Transcriptome Sequencing and Differential Analysis of Ovaries Across Diverse States (Follicular and Non-Follicular Phases)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Sample Collection
2.2. Experimental Methods
2.2.1. RNA Extraction, Library Construction, and Sequencing
2.2.2. Sequence Analysis
2.2.3. Selection of DEGs
2.2.4. Gene Function Enrichment Analysis
2.2.5. Quantitative Real-Time PCR (RT-qPCR) Validation
2.2.6. Protein–Protein Interaction
2.2.7. Data Analysis
3. Results and Analysis
3.1. Sequencing Data Quality Control and Mapping
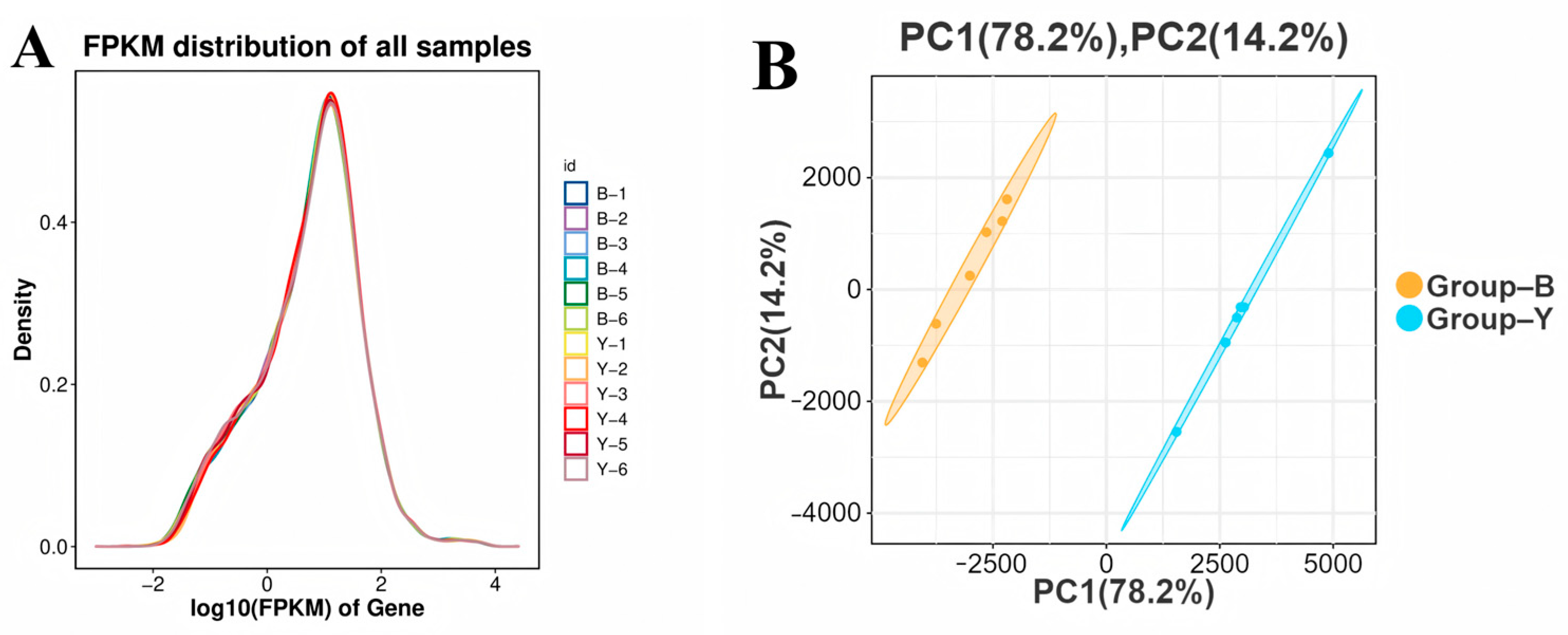
3.2. Evaluation of Sequencing Results
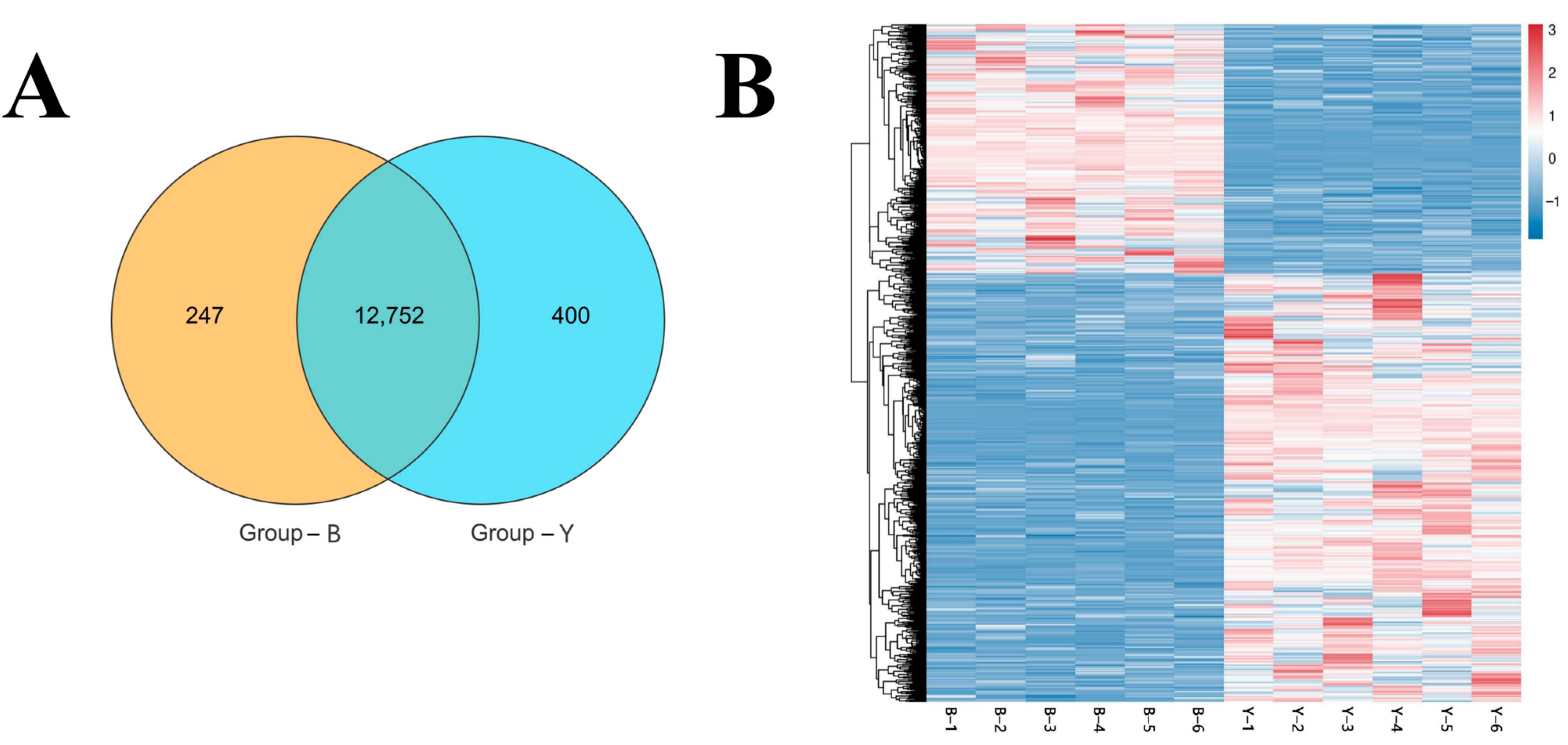
3.3. Gene Transcription, Expression, and Clustering Pattern Analysis
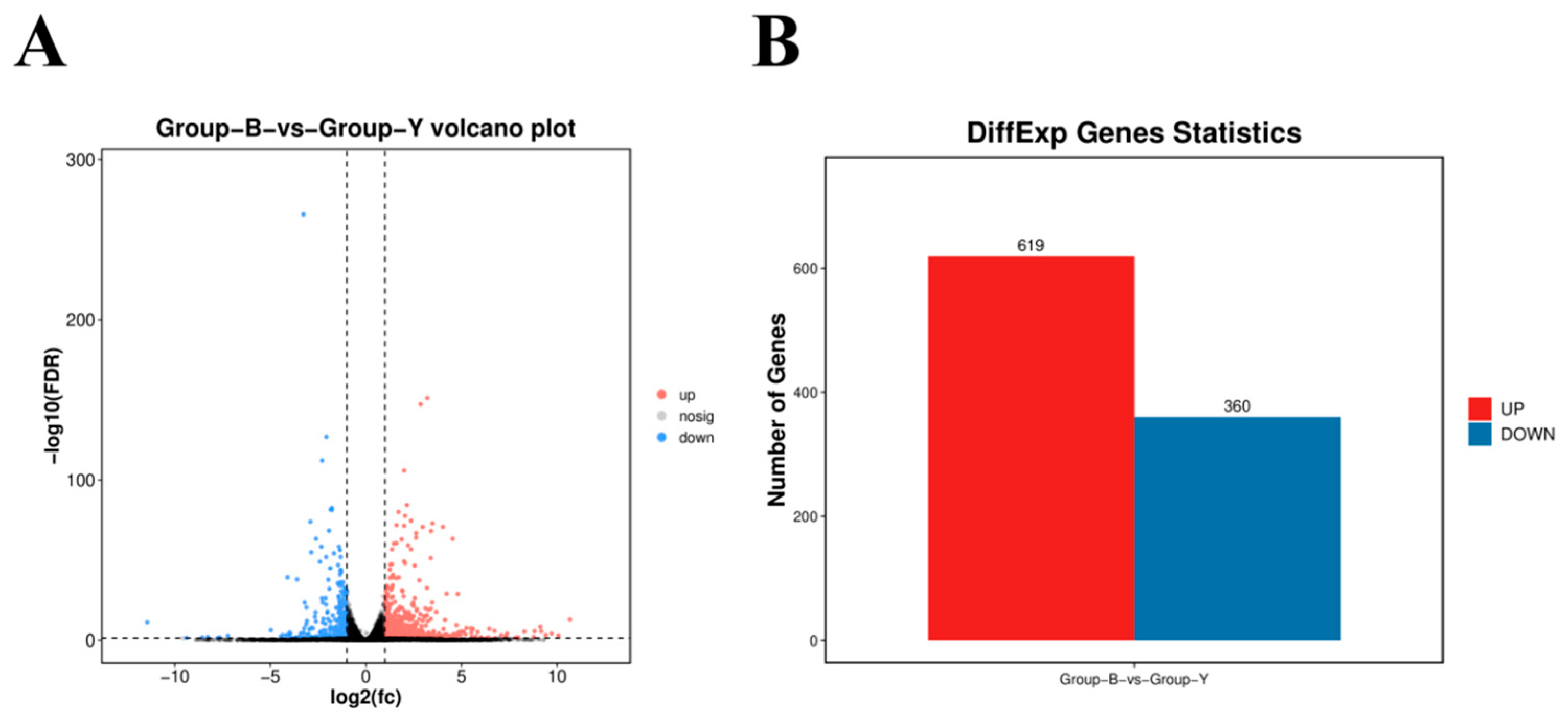
3.4. Analysis of DifferEntially Expressed Genes
3.5. GO Functional Annotation and KEGG Pathway Enrichment Analysis of DEGs
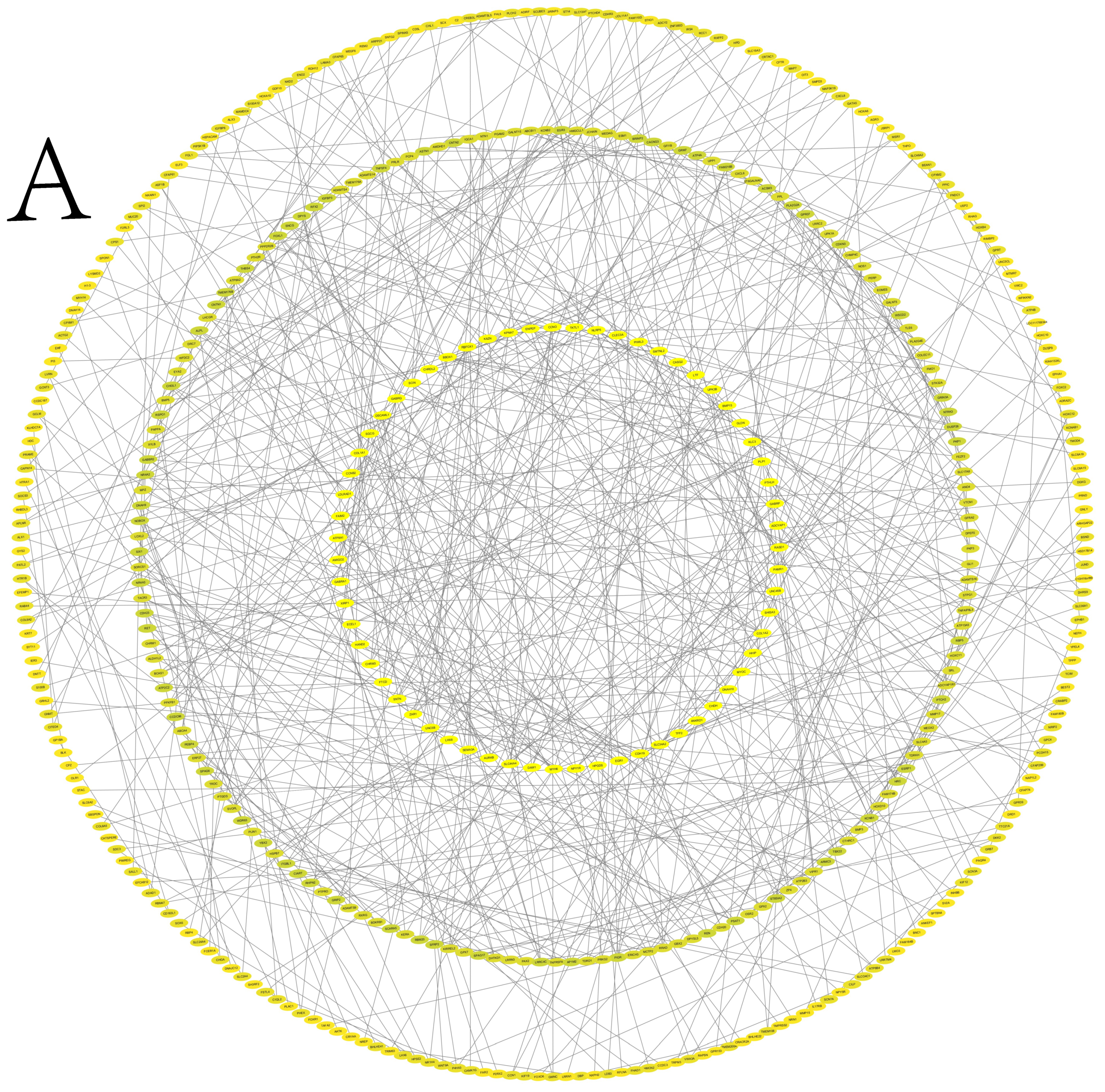
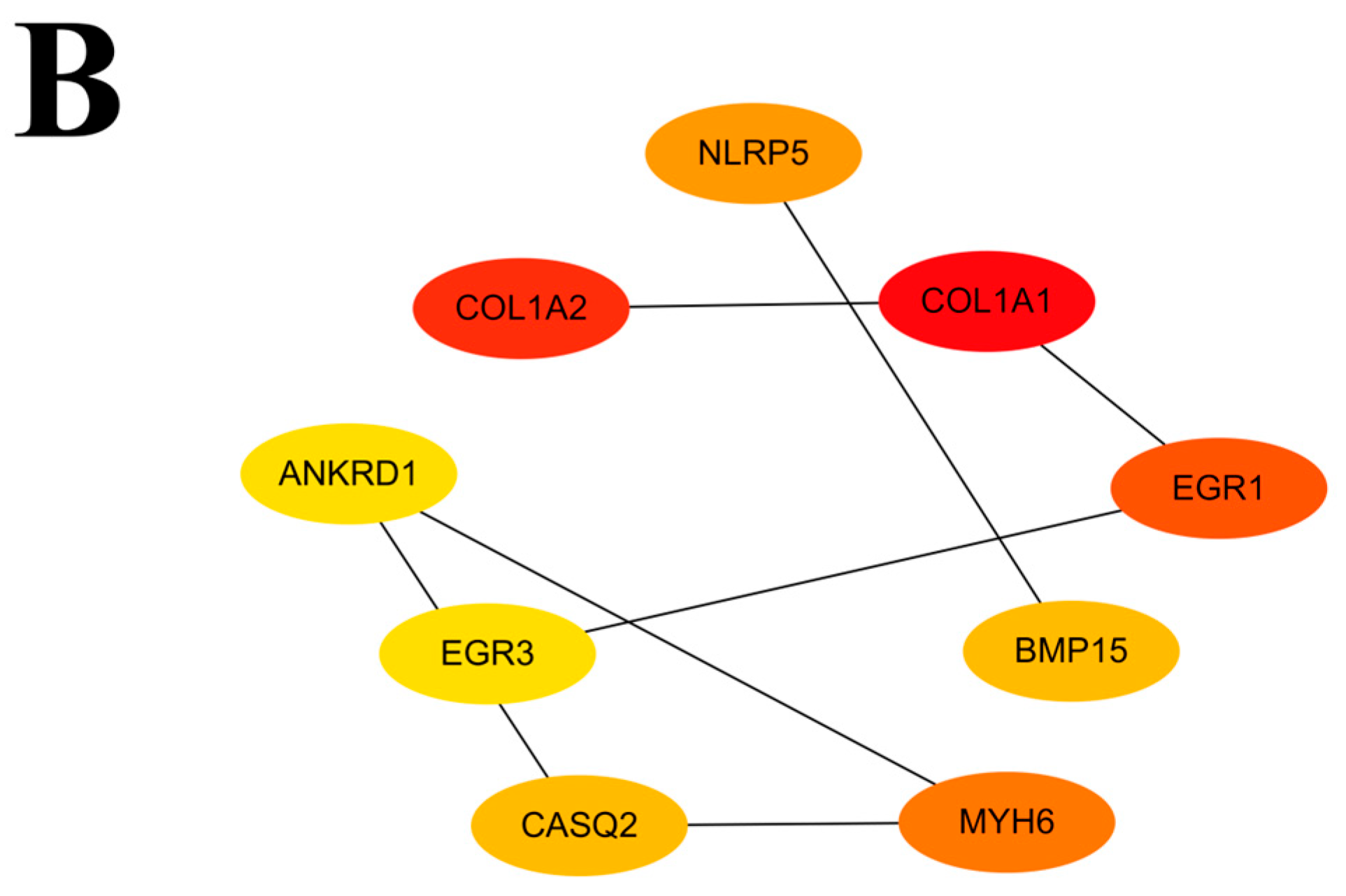
3.6. Protein–Protein Interaction Network Analysis
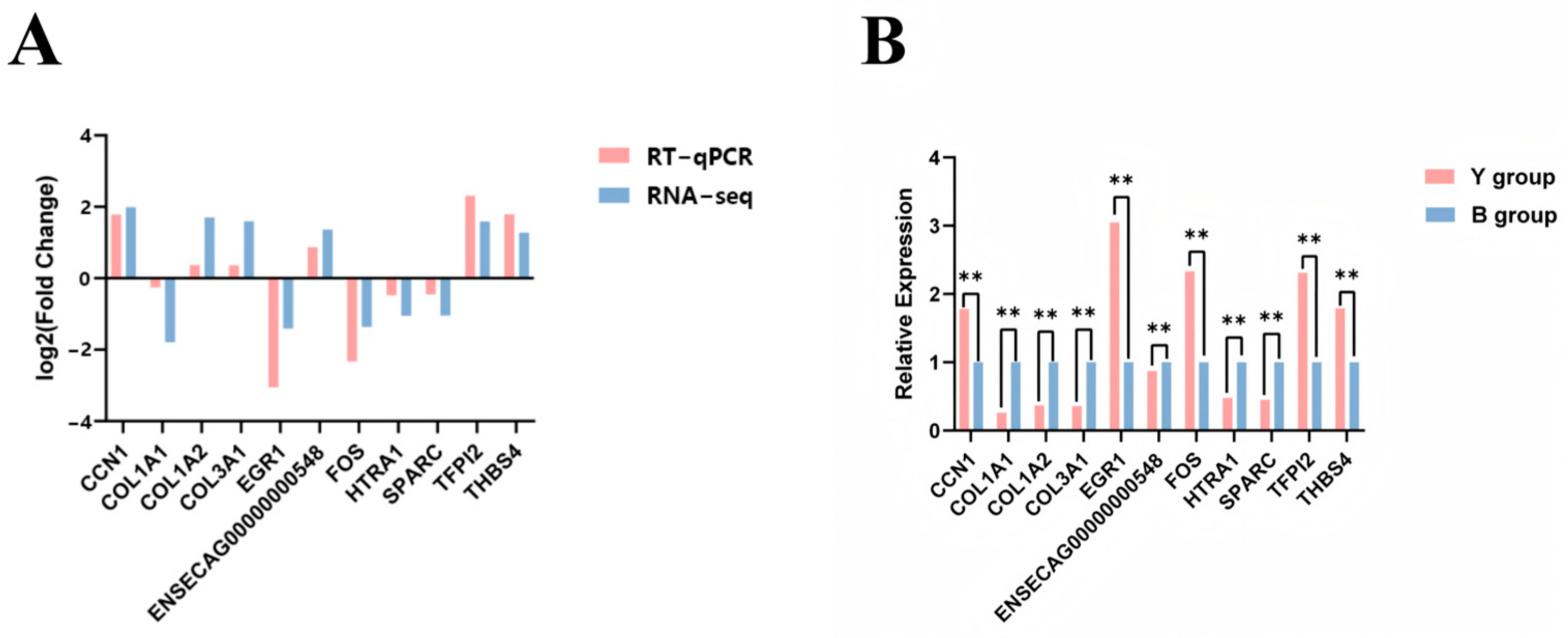
3.7. RT-qPCR Validation
4. Discussion
4.1. The Process of Follicular Development
4.2. Differential Gene Expression Analysis
4.3. KEGG Analysis of DEG
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, X.; Fang, C.; Liu, L.; Zhao, X.; Liu, W.; Cao, H.; Lv, S. Transcriptome study underling difference of milk yield during peak lactation of Kazakh horse. J. Equine Veter-Sci. 2021, 102, 103424. [Google Scholar] [CrossRef] [PubMed]
- Converse, A.; Zaniker, E.J.; Amargant, F.; Duncan, F.E. Recapitulating folliculogenesis and oogenesis outside the body: Encapsulated in vitro follicle growth. Biol. Reprod. 2023, 108, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Li, J. The art of oocyte meiotic arrest regulation. Reprod. Biol. Endocrinol. 2019, 17, 8. [Google Scholar] [CrossRef]
- Zhou, J.; Peng, X.; Mei, S. Autophagy in ovarian follicular development and atresia. Int. J. Biol. Sci. 2019, 15, 726–737. [Google Scholar] [CrossRef]
- Biswas, A.; Ng, B.H.; Prabhakaran, V.S.O.; Chan, C.J. Squeezing the eggs to grow: The mechanobiology of mammalian folliculogenesis. Front. Cell Dev. Biol. 2022, 10, 1038107. [Google Scholar] [CrossRef]
- Rybska, M.; Knap, S.; Jankowski, M.; Jeseta, M.; Bukowska, D.; Antosik, P.; Nowicki, M.; Zabel, M.; Kempisty, B.; Jaśkowski, J.M. Characteristic of factors influencing the proper course of folliculogenesis in mammals. Med. J. Cell Biol. 2018, 6, 33–38. [Google Scholar] [CrossRef]
- Young, J.M.; McNeilly, A.S. Theca: The forgotten cell of the ovarian follicle. Reproduction 2010, 140, 489. [Google Scholar] [CrossRef]
- Zhao, Z.; Zou, X.; Lu, T.; Deng, M.; Li, Y.; Guo, Y.; Sun, B.; Liu, G.; Liu, D. Identification of mRNAs and lncRNAs Involved in the Regulation of Follicle Development in Goat. Front. Genet. 2020, 11, 589076. [Google Scholar] [CrossRef]
- Ren, W.; Wang, J.; Zeng, Y.; Wang, T.; Meng, J.; Yao, X. Transcriptome identification of differential mammary genes of Kazakh horses during early pregnancy. Gene 2024, 902, 148189. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Wang, J.; Zeng, Y.; Wang, T.; Meng, J.; Yao, X. Differential age-related transcriptomic analysis of ovarian granulosa cells in Kazakh horses. Front. Endocrinol. 2024, 15, 1346260. [Google Scholar] [CrossRef]
- Botta, D.; Fuller, M.J.; Marquez-Lago, T.T.; Bachus, H.; Bradley, J.E.; Weinmann, A.S.; Zajac, A.J.; Randall, T.D.; Lund, F.E.; León, B.; et al. Dynamic regulation of T follicular regulatory cell responses by interleukin 2 during influenza infection. Nat. Immunol. 2017, 18, 1249–1260. [Google Scholar] [CrossRef]
- Kere, M.; Liu, P.-C.; Chen, Y.-K.; Chao, P.-C.; Tsai, L.-K.; Yeh, T.-Y.; Siriboon, C.; Intawicha, P.; Lo, N.-W.; Chiang, H.-I.; et al. Ultrastructural Characterization of Porcine Growing and In Vitro Matured Oocytes. Animals 2020, 10, 664. [Google Scholar] [CrossRef]
- Jones, A.S.K.; Shikanov, A. Follicle development as an orchestrated signaling network in a 3D organoid. J. Biol. Eng. 2019, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Dipaz-Berrocal, D.; Rojas, G.; Mamani, C.; Mellisho, E. 128 Study of preantral ovarian follicular population in fetal alpaca (Vicugna pacos) ovaries. Reprod. Fertil. Dev. 2020, 32, 191. [Google Scholar] [CrossRef]
- Fountas, S.; Petinaki, E.; Bolaris, S.; Kargakou, M.; Dafopoulos, S.; Zikopoulos, A.; Moustakli, E.; Sotiriou, S.; Dafopoulos, K. The Roles of GDF-9, BMP-15, BMP-4 and EMMPRIN in Folliculogenesis and In Vitro Fertilization. J. Clin. Med. 2024, 13, 3775. [Google Scholar] [CrossRef]
- Lee, E.B.; Chakravarthi, V.P.; Wolfe, M.W.; Rumi, M.A.K. ERβ Regulation of Gonadotropin Responses during Folliculogenesis. Int. J. Mol. Sci. 2021, 22, 10348. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, A.; Kim, B.; Yeh, J. Luteinizing hormone action in human oocyte maturation and quality: Signaling pathways, regulation, and clinical impact. Reprod. Sci. 2020, 27, 1223–1252. [Google Scholar] [CrossRef]
- Uyar, A.; Torrealday, S.; Seli, E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil. Steril. 2013, 99, 979–997. [Google Scholar] [CrossRef]
- Sudiman JSutton-McDowall, M.L.; Ritter, L.J.; White, M.A.; Mottershead, D.G.; Thompson, J.G.; Gilchrist, R.B. Bone morphogenetic protein 15 in the pro-mature complex form enhances bovine oocyte developmental competence. PLoS ONE 2014, 9, e103563. [Google Scholar] [CrossRef]
- Alam, M.H.; Lee, J.; Miyano, T. GDF9 and BMP15 induce development of antrum-like structures by bovine granulosa cells without oocytes. J. Reprod. Dev. 2018, 64, 423–431. [Google Scholar] [CrossRef]
- Mottershead, D.G.; Harrison, C.A.; Mueller, T.D.; Stanton, P.G.; Gilchrist, R.B.; McNatty, K.P. Growth differentiation factor 9:bone morphogenetic protein 15 (GDF9:BMP15) synergism and protein heterodimerization. Proc. Natl. Acad. Sci. USA 2013, 110, E2257. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.M.; Qiao, J.; Leung, P.C.K. Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors. Hum. Reprod. Update 2017, 23, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Portela, A.; Passos, J.R.S.; Cunha, E.V.; Silva, A.W.B.; Costa, J.J.N.; Saraiva, M.V.A.; Donato, M.A.M.; Peixoto, C.A.; Van Den Hurk, R.; et al. Effects of BMP-4 and FSH on growth, morphology, and mRNA expression of oocyte-secreted factors in cultured bovine secondary follicles. Anim. Reprod. (AR) 2018, 12, 910–919. [Google Scholar]
- Magro-Lopez, E.; Muñoz-Fernández, M.Á. The role of BMP signaling in female reproductive system development and function. Int. J. Mol. Sci. 2021, 22, 11927. [Google Scholar] [CrossRef]
- Dalbies-Tran, R.; Cadoret, V.; Desmarchais, A.; Elis, S.; Maillard, V.; Monget, P.; Monniaux, D.; Reynaud, K.; Saint-Dizier, M.; Uzbekova, S. A comparative analysis of oocyte development in mammals. Cells 2020, 9, 1002. [Google Scholar] [CrossRef]
- Qin, Y.; Tang, T.; Li, W.; Liu, Z.; Yang, X.; Shi, X.; Sun, G.; Liu, X.; Wang, M.; Liang, X.; et al. Bone morphogenetic protein 15 knockdown inhibits porcine ovarian follicular development and ovulation. Front. Cell Dev. Biol. 2019, 7, 286. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, W.; Zhou, J.; Zhang, R.; Lin, X.; Sooranna, S.R.; Deng, Y.; Shi, D. Epigenetic modifications of gonadotropin receptors can regulate follicular development. Anim. Reprod. Sci. 2024, 268, 107534. [Google Scholar] [CrossRef]
- Wang, X.; Di, R.; Liu, Q.; Hu, W.; Ma, L.; Chu, M. The role of BMP15 and GDF9 mutations in follicular development in mice and sheep and their impact on ovulation number. J. Agric. Biotechnol. 2020, 28, 1870–1880. [Google Scholar]
- Giroto, A.B.; Chaves, M.P.; dos Santos, P.H.; Fontes, P.K.; Nunes, S.G.; Manssur, T.S.B.; Mendes, L.O.; Castilho, A.C.d.S. Expression of luteinizing hormone receptor during development of bovine fetal ovary. Anim. Reprod. 2024, 21, e20230112. [Google Scholar] [CrossRef]
- Convissar, S.; Winston, N.J.; Fierro, M.A.; Scoccia, H.; Zamah, A.M.; Stocco, C. Sp1 regulates steroidogenic genes and LHCGR expression in primary human luteinized granulosa cells. J. Steroid Biochem. Mol. Biol. 2019, 190, 183–192. [Google Scholar] [CrossRef]
- Rawan, A.F.; Langar, H.; Munetomo, M.; Yamamoto, Y.; Kawano, K.; Kimura, K. Effects of insulin-like growth factor-1 on the mRNA expression of estradiol receptors, steroidogenic enzymes, and steroid production in bovine follicles. J. Reprod. Dev. 2023, 69, 337–346. [Google Scholar] [CrossRef]
- Pastorello, M. Hormone Receptors and Steroidogenic Enzymes in Oocytes, Cumulus and Granulosa Cells, Follicular Fluid Hormone Milieu, and Follicle Blood Perfusion in Mares. Master’s Thesis, Southern Illinois University at Carbondale, Carbondale, IL, USA, 2021. [Google Scholar]
- Casarini, L.; Lispi, M.; Longobardi, S.; Milosa, F.; La Marca, A.; Tagliasacchi, D.; Pignatti, E.; Simoni, M.; Gromoll, J. LH and hCG action on the same receptor results in quantitatively and qualitatively different intracellular signalling. PLoS ONE 2012, 7, e46682. [Google Scholar] [CrossRef]
- Gulevskyy, A.K.; Akhatova, Y.S. Chorionic Gonadotropine: Structural Heterogeneity, Metabolic Pathway, Functions, Obtaining and Possibilities of Clinical Application. Biotechnol. Acta 2021, 14, 5–21. [Google Scholar] [CrossRef]
- Sun, T.; Xiao, C.; Yang, Z.; Deng, J.; Yang, X. Grade follicles transcriptional profiling analysis in different laying stages in chicken. BMC Genom. 2022, 23, 492. [Google Scholar] [CrossRef]
- Shen, L.; Liu, J.; Luo, A.; Wang, S. The stromal microenvironment and ovarian aging: Mechanisms and therapeutic opportunities. J. Ovarian Res. 2023, 16, 237. [Google Scholar] [CrossRef]
- Grosbois, J.; Bailie, E.C.; Kelsey, T.W.; Anderson, R.A.; Telfer, E.E. Spatio-temporal remodelling of the composition and architecture of the human ovarian cortical extracellular matrix during in vitro culture. Hum. Reprod. 2023, 38, 444–458. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, L.; Zeng, T.; Du, X.; Tao, Z.; Li, G.; Zhong, S.; Wen, J.; Zhou, C.; Xu, X.; et al. Transcriptome analyses of potential regulators of pre-and post-ovulatory follicles in the pigeon (Columba livia). Reprod. Fertil. Dev. 2022, 34, 689–697. [Google Scholar] [CrossRef]
- Gross, J.; Knipper, M.; Mazurek, B. Candidate Key Proteins in Tinnitus-A Bioinformatic Study of Synaptic Transmission in the Cochlear Nucleus. Biomedicines 2024, 12, 1615. [Google Scholar] [CrossRef]
- Li, L.; Shi, X.; Shi, Y.; Wang, Z. The signaling pathways involved in ovarian follicle development. Front. Physiol. 2021, 12, 730196. [Google Scholar] [CrossRef]
- Monte, A.P.O.; Bezerra, M.É.S.; Menezes, V.G.; Gouveia, B.B.; Barberino, R.S.; Lins, T.L.B.G.; Barros, V.R.P.; Santos, J.M.S.; Donfack, N.J.; Matos, M.H.T. Involvement of phosphorylated Akt and FOXO3a in the effects of growth and differentiation Factor-9(GDF-9)on inhibition of follicular apoptosis and induction of granulosa cell proliferation after in vitro culture of sheep ovarian tissue. Reprod. Sci. 2021, 28, 2174–2185. [Google Scholar] [CrossRef]
- Dorfman, M.D.; Garcia-Rudaz, C.; Alderman, Z.; Kerr, B.; Lomniczi, A.; Dissen, G.A.; Castellano, J.M.; Garcia-Galiano, D.; Gaytan, F.; Xu, B.; et al. Loss of Ntrk2/Kiss1r signaling in oocytes causes premature ovarian failure. Endocrinology 2014, 155, 3098–3111. [Google Scholar] [CrossRef]
- Song, P.; Yue, Q.; Fu, Q.; Li, X.; Li, X.; Zhou, R.; Chen, X.; Tao, C. Integrated analysis of miRNA–mRNA interaction in ovaries of Turpan Black Sheep during follicular and luteal phases. Reprod. Domest. Anim. 2021, 56, 46–57. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Du, H.; Ruan, R.; Luo, J.; Qiao, X.; Liu, Z.; Liu, Y.; Xu, Q.; Yu, T.; et al. Transcriptome and lipidomics profiling of F2 generation female Chinese sturgeon (Acipenser sinensis) in response to different arachidonic acid diets. Aquac. Rep. 2022, 23, 101020. [Google Scholar] [CrossRef]
- Tahir, M.S.; Nguyen, L.T.; Schulz, B.L.; Boe-Hansen, G.A.; Thomas, M.G.; Moore, S.S.; Lau, L.Y.; Fortes, M.R.S. Proteomics recapitulates ovarian proteins relevant to puberty and fertility in Brahman Heifers (Bos indicus L.). Genes 2019, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Cheng, X.; Zhang, H.; Li, J.; Li, L.; Wei, H.; Zhang, S. Cholic acid inhibits ovarian steroid hormone synthesis and follicular development through farnesoid X receptor signaling in mice. Int. J. Biol. Macromol. 2025, 301, 140458. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zou, X.; Yang, Z.; Lei, F.; Chen, Y.; Liu, G.; Sun, B.; Liu, D.; Li, Y. Analysis of follicular transcriptome in goat ovarian matrix based on high-throughput sequencing. J. South China Agric. Univ. 2020, 41, 23–32. [Google Scholar]
- Liu, S.; Chen, J.; Liu, M.; Zhang, C.; Chao, X.; Yang, H.; Wang, T.; Bi, H.; Ding, Y.; Wang, Z.; et al. miR-107 suppresses porcine granulosa cell proliferation and estradiol synthesis while promoting apoptosis via targeting PTGS2. Theriogenology 2025, 238, 117367. [Google Scholar] [CrossRef] [PubMed]
- Naser, R.; Fakhoury, I.; El-Fouani, A.; Abi-Habib, R.; El-Sibai, M. Role of the tumor microenvironment in cancer hallmarks and targeted therapy. Int. J. Oncol. 2022, 62, 23. [Google Scholar] [CrossRef]
- Zhao, J.; Pan, H.; Liu, Y.; He, Y.; Shi, H.; Ge, G. Interacting networks of the hypothalamic–pituitary–ovarian axis regulate layer hens’ performance. Genes 2023, 14, 141. [Google Scholar] [CrossRef]
- Young Park, E.; Hee Park, J.; Mai, N.T.Q.; Moon, B.S.; Choi, J.K. Control of the growth and development of murine preantral follicles in a biomimetic ovary using a decellularized porcine scaffold. Mater. Today Bio 2023, 23, 100824. [Google Scholar] [CrossRef]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef]
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012, 58, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulou, K.; Piperigkou, Z.; Tzaferi, K.; Karamanos, N.K. Trends in extracellular matrix biology. Mol. Biol. Rep. 2023, 50, 853–863. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Zeng, X. Identification of hub genes associated with follicle development in multiple births sheep by WGCNA. Front. Vet. Sci. 2022, 9, 1057282. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.M.; da Silveira, J.C.; Perrini, C.; Del Collado, M.; Gebremedhn, S.; Tesfaye, D.; Meirelles, F.V.; Perecin, F.; Zhang, M. The role of the PI3K-Akt signaling pathway in the developmental competence of bovine oocytes. PLoS ONE 2017, 12, e0185045. [Google Scholar] [CrossRef] [PubMed]



| Group | Sample | Raw Reads | Clean Reads | N (%) | Q20 (%) | Q30 (%) | Clean Reads% |
|---|---|---|---|---|---|---|---|
| B Group | B-1 | 37,829,074 | 37,774,698 | 0.03 | 98.35 | 96.00 | 99.86 |
| B-2 | 44,282,326 | 44,229,932 | 0.03 | 98.58 | 96.51 | 99.88 | |
| B-3 | 45,315,170 | 45,185,390 | 0.15 | 98.82 | 96.83 | 99.71 | |
| B-4 | 43,952,288 | 43,784,598 | 0.19 | 98.91 | 97.15 | 99.62 | |
| B-5 | 47,014,742 | 46,965,604 | 0.02 | 98.41 | 96.15 | 99.90 | |
| B-6 | 37,822,500 | 37,786,314 | 0.02 | 98.38 | 96.10 | 99.90 | |
| Y Group | Y-1 | 50,051,426 | 49,991,394 | 0.04 | 98.51 | 96.39 | 99.88 |
| Y-2 | 36,019,928 | 35,975,982 | 0.02 | 98.65 | 96.68 | 99.88 | |
| Y-3 | 43,578,570 | 43,257,576 | 0.03 | 98.50 | 96.42 | 99.26 | |
| Y-4 | 43,430,892 | 43,270,734 | 0.20 | 98.91 | 97.15 | 99.63 | |
| Y-5 | 45,454,976 | 45,356,876 | 0.08 | 98.83 | 96.90 | 99.78 | |
| Y-6 | 47,604,614 | 47,558,110 | 0.02 | 98.28 | 95.85 | 99.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Wang, T.; Xue, Y.; Shen, Z.; Meng, C.; Yao, X.; Meng, J.; Wang, J.; Chu, H.; Ren, W.; et al. Transcriptome Sequencing and Differential Analysis of Ovaries Across Diverse States (Follicular and Non-Follicular Phases). Animals 2025, 15, 2436. https://doi.org/10.3390/ani15162436
Sun J, Wang T, Xue Y, Shen Z, Meng C, Yao X, Meng J, Wang J, Chu H, Ren W, et al. Transcriptome Sequencing and Differential Analysis of Ovaries Across Diverse States (Follicular and Non-Follicular Phases). Animals. 2025; 15(16):2436. https://doi.org/10.3390/ani15162436
Chicago/Turabian StyleSun, Jiabei, Tongliang Wang, Yuheng Xue, Zhehong Shen, Chen Meng, Xinkui Yao, Jun Meng, Jianwen Wang, Hongzhong Chu, Wanlu Ren, and et al. 2025. "Transcriptome Sequencing and Differential Analysis of Ovaries Across Diverse States (Follicular and Non-Follicular Phases)" Animals 15, no. 16: 2436. https://doi.org/10.3390/ani15162436
APA StyleSun, J., Wang, T., Xue, Y., Shen, Z., Meng, C., Yao, X., Meng, J., Wang, J., Chu, H., Ren, W., Li, L., & Zeng, Y. (2025). Transcriptome Sequencing and Differential Analysis of Ovaries Across Diverse States (Follicular and Non-Follicular Phases). Animals, 15(16), 2436. https://doi.org/10.3390/ani15162436





