Reduced Dietary Protein Levels Improved Growth Performance, Promoted Efficient Nutrient Utilization, Increased Fecal Lactobacillus, and Reduced Fecal Malodorous Compounds in Late-Fattening Barrows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design and Feeding Management
2.3. Measurement of Growth Performance
2.4. Measurement of Nutrient Digestibility
2.5. Sample Collection and Preparation
2.6. Measurement of Serum Biochemical Indices
2.7. Measurement of Carcass Traits and Meat Quality
2.8. Analysis of Fecal Microbiota
2.9. Determination of Fecal Malodorous Compounds
2.9.1. Ammonia Nitrogen
2.9.2. Biogenic Amines
2.9.3. VFAs
2.9.4. Phenolic and Indole-Related Compounds
2.10. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Nutrient Digestibility
3.3. Effects of LP Diet on Serum Biochemical Indices in Late-Fattening Barrows
3.4. Carcass Traits and Meat Quality
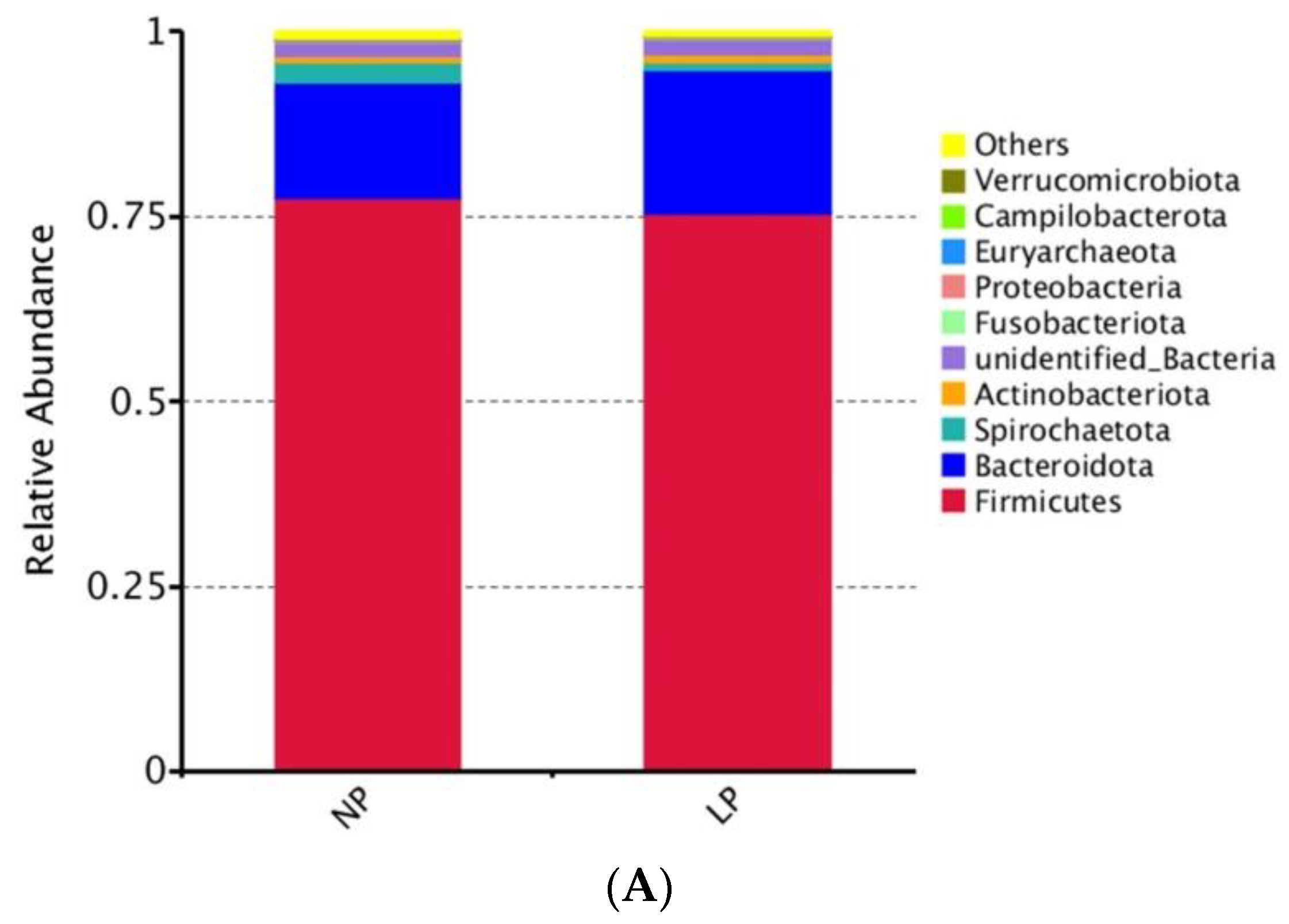
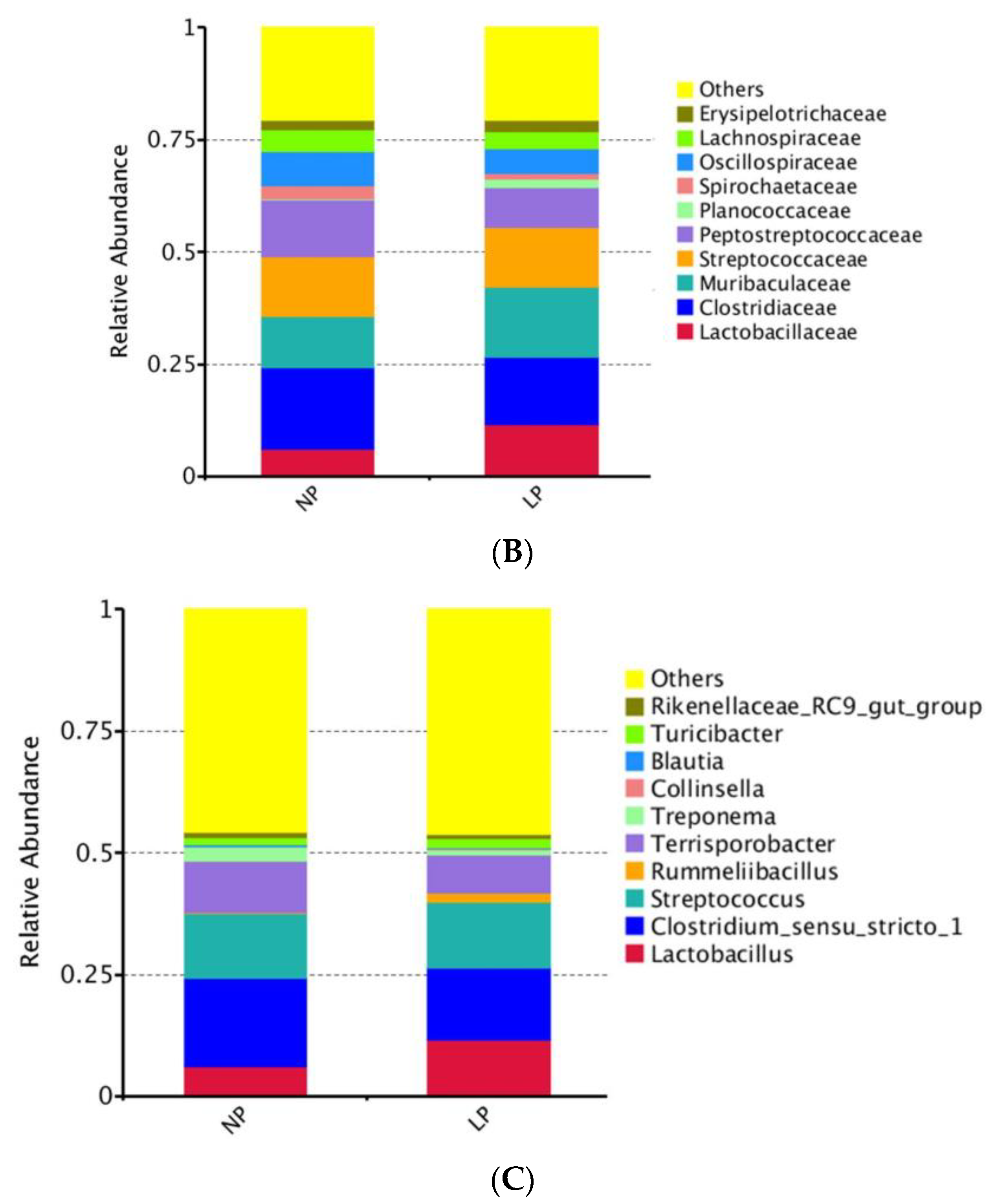
3.5. Fecal Microbiota
3.5.1. Analysis of OTUs, α-Diversity Indices, and Observed Microbial Species
3.5.2. Analysis of Differential Microbiota Composition
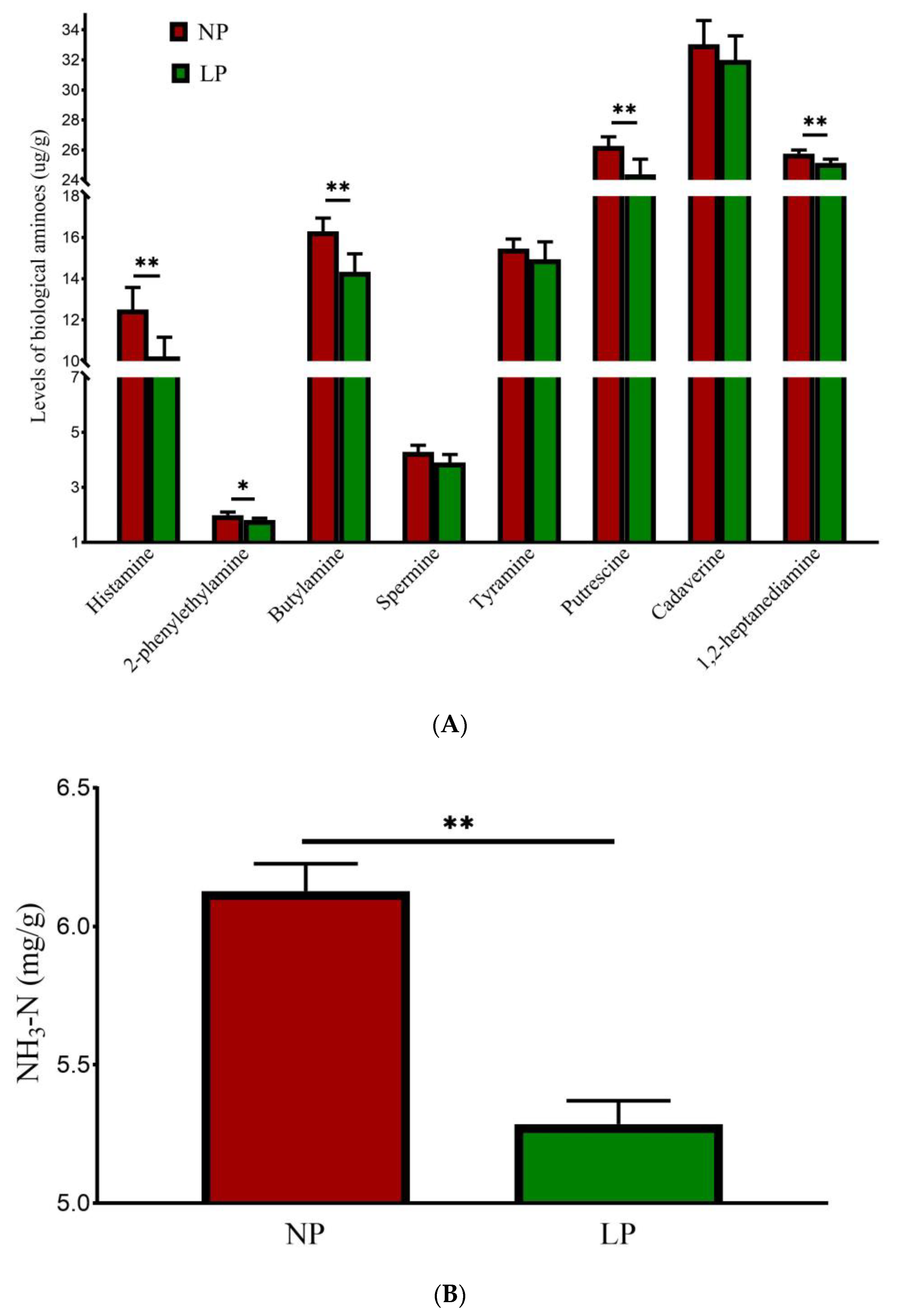
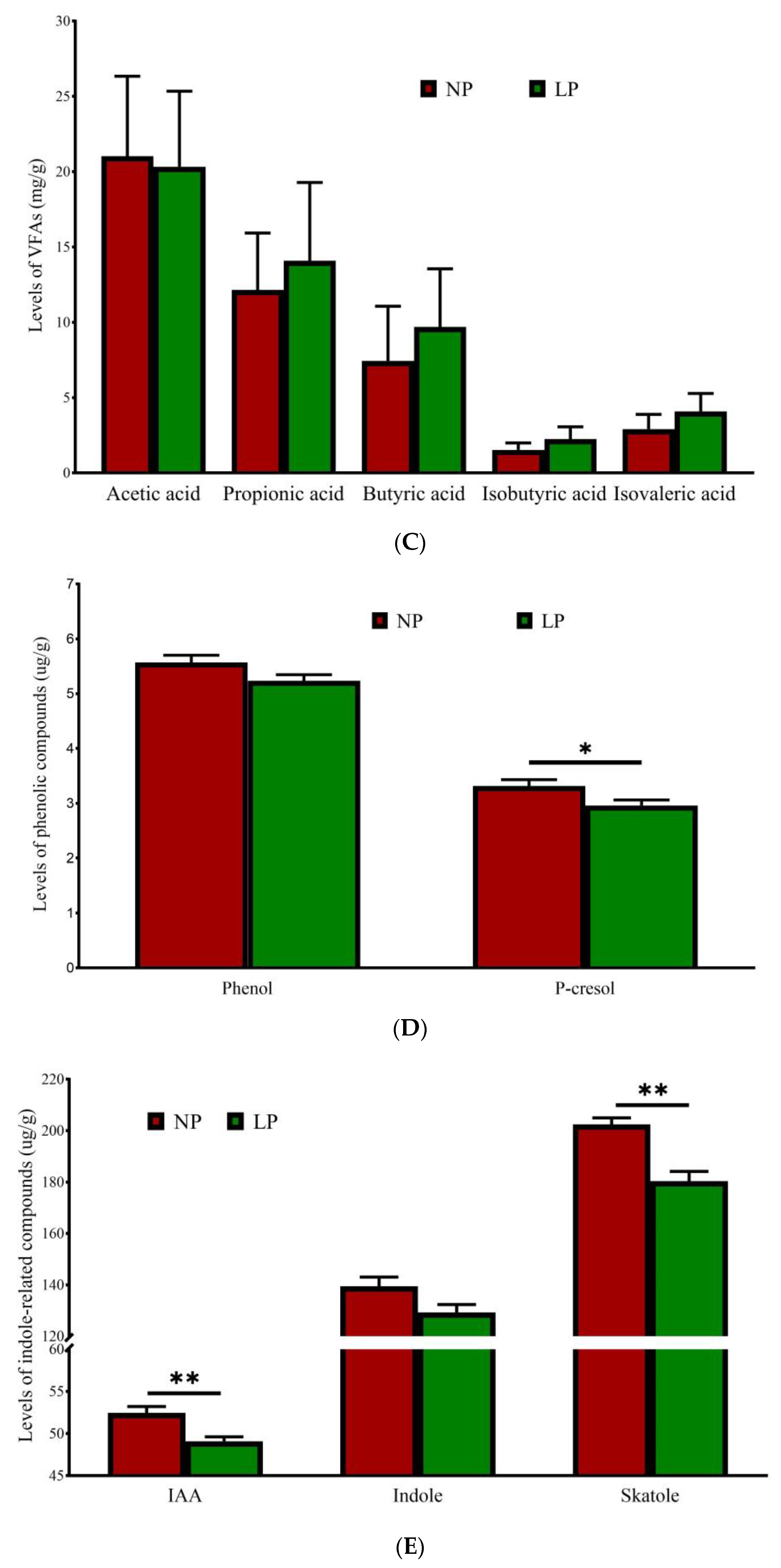
3.6. Fecal Malodorous Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhou, J.; Wang, G.; Cai, S.; Zeng, X.; Qiao, S. Advances in low-protein diets for swine. J. Anim. Sci. Biotechnol. 2018, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- van Heugten, E. Growing-Finishing Swine Nutrition Recommendations and Feeding Management. Pork Information Gateway. PIG 07-01-09. 2010. Available online: https://porkgateway.org/resource/growing-finishing-swine-nutrient-recommendations-and-feeding-management (accessed on 7 July 2025).
- National Research Council. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Qin, C.; Huang, P.; Qiu, K.; Sun, W.; Xu, L.; Zhang, X.; Yin, J. Influences of dietary protein sources and crude protein levels on intracellular free amino acid profile in the longissimus dorsi muscle of finishing gilts. J. Anim. Sci. Biotechnol. 2015, 6, 52. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Zhang, L.; Kong, X.; Li, F. Serine-to-glycine ratios in low-protein diets regulate intramuscular fat by affecting lipid metabolism and myofiber type transition in the skeletal muscle of growing-finishing pigs. Anim. Nutr. 2021, 7, 384–392. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine, 10th ed.; National Academies Press: Washington, DC, USA, 1998. [Google Scholar]
- Jiang, S.; Quan, W.; Luo, J.; Lou, A.; Zhou, X.; Li, F.; Shen, Q.W. Low-protein diets supplemented with glycine improves pig growth performance and meat quality: An untargeted metabolomic analysis. Front. Vet. Sci. 2023, 10, 1170573. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.E.; Parnsen, W.; Zhang, S.; Abreu, M.L.T.; Kim, S.W. Low crude protein formulation with supplemental amino acids for its impacts on intestinal health and growth performance of growing-finishing pigs. J. Anim. Sci. Biotechnol. 2024, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yi, X.; Zhang, G.; Lu, N.; Chu, L.; Thacker, P.A.; Qiao, S. Studies on reducing nitrogen excretion: I. Net energy requirement of finishing pigs maximizing performance and carcass quality fed low crude protein diets supplemented with crystalline amino acids. J. Anim. Sci. Biotechnol. 2011, 2, 84–93. [Google Scholar]
- Le Bellego, L.; Noblet, J. Performance and utilization of dietary energy and amino acids in piglets fed low protein diets. Livest. Prod. Sci. 2002, 76, 45–58. [Google Scholar] [CrossRef]
- Le Bellego, L.; van Milgen, J.; Dubois, S.; Noblet, J. Energy utilization of low-protein diets in growing pigs. J. Anim. Sci. 2001, 79, 1259–1271. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- McCarthy, J.F.; Aherene, F.X.; Okai, D.B. Use of HCl insoluble ash as an index material for determining apparent digestibility with pigs. Can. J. Anim. Sci. 1974, 54, 107–109. [Google Scholar] [CrossRef]
- Diao, H.; Zheng, P.; Yu, B.; He, J.; Mao, X.B.; Yu, J.; Chen, D.W. Effects of dietary supplementation with benzoic acid on intestinal morphological structure and microflora in weaned piglets. Livest. Sci. 2014, 167, 249–256. [Google Scholar] [CrossRef]
- Yang, Y.X.; Mu, C.L.; Zhang, J.F.; Zhu, W.Y. Determination of biogenic amines in digesta by high performance liquid chromatography with precolumn dansylation. Anal. Lett. 2014, 47, 1290–1298. [Google Scholar] [CrossRef]
- Wang, X.F.; Mao, S.Y.; Liu, J.H.; Zhang, L.; Cheng, Y.; Jin, W.; Zhu, W. Effect of the gynosaponin on methane production and microbe numbers in a fungus-methanogen co-culture. J. Anim. Feed Sci. 2011, 20, 272–284. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Ma, X. Hermetia illucens larvae as a potential dietary protein source altered the microbiota and modulated mucosal immune status in the colon of finishing pigs. J. Anim. Sci. Biotechnol. 2019, 10, 50. [Google Scholar] [CrossRef]
- Yang, Z.; Urriola, E.P.; Johnston, J.L.; Shurson, G.C. A systems approach to evaluate nitrogen utilization efficiency and environmental impacts of swine growing-finishing feeding programs in U.S. pork production systems. J. Anim. Sci. 2023, 101, skad188. [Google Scholar] [CrossRef]
- Song, W.; Wu, Z.; Li, W.; Li, Y. Multiple amino acid supplementations to reduce dietary protein for pigs during early and late finisher periods under commercial conditions. J. Sci. Food Agric. 2023, 103, 3205–3209. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.C.; Duarte, M.E.; Kim, S.W. Advances, implications, and limitations of low-crude-protein diets in pig production. Animals 2022, 12, 3478. [Google Scholar] [CrossRef]
- Soto, J.A.; Tokach, M.D.; Dritz, S.S.; Woodworth, J.C.; Derouchey, J.M.; Goodband, B.D.; Wu, F. Optimal dietary standardized ileal digestible lysine and crude protein concentration for growth and carcass performance in finishing pigs weighing greater than 100 kg. J. Anim. Sci. 2019, 97, 1701–1711. [Google Scholar] [CrossRef]
- Soto, J.A.; Tokach, M.D.; Dritz, S.S.; Woodworth, J.C.; Derouchey, J.M.; Goodband, B.D. Dietary supplementation of choline and potassium in low crude protein diets on growth and carcass performance of finishing pigs. J. Anim. Sci. 2018, 96 (Suppl. S2), 126. [Google Scholar] [CrossRef]
- Fang, L.H.; Jin, Y.H.; Do, S.H.; Hong, J.S.; Kim, B.O.; Han, T.H.; Kim, Y.Y. Effects of dietary energy and crude protein levels on growth performance, blood profiles, and nutrient digestibility in weaning pigs. Asian Austral. J. Anim. 2019, 32, 556–563. [Google Scholar] [CrossRef]
- Niyonsaba, N.; Jin, X.J.; Kim, Y.K. Effect of reducing dietary crude protein level on growth performance, blood profiles, nutrient digestibility, carcass traits, and odor emissions in growing-finishing pigs. Anim. Biosci. 2023, 36, 1584–1595. [Google Scholar] [CrossRef]
- Munezero, O.; Kim, I.H. Effects of protease enzyme supplementation in weanling pigs’ diet with different crude protein levels on growth performance and nutrient digestibility. J. Anim. Sci. Technol. 2022, 64, 854–862. [Google Scholar] [CrossRef]
- Han, Y.G.; Lee, G.I.; Do, S.H.; Jang, J.C.; Kim, Y.Y. The effect of reduced crude protein on growth performance, nutrient digestibility, and meat quality in weaning to finishing pigs. Animals 2023, 13, 1938. [Google Scholar] [CrossRef]
- Lala, A.O.; Oso, A.O.; Osafo, E.L.K.; Houdijk, J.G.M.; Eyarefe, D.O. Effect of high concentration of phytase supplementation on energy and nutrient digestibility of growing pigs fed with reduced dietary crude protein balanced with limiting amino acids. Livest. Sci. 2020, 240, 104143. [Google Scholar] [CrossRef]
- Kohn, R.A.; Dinneen, M.M.; Russek-Cohen, E. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats. J. Anim. Sci. 2005, 83, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Niyazov, N.S.A.; Cherepanov, G.G.; Ostrenko, K.S. Use of low-protein diets for growing pigs to reduce fecal nitrogen excretion. Ukr. J. Ecol. 2020, 10, 313–316. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, J.; Wu, Q.; Chen, J.; Yang, X.; Wang, L.; Jiang, Z. Low protein diet improves meat quality and modulates the composition of gut microbiota in finishing pigs. Front. Vet. Sci. 2022, 9, 603. [Google Scholar] [CrossRef]
- Molist, F.; Pijlman, J.; van der Aar, P.J.; Rover, M.; Ensink, J.; Corrent, E. Effect of low crude protein diets on growth performance and carcass characteristics of grower-finisher pigs. J. Anim. Sci. 2016, 94, 226–229. [Google Scholar] [CrossRef]
- Zhang, L.; Li, F.; Guo, Q.; Duan, Y.; Wang, W.; Yang, Y.; Yin, Y.; Gong, S.; Han, M.; Yin, Y. Balanced branched-chain amino acids modulate meat quality by adjusting muscle fiber type conversion and intramuscular fat deposition in finishing pigs. J. Sci. Food Agric. 2022, 102, 3796–3807. [Google Scholar] [CrossRef]
- Wang, D.; Chen, G.; Chai, M.; Shi, C.; Geng, Y.; Che, Y.; Li, Y.; Liu, S.; Gao, Y.; Hou, H. Effects of dietary protein levels on production performance, meat quality and flavor of fattening pigs. Front. Nutr. 2022, 9, 910519. [Google Scholar] [CrossRef]
- Liu, S.H.; Xie, J.Y.; Fan, Z.Y.; Ma, X.K.; Yin, Y.L. Effects of low protein diet with a balanced amino acid pattern on growth performance, meat quality and cecal microflora of finishing pigs. J. Sci. Food Agric. 2023, 103, 957–967. [Google Scholar] [CrossRef]
- Wang, D.; Chen, G.; Li, W.; Chai, M.; Zhang, H.; Su, Y. Effects of low protein diet on production performance and intestinal microbial composition in pigs. Vet. Sci. 2023, 10, 655. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, S.M.; Song, X.L.; Pan, H.B.; Li, W.Z.; Zhang, Y.Y.; Gao, S.Z.; Chen, D.W. Low protein diet up-regulate intramuscular lipogenic gene expression and down-regulate lipolytic gene expression in growth-finishing pigs. Livest. Sci. 2012, 148, 119–128. [Google Scholar] [CrossRef]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Paul, R.R.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, G.; Chen, D.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Huang, Z.; Luo, Y.; Yu, B. Dietary protein levels and amino acid supplementation patterns alter the composition and functions of colonic microbiota in pigs. Anim. Nutr. 2020, 6, 143–151. [Google Scholar] [CrossRef]
- Wang, Y.; Han, S.; Zhou, J.; Li, P.; Wang, G.; Yu, H.; Cai, S.; Zeng, X.; Johnston, L.J.; Levesque, C.L.; et al. Effects of dietary crude protein level and N-carbamylglutamate supplementation on nutrient digestibility and digestive enzyme activity of jejunum in growing pigs. J. Anim. Sci. 2020, 98, skaa088. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.Y.; Cai, M.Y.; Wang, S.S.; Lu, Y.Y.; Tang, Z.R.; Tang, Q.S.; Gao, J.C.; Xu, Y.T.; Peng, X.; Sun, Z.H. Effects of adding niacinamide to diets with normal and low protein levels on the immunity, antioxidant, and intestinal microbiota in growing-finishing pigs. J. Nutr. Biochem. 2025, 136, 109809. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.; Broomfield, M.; Jones, S.; Donovan, B. Ammonia and odour emissions from UK pig farms and nitrogen leaching from outdoor pig production. a review. Sci. Total Environ. 2014, 470, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Li, L.; Rezaei, A.; Eslamfam, S.; Che, D.; Ma, X. Metabolites of dietary protein and peptides by intestinal microbes and their impacts on gut. Curr. Protein Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhu, W.; Hang, S. Effects of low-protein diet on the intestinal morphology, digestive enzyme activity, blood urea nitrogen, and gut microbiota and metabolites in weaned pigs. Arch. Anim. Nutr. 2019, 73, 287–305. [Google Scholar] [CrossRef]
- Lee, J.Y.; Htoo, J.K.; Kluenemann, M.; González-Vega, J.C.; Nyachoti, C.M. Effects of dietary protein content and crystalline amino acid supplementation patterns in low protein diets on intestinal bacteria and their metabolites in weaned pigs raised under different sanitary conditions. J. Anim. Sci. 2023, 101, skad252. [Google Scholar] [CrossRef]
- Lee, J.Y.; González-Vega, J.C.; Htoo, J.K.; Nyachoti, C.M. Effects of dietary crude protein content and resistant starch supplementation on growth performance, intestinal histomorphology and microbial metabolites in weaned pigs. Arch. Anim. Nutr. 2024, 78, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Hwang, O.; Park, S. Effect of dietary protein levels on composition of odorous compounds and bacterial ecology in pig manure. Asian. Austral. J. Anim. 2015, 28, 1362–1370. [Google Scholar]
- Zhang, C.; Yu, M.; Yang, Y.; Mu, C.; Su, Y.; Zhu, W. Effect of early antibiotic administration on cecal bacterial communities and their metabolic profiles in pigs fed diets with different protein levels. Anaerobe 2016, 42, 188–196. [Google Scholar] [CrossRef] [PubMed]
| Item | Phase Ⅰ | Phase Ⅱ | ||
|---|---|---|---|---|
| NP | LP | NP | LP | |
| Ingredients | ||||
| Corn, % | 71.80 | 78.50 | 74.00 | 81.50 |
| Soybean meal, % | 13.35 | 5.40 | 10.90 | 3.10 |
| Wheat middling, % | 4.00 | 4.00 | 4.00 | 4.00 |
| Rapeseed meal, % | 2.00 | 2.00 | 2.00 | 2.00 |
| Extruded corn, % | 3.95 | 3.74 | 3.79 | 2.92 |
| Soybean oil, % | 1.97 | 1.87 | 1.89 | 1.46 |
| Zeolite, % | 1.71 | 2.74 | 2.24 | 2.92 |
| Limestone, % | 0.90 | 0.90 | 0.90 | 0.90 |
| CaHPO4, % | 0.50 | 0.50 | 0.50 | 0.50 |
| Salt, % | 0.40 | 0.40 | 0.40 | 0.40 |
| L-Lys·HCl, % | 0.29 | 0.52 | 0.21 | 0.43 |
| L-Thr, % | 0.08 | 0.18 | 0.05 | 0.15 |
| L-Trp, % | 0.01 | 0.05 | 0.01 | 0.04 |
| DL-Met, % | 0.01 | 0.07 | 0.00 | 0.03 |
| L-Val, % | 0.00 | 0.00 | 0.00 | 0.06 |
| L-Ile, % | 0.00 | 0.00 | 0.00 | 0.05 |
| Premix 1,2, % | 1.00 | 1.00 | 1.00 | 1.00 |
| Total, % | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrient levels 3 | ||||
| NE, MJ/kg | 10.36 | 10.36 | 10.36 | 10.36 |
| CP, % | 13.86 | 11.42 | 12.68 | 10.46 |
| Calcium, % | 0.72 | 0.69 | 0.74 | 0.68 |
| Total phosphorus, % | 0.62 | 0.59 | 0.58 | 0.54 |
| Standardized ileal digestible (SID) amino acid | ||||
| SID Lys, % | 0.73 | 0.73 | 0.61 | 0.61 |
| SID Met, % | 0.21 | 0.24 | 0.19 | 0.19 |
| SID Cys, % | 0.22 | 0.18 | 0.21 | 0.17 |
| SID Thr, % | 0.46 | 0.46 | 0.40 | 0.40 |
| SID Trp, % | 0.13 | 0.13 | 0.11 | 0.11 |
| SID Val, % | 0.49 | 0.48 | 0.46 | 0.41 |
| SID Ile, % | 0.43 | 0.39 | 0.40 | 0.33 |
| Item | Group | p-Value | |
|---|---|---|---|
| NP | LP | ||
| Initial body weight, kg | 76.23 ± 6.81 | 76.38 ± 6.46 | 0.935 |
| Phase Ⅰ (days 1–28) | |||
| Final body weight, kg | 107.3 ± 8.85 | 107.8 ± 9.19 | 0.834 |
| ADG, kg/d | 1.15 ± 0.16 | 1.16 ± 0.18 | 0.820 |
| ADFI, kg/d | 3.85 ± 0.31 | 3.97 ± 0.47 | 0.762 |
| Feed-to-gain ratio | 3.37 ± 0.33 | 3.40 ± 0.27 | 0.869 |
| Phase Ⅱ (days 29–55) | |||
| Final body weight, kg | 135.5 ± 10.31 | 138.6 ± 11.10 | 0.312 |
| ADG, kg/d | 1.05 ± 0.12 | 1.14 ± 0.18 | 0.036 |
| ADFI, kg/d | 4.01 ± 0.23 | 4.14 ± 0.24 | 0.437 |
| Feed-to-gain ratio | 3.83 ± 0.15 | 3.64 ± 0.32 | 0.260 |
| Whole period | |||
| ADG, kg/d | 1.10 ± 0.11 | 1.15 ± 0.15 | 0.150 |
| ADFI, kg/d | 3.93 ± 0.24 | 4.05 ± 0.34 | 0.549 |
| Feed-to-gain ratio | 3.58 ± 0.21 | 3.51 ± 0.23 | 0.626 |
| Items | Group | p-Value | |
|---|---|---|---|
| NP | LP | ||
| Dry matter, % | 94.15 ± 0.36 | 95.13 ± 0.42 | 0.004 |
| CP, % | 80.83 ± 0.79 | 84.24 ± 0.57 | 0.000 |
| EE, % | 74.96 ± 4.31 | 78.43 ± 2.63 | 0.163 |
| Ca, % | 54.24 ± 2.81 | 64.30 ± 3.40 | 0.001 |
| P, % | 47.98 ± 2.82 | 52.11 ± 2.16 | 0.032 |
| Item | Group | p-Value | |
|---|---|---|---|
| NP | LP | ||
| TP, g/L | 61.23 ± 4.34 | 62.34 ± 2.92 | 0.535 |
| BUN, mmol/L | 8.54 ± 1.12 | 6.67 ± 1.10 | 0.001 |
| GLU, mmol/L | 3.31 ± 0.65 | 3.52 ± 0.49 | 0.425 |
| TG, mmol/L | 0.48 ± 0.10 | 0.51 ± 0.06 | 0.393 |
| TCHO, mmol/L | 1.71 ± 0.14 | 1.20 ± 0.31 | 0.022 |
| NEFA, mmol/L | 0.12 ± 0.03 | 0.19 ± 0.03 | 0.000 |
| Item | Group | p-Value | |
|---|---|---|---|
| NP | LP | ||
| Dressing percentage, % | 81.91 ± 1.35 | 82.44 ± 1.15 | 0.370 |
| Average back fat, cm | 3.63 ± 0.54 | 3.66 ± 0.48 | 0.910 |
| Lean meat rate, % | 58.33 ± 0.33 | 58.44 ± 0.49 | 0.597 |
| pH24h value | 5.59 ± 0.16 | 5.67 ± 0.12 | 0.359 |
| Meat color L* value | 55.03 ± 3.99 | 55.34 ± 2.48 | 0.877 |
| Meat color a* value | 17.19 ± 0.94 | 17.45 ± 0.69 | 0.606 |
| Meat color b* value | 4.79 ± 0.34 | 4.46 ± 0.59 | 0.334 |
| Shear force, N | 36.10 ± 4.89 | 38.57 ± 4.93 | 0.477 |
| Drip loss, % | 4.35 ± 1.12 | 5.24 ± 1.67 | 0.371 |
| Cooking loss, % | 16.51 ± 3.96 | 14.54 ± 1.81 | 0.278 |
| Milling loss, % | 38.22 ± 4.61 | 38.93 ± 3.46 | 0.774 |
| Intramuscular fat, % | 1.71 ± 0.75 | 2.30 ± 0.86 | 0.285 |
| Item | Group | p-Value | |
|---|---|---|---|
| NP | LP | ||
| Shannon | 5.66 ± 0.22 | 5.42 ± 0.17 | 0.058 |
| Simpson | 0.93 ± 0.02 | 0.92 ± 0.01 | 0.751 |
| Chao1 | 734.49 ± 25.12 | 745.08 ± 29.07 | 0.480 |
| ACE | 743.63 ± 28.64 | 739.54 ± 32.02 | 0.800 |
| Observed species | 688.71 ± 28.08 | 679.78 ± 32.15 | 0.570 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, X.; Wu, J.; Liu, S.; Ma, Q.; Men, X.; Li, Y.; Xu, Z.; Deng, B. Reduced Dietary Protein Levels Improved Growth Performance, Promoted Efficient Nutrient Utilization, Increased Fecal Lactobacillus, and Reduced Fecal Malodorous Compounds in Late-Fattening Barrows. Animals 2025, 15, 2465. https://doi.org/10.3390/ani15162465
Tao X, Wu J, Liu S, Ma Q, Men X, Li Y, Xu Z, Deng B. Reduced Dietary Protein Levels Improved Growth Performance, Promoted Efficient Nutrient Utilization, Increased Fecal Lactobacillus, and Reduced Fecal Malodorous Compounds in Late-Fattening Barrows. Animals. 2025; 15(16):2465. https://doi.org/10.3390/ani15162465
Chicago/Turabian StyleTao, Xin, Jie Wu, Shujie Liu, Qianqian Ma, Xiaoming Men, Yongming Li, Ziwei Xu, and Bo Deng. 2025. "Reduced Dietary Protein Levels Improved Growth Performance, Promoted Efficient Nutrient Utilization, Increased Fecal Lactobacillus, and Reduced Fecal Malodorous Compounds in Late-Fattening Barrows" Animals 15, no. 16: 2465. https://doi.org/10.3390/ani15162465
APA StyleTao, X., Wu, J., Liu, S., Ma, Q., Men, X., Li, Y., Xu, Z., & Deng, B. (2025). Reduced Dietary Protein Levels Improved Growth Performance, Promoted Efficient Nutrient Utilization, Increased Fecal Lactobacillus, and Reduced Fecal Malodorous Compounds in Late-Fattening Barrows. Animals, 15(16), 2465. https://doi.org/10.3390/ani15162465






