Experimental Study on the Response Mechanisms of Drift Egg Transport and Adhesive Egg Hatching to Reservoir Impoundment in the Lower Jinsha River
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.1.1. Hydrodynamics–Microtopography Simulation Flume
2.1.2. Hatching Apparatus
2.1.3. Experimental Materials
2.2. Experimental Methods
2.2.1. Near-Bed Drift Threshold for Drifting Eggs
2.2.2. Effect of Sediment Deposition on the Hatching of Adhesive Eggs
3. Results
3.1. Settling Velocity of Model Eggs
3.2. Near-Bed Drift Threshold of Drifting Eggs
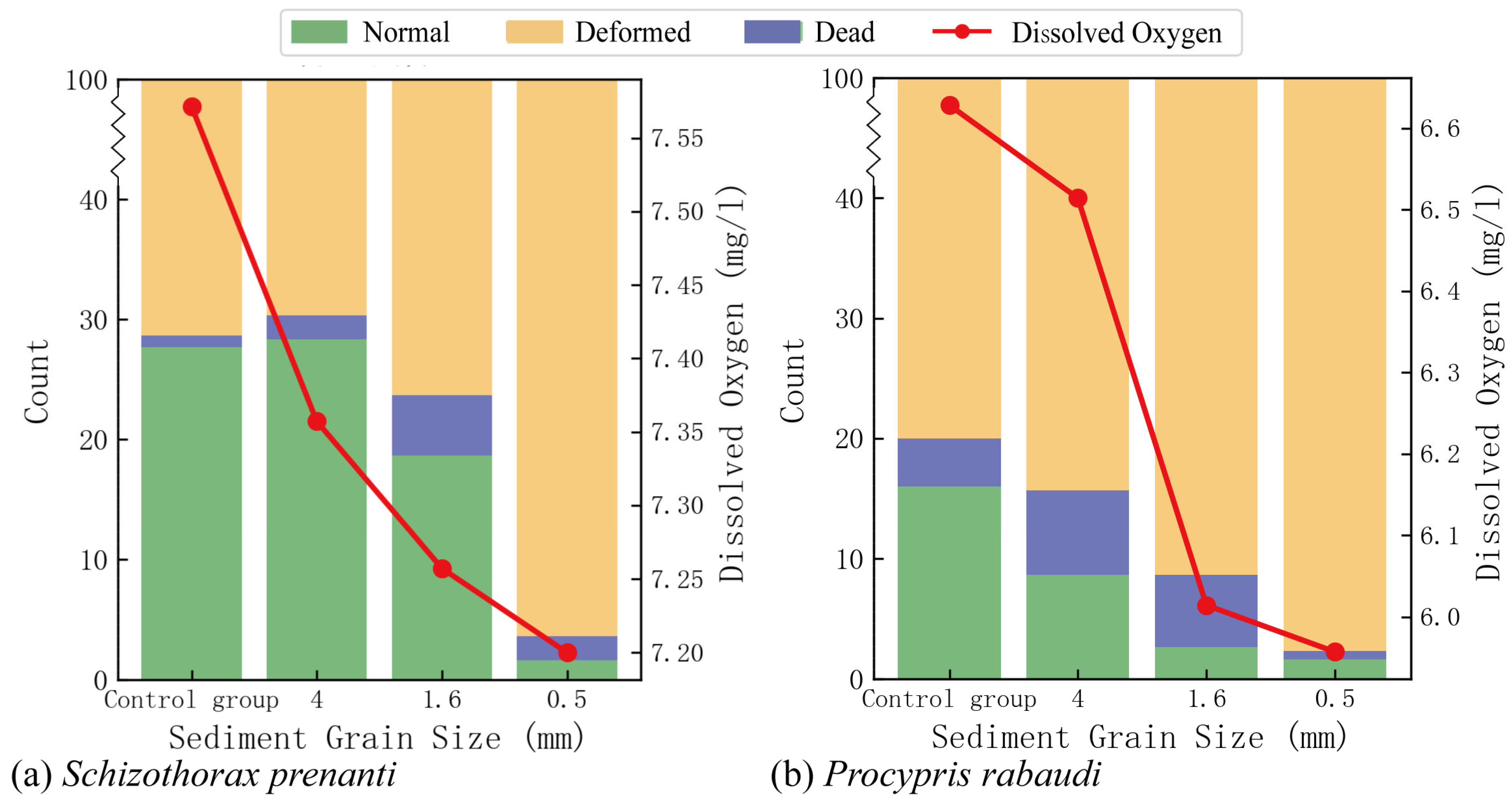
3.3. Effects of Sediment Deposition on Egg Hatching
4. Discussion
4.1. Low-Velocity Compensatory Mechanisms and Implications for Flow Management
4.2. Sediment Deposition Affects Fish Egg Hatching by Altering DO
5. Conclusions
- (1)
- Model eggs, with a density of 1.008 g/cm3 and diameters of 6 mm and 5 mm, were fabricated to simulate the drifting behavior of fish eggs. Through experiments, this study fitted the coefficient in the egg settling velocity formula as k = 0.553.
- (2)
- Using dimensional analysis (Π theorem), a threshold formula for near-bed drifting eggs was derived, i.e., . This formula comprehensively reflects the motion characteristics of drifting eggs under hydrodynamic forces.
- (3)
- Taking S. prenanti and P. rabaudi as examples, the study clarified the impact of sediment deposition on the hatching of adhesive eggs and its underlying mechanisms. The results showed that sediment deposition significantly reduced the hatching rate of adhesive eggs, mainly through changes in DO levels.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.; Li, Q.; Lin, Y.; Zhang, J.; Xia, J.; Ni, J.; Cooke, S.J.; Best, J.; He, S.; Feng, T.; et al. River Damming Impacts on Fish Habitat and Associated Conservation Measures. Rev. Geophys. 2023, 61, e2023RG000819. [Google Scholar] [CrossRef]
- He, F.; Zarfl, C.; Tockner, K.; Olden, J.D.; Campos, Z.; Muniz, F.; Svenning, J.-C.; Jähnig, S.C. Hydropower impacts on riverine biodiversity. Nat. Rev. Earth Environ. 2024, 5, 755–772. [Google Scholar] [CrossRef]
- Csiki, S.J.; Rhoads, B.L. Influence of four run-of-river dams on channel morphology and sediment characteristics in Illinois, USA. Geomorphology 2014, 206, 215–229. [Google Scholar] [CrossRef]
- Kuriqi, A.; Pinheiro, A.N.; Sordo-Ward, A.; Bejarano, M.D.; Garrote, L. Ecological impacts of run-of-river hydropower plants—Current status and future prospects on the brink of energy transition. Renew. Sustain. Energy Rev. 2021, 142, 110833. [Google Scholar] [CrossRef]
- Li, T.; Wang, S.; Liu, Y.; Fu, B.; Zhao, W. Driving forces and their contribution to the recent decrease in sediment flux to ocean of major rivers in China. Sci. Total Environ. 2018, 634, 534–541. [Google Scholar] [CrossRef]
- Cao, W.; Chang, J.; Qiao, Y.; Duan, Z. Fish Resources of Early Life History Stages in Yangtze River; China WaterPower Press: Beijing, China, 2007. [Google Scholar]
- Zhu, L.; Chen, C.; Zhang, J. Study on variations of runoff and sediment and effect to the lower Jinsha River. J. Sediment Res. 2016, 5, 20–27. [Google Scholar]
- Zhu, L.; Chen, D.; Yang, C.; Chen, K.; Li, S. Sediment deposition of cascade reservoirs in the lower Jinsha River and scouring of river channel under dam. J. Lake Sci. 2023, 35, 1097–1110. [Google Scholar] [CrossRef]
- Du, Z.; Dong, X.; Zhang, F.; Qin, L. Study on runoff and sediment characteristics and reservoir deposition in Xiluodu Reservoir of the Jinsha River. J. Sediment Res. 2022, 47, 22–28. [Google Scholar]
- Yue, L. The Evolution of Flow and Sediment Regime in the Yangtze River and Its Fish Response Mechanism. Ph.D. Thesis, North China University of Water Resources and Hydropower, Zhengzhou, China, 2021. [Google Scholar]
- Liu, Q.; Zhang, P.; Li, H.; You, L.; Li, Y.; Li, J.; Liu, M.; Zhao, P.; Wang, K.; Zhu, Z. Assessment and conservation strategies for endemic fish with drifting eggs threatened by the cascade barrier effect: A case study in the Yalong River, China. Ecol. Eng. 2021, 170, 106364. [Google Scholar] [CrossRef]
- Garcia, T.; Jackson, P.R.; Murphy, E.A.; Valocchi, A.J.; Garcia, M.H. Development of a Fluvial Egg Drift Simulator to evaluate the transport and dispersion of Asian carp eggs in rivers. Ecol. Model. 2013, 263, 211–222. [Google Scholar] [CrossRef]
- Yu, Z.T.; Liang, Z.S.; Yi, B.L. The early development of Coreius heterodon and Coreius guichenoti. Acta Hydrobiol. Sin. 1984, 8, 371–388. [Google Scholar] [CrossRef]
- Li, T.; Tang, L.; Wang, L.; An, L.; Wang, J.; Mo, K.L.; Chen, Q.W. Distribution characteristics and ecological types changes in fish communities under hydropower development from Xiluodu to Xiangjiaba reach. Acta Ecol. Sin. 2020, 40, 1473–1485. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Q.; Wang, Y.; Li, K.; Qin, L.; Liang, R.; Li, J. Does drifting passage need to be linked to fish habitat assessment? Assessing environmental flow for multiple fish species with different spawning patterns with a framework integrating habitat connectivity. J. Hydrol. 2022, 612, 128247. [Google Scholar] [CrossRef]
- Stanley, J.G.; Miley, W.W.; Sutton, D.L. Reproductive requirements and likelihood for naturalization of escaped grass carp in the United States. Trans. Am. Fish. Soc. 1978, 107, 119–128. [Google Scholar] [CrossRef]
- Leslie, A.J., Jr.; Van Dyke, J.M.; Nall, L.E.; Miley, W.W. Current velocity for transport of grass carp eggs. Trans. Am. Fish. Soc. 1982, 111, 99–101. [Google Scholar] [CrossRef]
- Kolar, C.S.; Chapman, D.; Courtenay, W.R., Jr.; Housel, C.M.; Williams, J.D.; Jennings, D.P. Bigheaded Carps: A Biological Synopsis and Environmental Risk Assessment; American Fisheries Society: Bethesda, MD, USA, 2007. [Google Scholar]
- Kocovsky, P.M.; Chapman, D.C.; McKenna, J.E. Thermal and hydrologic suitability of Lake Erie and its major tributaries for spawning of Asian carps. J. Great Lakes Res. 2012, 38, 159–166. [Google Scholar] [CrossRef]
- Li, C. A Preliminary Analysis of the Impacts of the Cascade Hydropower Development on the Fish Biodiversity in the Upper Reach of the Yangtze River. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2012. (In Chinese). [Google Scholar]
- George, A.E.; Chapman, D.C.; Deters, J.E.; Erwin, S.O.; Hayer, C.A. Effects of sediment burial on grass carp, Ctenopharyngodon idella (Valenciennes, 1844), eggs. J. Appl. Ichthyol. 2015, 31, 1120–1126. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, W.-Y.; Liu, Q.-Y.; Li, Y.; Tian, H.-W.; Cheng, B.-X.; Zhang, Z.-Y.; Wu, Z.-H.; Qing, J.; Sun, G.; et al. Impact of Low-Head Dam Removal on River Morphology and Habitat Suitability in Mountainous Rivers. Int. J. Environ. Res. Public Health 2022, 19, 11743. [Google Scholar] [CrossRef]
- Wei, Q. Reproductive Behavioral Ecology of Chinese Sturgeon (Acipenser sinensis Gray) with Its Stock Assessment. Ph.D. Thesis, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China, 2003. [Google Scholar]
- Qiang, J.; Zhong, C.Y.; Bao, J.W.; Liang, M.; Liang, C.; Tao, Y.F.; Li, H.X.; He, J.; Xu, P. Synergistic effect of water temperature and dissolved oxygen concentration on rates of fertilization, hatching and deformity of hybrid yellow catfish (Tachysurus fulvidraco♀ × Pseudobagrus vachellii♂). J. Therm. Biol. 2019, 83, 47–53. [Google Scholar] [CrossRef]
- Guo, J.; Liao, B.; Zhou, Q.; Nie, R. Experimental study on influence of sediment concentration to survival of Schizothorax prenanti. J. Sediment Res. 2020, 45, 7–12. [Google Scholar]
- Qin, X.H.; Liu, G.Y.; Wu, Y.J.; Shi, X.T.; Wang, X.L. Effects of Light Intensity on the Hatching Rate of Fertilized Schizothorax chongi Eggs and on the Growth and Feeding of Larvae. J. Hydroecol. 2017, 38, 97–102. [Google Scholar] [CrossRef]
- Chojnacki, K.A.; George, A.E.; DeLonay, A.J. The effects of substrate and sediment burial on survival of developing pallid sturgeon (Scaphirhynchus albus) and shovelnose sturgeon (S. platorynchus) embryos. Environ. Biol. Fishes 2023, 106, 527–539. [Google Scholar] [CrossRef]
- Julien, H. The Impact of Winter Fluvial Processes on Spawning Grounds and Intergranular Survival of Atlantic Salmon (Salmo salar L.). Master’s Thesis, Institut National de la Recherche Scientifique, Quebec, QC, Canada, 2000. [Google Scholar]
- Kähler, C.J.; Astarita, T.; Vlachos, P.P.; Sakakibara, J.; Hain, R.; Discetti, S.; La Foy, R.; Cierpka, C. Main results of the 4th International PIV Challenge. Exp. Fluids 2016, 57, 97. [Google Scholar] [CrossRef]
- Guo, J. Modelling river bedform evolution. In Proceedings of the 15th International Symposium on River Sedimentation, Florence, Italy, 5–8 September 2023. [Google Scholar]
- Wang, Q.; Han, Y.; Li, P.; Zhang, W.; Wang, Y.; Xi, Y.; Yao, W. Ecohydraulic modelling to evaluate cascade dam construction impact and support fish habitat restoration. Ecol. Eng. 2023, 192, 106974. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, K.; Yang, W.; Wang, L.; Yang, S.; Li, W.; Zhang, P. Hydrostatic Settling Characteristics of Drifting Fish Eggs in Main Stream of Yangtze River from Yibin to Fengdu. Resour. Environ. Yangtze Basin 2022, 31, 1918–1925. [Google Scholar]
- Garcia, T.; Zamalloa, C.Z.; Jackson, P.R.; Murphy, E.A.; Garcia, M.H. A Laboratory Investigation of the Suspension, Transport, and Settling of Silver Carp Eggs Using Synthetic Surrogates. PLoS ONE 2015, 10, e0145775. [Google Scholar] [CrossRef]
- Zhang, X. Study on the Movement Characteristics of Drifting Fish Eggs in the Upper Reaches of the Yangtze River. Ph.D. Thesis, Chongqing Jiaotong University, Chongqing, China, 2022. [Google Scholar]
- Wang, B.H.; Wang, X.Z. New Method for Calculation of Settling Velocity of a Spherodial Particle. China Powder Sci. Technol. 1996, 2, 30–39. [Google Scholar]
- Xiong, M.H.; Shao, K.; Li, W.T.; Zhu, B. Research progress on resources variation and protection of Coreius guichenoti. Yangtze River 2023, 54, 63–71. [Google Scholar] [CrossRef]
- Liu, L.; Wu, G.; Wang, Z. Reproduction ecology of Coreius heterodon (Bleeker) and Coreius guichenoti (Sauvage et Dabry) in the mainstream of the Changjiang River after the construction of Gezhouba Dam. Acta Hydrobiol. Sin. 1990, 14, 205–215. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, L.; Cao, H.; Ruan, Y. Influence of ecological regulation of cascade reservoirs in the lower Jinsha River. Ecol. Environ. Monit. Three Gorges 2020, 5, 8–13. [Google Scholar]
- Zhang, D.; Fan, H.; Wang, M.; Li, F.; Ruan, Y. Target Fish Screening for the Ecological Operation of Wudongde Hydropower Station on Jinsha River. J. Hydroecol. 2022, 43, 73–82. [Google Scholar]
- Yang, Z.; Zhang, P.; Tang, H.; Gong, Y.; Dong, C.; Chen, X.; Zhao, N. The formation of habitat suitability curves for Coreius guichenoti (Sauvage & Dabry de Thiersant, 1874) of the lower Jinsha River. Ecol. Sci. 2017, 36, 129–137. [Google Scholar]
- Zhang, K. Study on Drifting Fish Egg Movement Patterns in Non-Uniform Water Flows. Ph.D. Thesis, Chongqing Jiaotong University, Chongqing, China, 2023. [Google Scholar]
- Curtis, W.; Logan, J.D.; Parker, W. Dimensional analysis and the pi theorem. Linear Algebra Appl. 1982, 47, 117–126. [Google Scholar] [CrossRef]
- Evans, E.; Petts, G.E. Hyporheic temperature patterns within riffles. Hydrol. Sci. J. 1997, 42, 199–213. [Google Scholar] [CrossRef]
- Sawyer, A.H.; Bayani Cardenas, M.; Buttles, J. Hyporheic temperature dynamics and heat exchange near channel-spanning logs. Water Resour. Res. 2012, 48, 1529. [Google Scholar] [CrossRef]
- Higashino, M.; O’Connor, B.L.; Hondzo, M.; Stefan, H.G. Oxygen transfer from flowing water to microbes in an organic sediment bed. Hydrobiologia 2008, 614, 219–231. [Google Scholar] [CrossRef]
- Reeder, W.J.; Quick, A.M.; Farrell, T.B.; Benner, S.G.; Feris, K.P.; Tonina, D. Spatial and temporal dynamics of dissolved oxygen concentrations and bioactivity in the hyporheic zone. Water Resour. Res. 2018, 54, 2112–2128. [Google Scholar] [CrossRef]
- Shumilova, O.O.; Sukhodolov, A.N.; Constantinescu, G.S.; MacVicar, B.J. Dynamics of shallow wakes on gravel-bed floodplains: Dataset from field experiments. Earth Syst. Sci. Data 2021, 13, 1519–1529. [Google Scholar] [CrossRef]
- Grant, S.B.; Gomez-Velez, J.D.; Ghisalberti, M. Modeling the effects of turbulence on hyporheic exchange and local-to-global nutrient processing in streams. Water Resour. Res. 2018, 54, 5883–5889. [Google Scholar] [CrossRef]
- Dressing, S.A. National Management Measures to Control Nonpoint Source Pollution from Agriculture; US Environmental Protection Agency, Office of Water: Washington, DC, USA, 2003. [Google Scholar]
- Hu, P.; Tang, J.; Yang, Z.; Zeng, Q.; Yang, M. Experimental study on critical hydrodynamic conditions for safe drifting of semi-buoyant eggs. J. Hydraul. Eng. 2021, 52, 1430–1438. [Google Scholar]
- Liu, X.; Lin, J.; Peng, Q.; Yu, K.; Chen, Y.; Zhuang, J. Experimental research on fish eggs’ movement using particle tracking velocimetry technique. J. Hydraul. Eng. 2018, 49, 501–511. [Google Scholar]
- Jun, X.; Pan, L.; Qian, C. Water supply safety and technological innovation safeguarding for the Three Gorges Project. China Water Resour. 2024, 22, 13–16+104. [Google Scholar]
- Du, H.; Wei, Q.; Zhang, H.; Liu, Z.; Wang, C.; Li, Y. Bottom substrate attributes relative to bedform morphology of spawning site of Chinese sturgeon Acipenser sinensis below the Gezhouba dam. J. Appl. Ichthyol. 2011, 27, 257–262. [Google Scholar] [CrossRef]
- Duerregger, A.; Pander, J.; Palt, M.; Mueller, M.; Nagel, C.; Geist, J. The importance of stream interstitial conditions for the early-life-stage development of the European nase (Chondrostoma nasus L.). Ecol. Freshw. Fish 2018, 27, 920–932. [Google Scholar] [CrossRef]
- Merz, J.E.; Setka, J.D.; Pasternack, G.B.; Wheaton, J.M. Predicting benefits of spawning-habitat rehabilitation to salmonid (Oncorhynchus spp.) fry production in a regulated California river. Can. J. Fish. Aquat. Sci. 2004, 61, 1433–1446. [Google Scholar] [CrossRef]
- Chapman, J.M.; Proulx, C.L.; Veilleux, M.A.; Levert, C.; Bliss, S.; Andre, M.-E.; Lapointe, N.W.; Cooke, S.J. Clear as mud: A meta-analysis on the effects of sedimentation on freshwater fish and the effectiveness of sediment-control measures. Water Res. 2014, 56, 190–202. [Google Scholar] [CrossRef]
- Hao, D.; Qiwei, W.; Hui, Z.; Chengyou, W.; Jinming, W.; Li, S. Changes of bottom substrate characteristics in spawning ground of Chinese sturgeon downstream the Gezhouba dam from impounding of Three Gorge reservoir. Acta Ecol. Sin. 2015, 35, 3124–3131. [Google Scholar] [CrossRef][Green Version]
- Kochhann, D.; Chapman, L. A foundational exploration of respiration in fish eggs and larvae. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2023; Volume 40, pp. 557–566. [Google Scholar][Green Version]
- Harter, T. Warm fish eggs gasp for oxygen. J. Exp. Biol. 2021, 224, jeb235150. [Google Scholar] [CrossRef]
- Decent, Q. Factors Controlling Dissolved Oxygen in Spawning Gravels: Evaluation of the Sediment Intrusion and Dissolved Oxygen Model (SIDO) for Fisheries Management. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2020. [Google Scholar]
- Broman, E.; Brüsin, M.; Dopson, M.; Hylander, S. Oxygenation of anoxic sediments triggers hatching of zooplankton eggs. Proc. R. Soc. B Biol. Sci. 2015, 282, 20152025. [Google Scholar] [CrossRef]
- Chen, K.; Guo, Z.; Zhan, Y.; Roden, E.E.; Zheng, C. Heterogeneity in permeability and particulate organic carbon content controls the redox condition of riverbed sediments at different timescales. Geophys. Res. Lett. 2024, 51, e2023GL107761. [Google Scholar] [CrossRef]
- Shrivastava, S.; Stewardson, M.; Arora, M. Sediment reworking in streambeds with fine sediment deposits and its influence on hyporheic flow regime. Water Resour. Res. 2021, 57, e2021WR030360. [Google Scholar] [CrossRef]
- Greig, S.; Sear, D.; Carling, P. The impact of fine sediment accumulation on the survival of incubating salmon progeny: Implications for sediment management. Sci. Total Environ. 2005, 344, 241–258. [Google Scholar] [CrossRef] [PubMed]
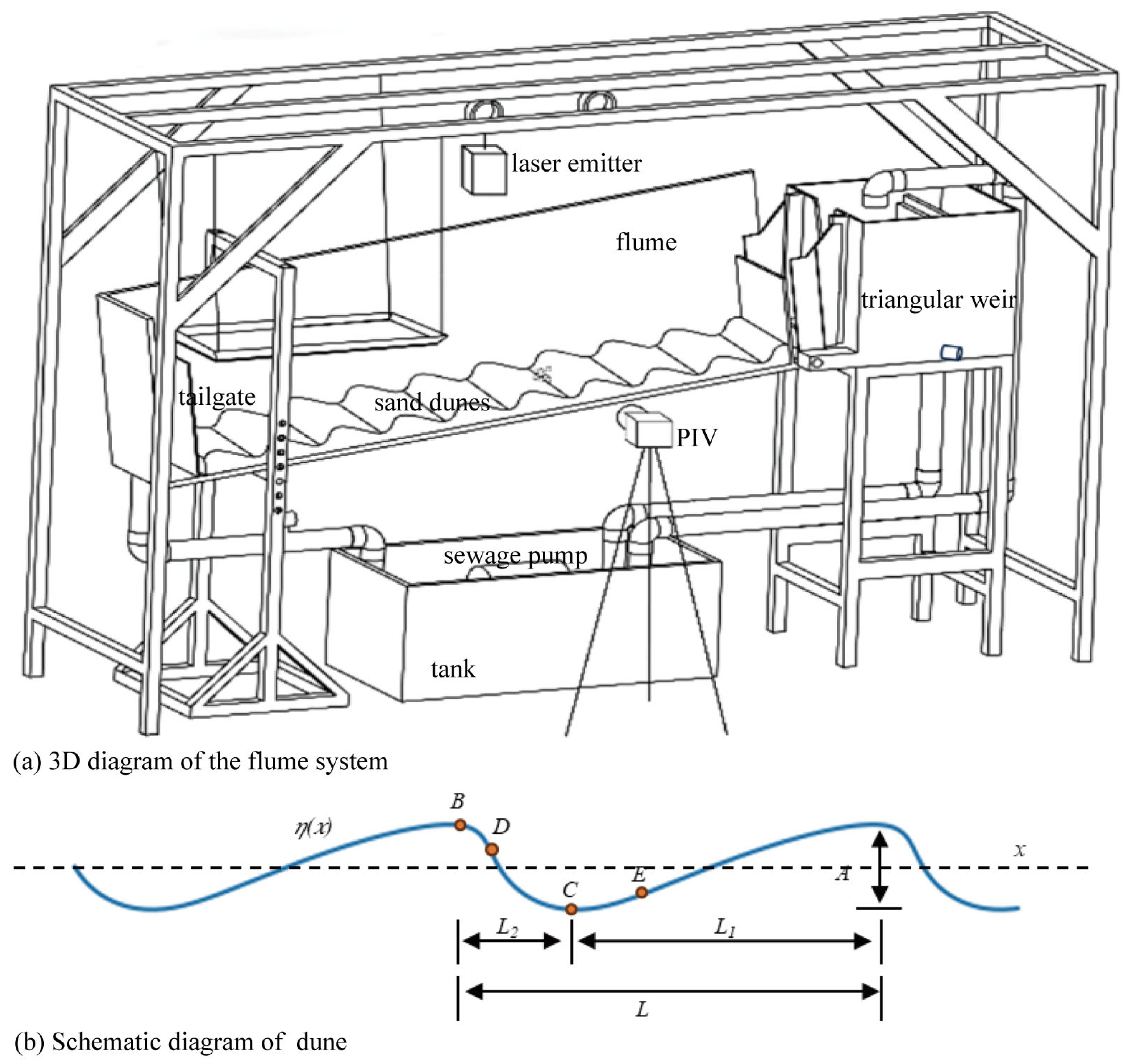
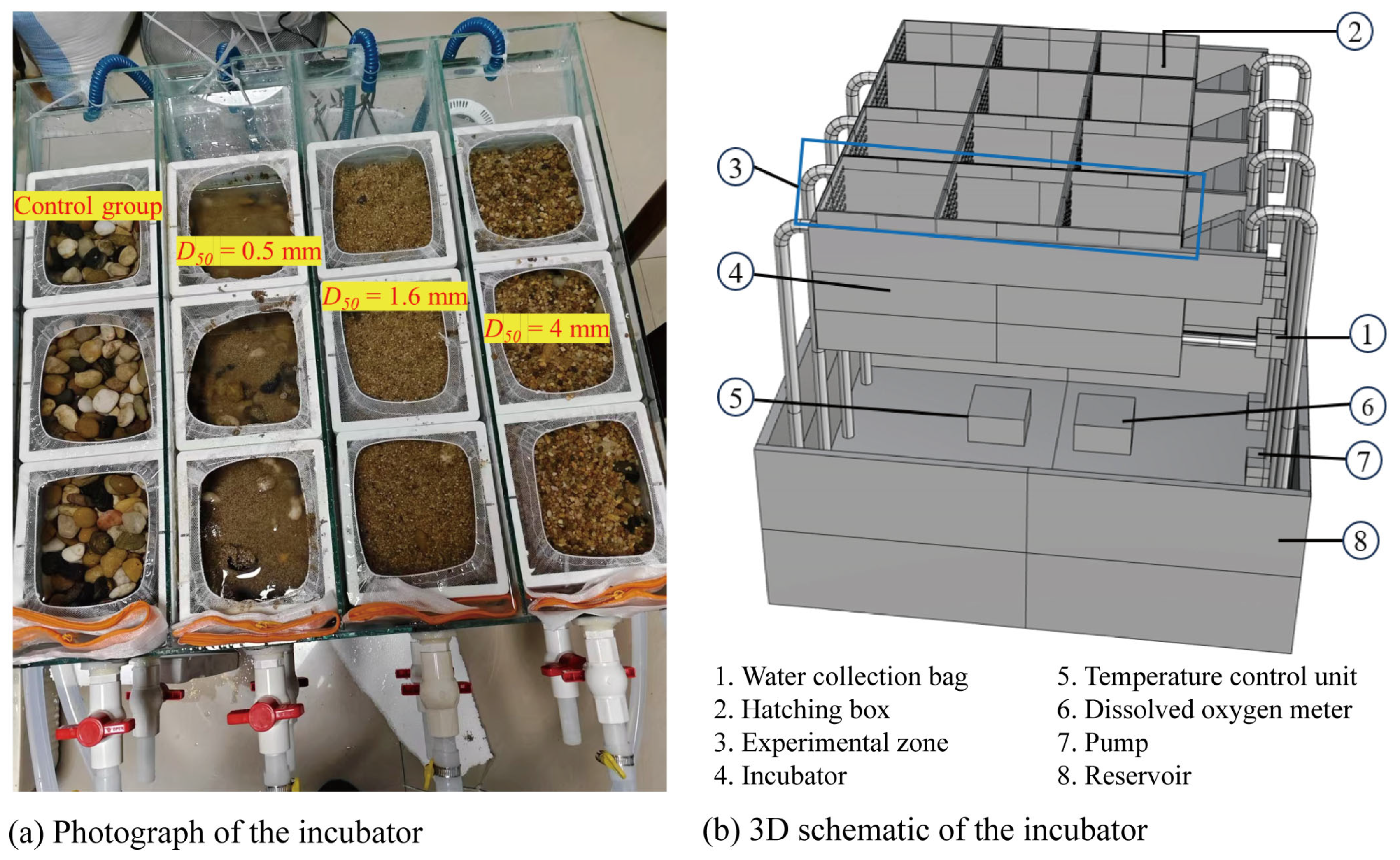
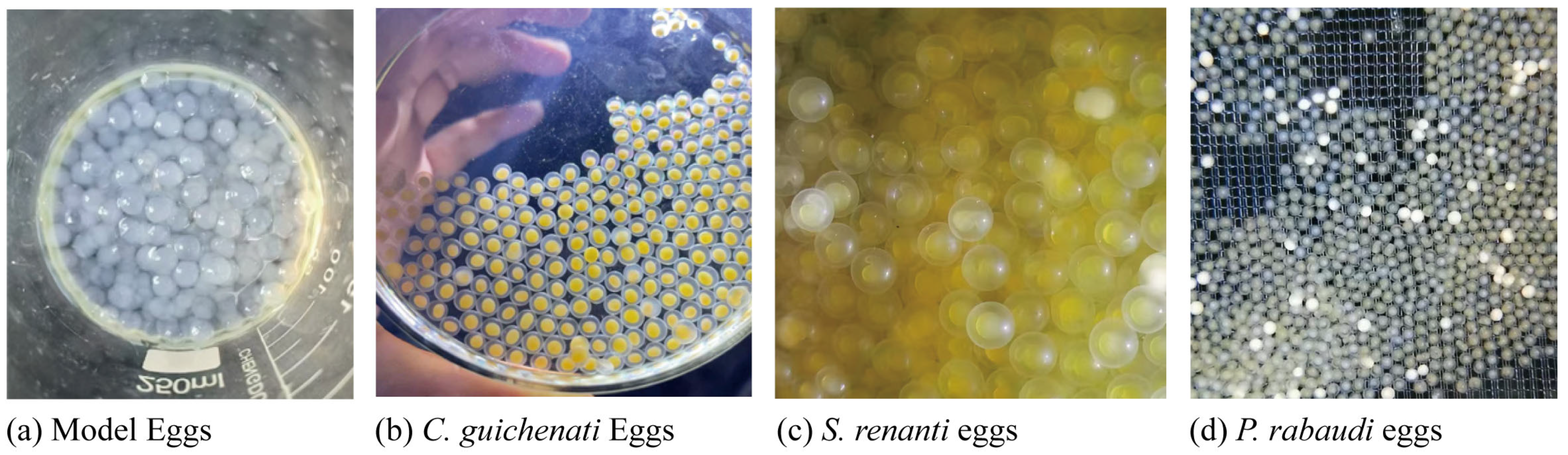

| Dune | F | L | A | a |
|---|---|---|---|---|
| Large | 0.2 | 18 | 1.8 | 0.782 |
| Small | 0.2 | 9 | 0.9 | 0.782 |
| Test Condition | Slope | Egg Diameter (mm) | Dune Size |
|---|---|---|---|
| 1 | 6.07% | 5 | Large |
| 2 | 6.07% | 5 | Small |
| 3 | 6.07% | 6 | Large |
| 4 | 6.07% | 6 | Small |
| 5 | 8.20% | 5 | Large |
| 6 | 8.20% | 5 | Small |
| 7 | 8.20% | 6 | Large |
| 8 | 8.20% | 6 | Small |
| 9 | 10.79% | 5 | Large |
| 10 | 10.79% | 5 | Small |
| 11 | 10.79% | 6 | Large |
| 12 | 10.79% | 6 | Small |
| 13 | 13.03% | 5 | Large |
| 14 | 13.03% | 5 | Small |
| 15 | 13.03% | 6 | Large |
| 16 | 13.03% | 6 | Small |
| 17 | 15.45% | 5 | Large |
| 18 | 15.45% | 5 | Small |
| 19 | 15.45% | 6 | Large |
| 20 | 15.45% | 6 | Small |
| Egg Diameter | k | Free Settling Velocity (m/s) | Relative Difference (%) | |
|---|---|---|---|---|
| Egg Diameter: 6 mm | Egg Diameter: 5 mm | |||
| Current study | 0.553 | 0.011994 | 0.010949 | |
| Zhang Xun (2022) [34] | 0.735 | 0.015941 | 0.014552 | +28.9–32.7% |
| Zhang Kanrui (2022) [41] | 0.609 | 0.013208 | 0.012058 | +8.7–9.1% |
| Species | Model | Variable | Coefficient | Standard Error | t | p |
|---|---|---|---|---|---|---|
| P. rabaudi | Model 1 | Intercept | 6.1021 | 0.076 | 80.167 | 0.000 |
| Sediment deposition | 0.0227 | 0.006 | 3.781 | 0.004 | ||
| Model 2 | Intercept | −1.0981 | 0.378 | −2.905 | 0.017 | |
| DO | 0.1923 | 0.062 | 3.106 | 0.013 | ||
| Sediment deposition | 0.0010 | 0.002 | 0.537 | 0.604 | ||
| Model 3 | Intercept | 0.0751 | 0.020 | 3.692 | 0.004 | |
| Sediment deposition | 0.0053 | 0.002 | 3.333 | 0.008 | ||
| S. prenanti | Model 1 | Intercept | 7.2409 | 0.016 | 459.198 | 0.000 |
| Sediment deposition | 0.0135 | 0.001 | 10.899 | 0.000 | ||
| Model 2 | Intercept | −15.2564 | 2.930 | −5.207 | 0.001 | |
| DO | 2.1313 | 0.405 | 5.267 | 0.001 | ||
| Sediment deposition | −0.0237 | 0.006 | −4.159 | 0.002 | ||
| Model 3 | Intercept | 0.1759 | 0.039 | 4.548 | 0.001 | |
| Sediment deposition | 0.0051 | 0.003 | 1.685 | 0.123 |
| Species | Effect Type | Estimated Value | Standard Error | t | p |
|---|---|---|---|---|---|
| P. rabaudi | Indirect effect | 0.1292 | 0.0416 | 3.1052 | 0.0112 |
| Direct effect | 0.0010 | 0.0018 | 0.5370 | 0.6043 | |
| Total effect | 0.1302 | 0.0416 | 3.1258 | 0.0122 | |
| Contribution rate of DO | 99.24% | - | - | - | |
| Direct contribution rate of sediment | 0.76% | - | - | - | |
| S. prenanti | Indirect effect | 0.7907 | 0.1501 | 5.2664 | 0.0004 |
| Direct effect | −0.0237 | 0.0057 | −4.1588 | 0.0025 | |
| Total effect | 0.7670 | 0.1502 | 5.1046 | 0.0006 | |
| Contribution rate of DO | 103.09% | - | - | - | |
| Direct contribution rate of sediment | −3.09% | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Li, W.; Chen, D.; Gao, Y.; Han, R. Experimental Study on the Response Mechanisms of Drift Egg Transport and Adhesive Egg Hatching to Reservoir Impoundment in the Lower Jinsha River. Animals 2025, 15, 2488. https://doi.org/10.3390/ani15172488
Zhu L, Li W, Chen D, Gao Y, Han R. Experimental Study on the Response Mechanisms of Drift Egg Transport and Adhesive Egg Hatching to Reservoir Impoundment in the Lower Jinsha River. Animals. 2025; 15(17):2488. https://doi.org/10.3390/ani15172488
Chicago/Turabian StyleZhu, Lekui, Wenchao Li, Dong Chen, Yiheng Gao, and Rui Han. 2025. "Experimental Study on the Response Mechanisms of Drift Egg Transport and Adhesive Egg Hatching to Reservoir Impoundment in the Lower Jinsha River" Animals 15, no. 17: 2488. https://doi.org/10.3390/ani15172488
APA StyleZhu, L., Li, W., Chen, D., Gao, Y., & Han, R. (2025). Experimental Study on the Response Mechanisms of Drift Egg Transport and Adhesive Egg Hatching to Reservoir Impoundment in the Lower Jinsha River. Animals, 15(17), 2488. https://doi.org/10.3390/ani15172488





