of Trophoblast Proliferation and Migration in Placentas Associated with Porcine Intrauterine Growth Retardation Using Integrated Transcriptome Sequencing Analysis. Theriogenology 2024, 216, 127–136. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Hympanova, L.; Krofta, L. Cardiovascular and Cerebrovascular Disease Associated microRNAs Are Dysregulated in Placental Tissues Affected with Gestational Hypertension, Preeclampsia and Intrauterine Growth Restriction. PLoS ONE 2015, 10, e0138383. [Google Scholar] [CrossRef]
- Gallagher, L.T.; Bardill, J.; Sucharov, C.C.; Wright, C.J.; Karimpour-Fard, A.; Zarate, M.; Breckenfelder, C.; Liechty, K.W.; Derderian, S.C. Dysregulation of miRNA–mRNA Expression in Fetal Growth Restriction in a Caloric Restricted Mouse Model. Sci. Rep. 2024, 14, 5579. [Google Scholar] [CrossRef]
- Kyrgiafini, M.-A.; Kaltsas, A.; Chatziparasidou, A.; Mamuris, Z. The Small RNA Landscape in Azoospermia: Implications for Male Infertility and Sperm Retrieval—A Preliminary Study. Int. J. Mol. Sci. 2025, 26, 3537. [Google Scholar] [CrossRef]
- Cortes-Araya, Y.; Cheung, S.; Ho, W.; Stenhouse, C.; Ashworth, C.J.; Esteves, C.L.; Donadeu, F.X. Effects of Foetal Size, Sex and Developmental Stage on Adaptive Transcriptional Responses of Skeletal Muscle to Intrauterine Growth Restriction in Pigs. Sci. Rep. 2024, 14, 8500. [Google Scholar] [CrossRef]
- Ho, S.-Y.; Yuliana, M.E.; Chou, H.-C.; Huang, L.-T.; Chen, C.-M. Altered Purine and Pentose Phosphate Pathway Metabolism in Uteroplacental Insufficiency-Induced Intrauterine Growth Restriction Offspring Rats Impair Intestinal Function. J. Nutr. Biochem. 2024, 134, 109737. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Chen, Y.; Zhang, L.; Wang, T. N-Acetylcysteine Protects against Intrauterine Growth Retardation-Induced Intestinal Injury via Restoring Redox Status and Mitochondrial Function in Neonatal Piglets. Eur. J. Nutr. 2019, 58, 3335–3347. [Google Scholar] [CrossRef] [PubMed]
- Adam-Raileanu, A.; Miron, I.; Lupu, A.; Bozomitu, L.; Sasaran, M.O.; Russu, R.; Rosu, S.T.; Nedelcu, A.H.; Salaru, D.L.; Baciu, G.; et al. Fetal Growth Restriction and Its Metabolism-Related Long-Term Outcomes-Underlying Mechanisms and Clinical Implications. Nutrients 2025, 17, 555. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Yang, F.; Li, Y.; Gao, N.; Zeng, Q.; Ma, H.; He, J.; Zhang, Y. Characterization and Function Analysis of miRNA Editing During Fat Deposition in Chinese Indigenous Ningxiang Pigs. Vet. Sci. 2024, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ji, B.; Song, R.; Wang, S.; Li, T.; Zhang, X.; Chen, K.; Li, T.; Li, J. Accurate Detection for a Wide Range of Mutation and Editing Sites of microRNAs from Small RNA High-Throughput Sequencing Profiles. Nucleic Acids Res. 2016, 44, e123. [Google Scholar] [CrossRef]
- Ocłoń, E.; Latacz, A.; Zubel–Łojek, J.; Pierzchała–Koziec, K. Hyperglycemia-Induced Changes in miRNA Expression Patterns in Epicardial Adipose Tissue of Piglets. J. Endocrinol. 2016, 229, 259–266. [Google Scholar] [CrossRef]
- Liu, T.; Cheng, W.; Gao, Y.; Wang, H.; Liu, Z. Microarray Analysis of microRNA Expression Patterns in the Semen of Infertile Men with Semen Abnormalities. Mol. Med. Rep. 2012, 6, 535–542. [Google Scholar] [CrossRef]
- Ma, J.; Chen, Q.; Wang, S.; Ma, R.; Jing, J.; Yang, Y.; Feng, Y.; Zou, Z.; Zhang, Y.; Ge, X.; et al. Mitochondria-Related miR-574 Reduces Sperm ATP by Targeting ND5 in Aging Males. Aging 2020, 12, 8321–8338. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Yin, S.-P.; Ren, Q.; Lu, D.-W.; Tang, T.; Li, Y.; Sun, Y.-Z.; Mo, H.-B.; Yin, T.-J.; Yi, Z.-Y.; et al. BAHD1 Serves as a Critical Regulator of Breast Cancer Cell Proliferation and Invasion. Breast Cancer 2022, 29, 516–530. [Google Scholar] [CrossRef]
- Ma, M.-Y.; Wang, Q.; Wang, S.-M.; Feng, X.-J.; Xian, Z.-H.; Zhang, S.-H. Wogonin Inhibits Hepatoma Cell Proliferation by Targeting miR-27b-5p/YWHAZ Axis. J. Biochem. Mol. Toxicol. 2023, 37, e23508. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, G.; Yang, J.; Lu, R.; Hu, H. DLEU2 Modulates Proliferation, Migration and Invasion of Platelet-Derived Growth Factor-BB (PDGF-BB)-Induced Vascular Smooth Muscle Cells (VSMCs) via miR-212-5p/YWHAZ Axis. Cell Cycle 2022, 21, 2013–2026. [Google Scholar] [CrossRef] [PubMed]
- Gallo, K.; Srinageshwar, B.; Ward, A.; Diola, C.; Dunbar, G.; Rossignol, J.; Bakke, J. Inducible Knockout of 14-3-3β Attenuates Proliferation and Spheroid Formation in a Human Glioblastoma Cell Line U87MG. Brain Sci. 2023, 13, 868. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, A.; Zhang, T. YWHAB Knockdown Inhibits Cell Proliferation Whilst Promoting Cell Cycle Arrest and Apoptosis in Colon Cancer Cells through PIK3R2. Exp. Ther. Med. 2023, 25, 193. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, P.; Kumar, D.; Hasan, S. Role of 14-3-3 Protein Family in the Pathobiology of EBV in Immortalized B Cells and Alzheimer’s Disease. Front. Mol. Biosci. 2024, 11, 1353828. [Google Scholar] [CrossRef]
- Fazilat, A.; Mamalo, A.S.; Roshani, S.; Razmi, S.; Valilo, M. The Interaction between miRNAs and 14-3-3ζ Protein in Different Diseases. Protein Pept. Lett. 2025, 32, 1–9. [Google Scholar] [CrossRef]
- Han, Z.; Wang, R.; Chi, P.; Zhang, Z.; Min, L.; Jiao, H.; Ou, G.; Zhou, D.; Qin, D.; Xu, C.; et al. The Subcortical Maternal Complex Modulates the Cell Cycle during Early Mammalian Embryogenesis via 14-3-3. Nat. Commun. 2024, 15, 8887. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.; Liu, X.; Liu, H.; Sun, H.; Wang, L.; Yu, J.; Li, J.; Shi, Y. Catalytic Subunit of Protein Phosphatase 2A (PP2Ac) Influences the Meiosis Initiation During Spermatocyte Meiosis Prophase I. Reprod. Sci. 2022, 29, 3201–3211. [Google Scholar] [CrossRef]
- Fang, C.; Li, L.; Li, J. Conditional Knockout in Mice Reveals the Critical Roles of Ppp2ca in Epidermis Development. Int. J. Mol. Sci. 2016, 17, 756. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, K.; Ma, J.; Sun, L.; Niu, L.; Zhao, Y.; Chen, L.; Zhou, L.; Xue, J.; Zhou, X.; et al. Intrauterine Growth-Restricted Pig-Associated Testicular Transcriptome Analysis Reveals microRNA-mRNA Regulatory Networks. Animals 2025, 15, 2486. https://doi.org/10.3390/ani15172486
Li J, Wang K, Ma J, Sun L, Niu L, Zhao Y, Chen L, Zhou L, Xue J, Zhou X, et al. Intrauterine Growth-Restricted Pig-Associated Testicular Transcriptome Analysis Reveals microRNA-mRNA Regulatory Networks. Animals. 2025; 15(17):2486. https://doi.org/10.3390/ani15172486
Chicago/Turabian StyleLi, Jiaxin, Kai Wang, Jianfeng Ma, Lijun Sun, Lili Niu, Ye Zhao, Lei Chen, Lixin Zhou, Jia Xue, Xiaofeng Zhou, and et al. 2025. "Intrauterine Growth-Restricted Pig-Associated Testicular Transcriptome Analysis Reveals microRNA-mRNA Regulatory Networks" Animals 15, no. 17: 2486. https://doi.org/10.3390/ani15172486
APA StyleLi, J., Wang, K., Ma, J., Sun, L., Niu, L., Zhao, Y., Chen, L., Zhou, L., Xue, J., Zhou, X., Wang, Y., Shen, L., Zhu, L., & Gan, M. (2025). Intrauterine Growth-Restricted Pig-Associated Testicular Transcriptome Analysis Reveals microRNA-mRNA Regulatory Networks. Animals, 15(17), 2486. https://doi.org/10.3390/ani15172486








