Refractory Multiple Myeloma in a West Highland White Terrier: Clinical Presentations and Therapeutic Interventions
Simple Summary
Abstract
1. Introduction
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MM | Multiple myeloma |
| A | Doxorubicin |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| ASCT | Autologous stem cell transplantation |
| B | Bortezomib |
| BUN | Blood urea nitrogen |
| C | Carfilzomib |
| CR | Complete remission |
| CT | Computed tomography |
| CTV | Clinical target volume |
| Cy | Cyclophosphamide |
| D | Dexamethasone |
| FNA | Fine-needle aspiration |
| GLOB | Globulin |
| GTV | Gross tumor volume |
| HVS | Hyperviscosity syndrome |
| I | Ixazomib |
| L | Lomustine |
| M | Melphalan |
| MP | Melphalan and prednisolone |
| MRI | Magnetic resonance imaging |
| NSAID | Non-steroidal anti-inflammatory drug |
| ORRs | Overall response rates |
| P | Prednisolone |
| RT | Radiation therapy |
| S | Sorafenib |
| SINEs | Selective inhibitors of nuclear export |
| T | Toceranib |
| TKIs | Tyrosine kinase inhibitors |
| TP | Total protein |
| V | Vincristine |
| VAD | Vincristine-doxorubicin-dexamethasone |
| VCOG-CTCAE v2 | Veterinary Cooperative Oncology Group-Common Terminology Criteria for Adverse Events |
| Ver | Verdinexor |
| VMAT | Volumetric modulated arc therapy |
| XPO1 | Inhibitor of exportin-1 |
References
- Osborne, C.A.; Perman, V.; Sautter, J.H.; Stevens, J.B.; Hanlon, G.F. Multiple myeloma in the dog. J. Am. Vet. Med. Assoc. 1968, 153, 1300–1319. [Google Scholar]
- MacEwen, E.G.; Hurvitz, A.I. Diagnosis and management of monoclonal gammopathies. Vet. Clin. N. Am. 1977, 7, 119–132. [Google Scholar] [CrossRef]
- Thrall, M.A. Lymphoproliferative disorders. Lymphocytic leukemia and plasma cell myeloma. Vet. Clin. N. Am. Small. Anim. Pract. 1981, 11, 321–347. [Google Scholar] [CrossRef]
- Priester, W.A.; McKay, F.W. The Occurrence of Tumors in Domestic Animals; National Cancer Institute: Bethesda, MD, USA, 1980; pp. 1–210. [Google Scholar]
- Ettinger, S.J.; Feldman, E.C.; Côté, E. Ettinger’s Textbook of Veterinary Internal Medicine-eBook: Ettinger’s Textbook of Veterinary Internal Medicine-eBook, 9th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Matus, R.E.; Leifer, C.E.; MacEwen, E.G.; Hurvitz, A.I. Prognostic factors for multiple myeloma in the dog. J. Am. Vet. Med. Assoc. 1986, 188, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.; Chon, E. Comparison of two melphalan protocols and evaluation of outcome and prognostic factors in multiple myeloma in dogs. J. Vet. Intern. Med. 2018, 32, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R.; Bertolini, D.R. Bone destruction and hypercalcemia in plasma cell myeloma. Semin. Oncol. 1986, 13, 291–299. [Google Scholar]
- Teddy, L.; Sylvester, S.R.; O’Connor, K.S.; Hume, K.R. Cyclical 10-day dosing of melphalan for canine multiple myeloma. Vet. Comp. Oncol. 2023, 21, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Christensen, N.; Langova, V. Long-term survival of a dog with biclonal multiple myeloma. Aus. Vet. Pract. 2014, 44, 621. [Google Scholar]
- Vail, D.M.; Thamm, D.H.; Liptak, J.M. Withrow and MacEwen’s Small Animal Clinical Oncology-E-Book, 6th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Tsang, R.W.; Campbell, B.A.; Goda, J.S.; Kelsey, C.R.; Kirova, Y.M.; Parikh, R.R.; Ng, A.K.; Ricardi, U.; Suh, C.O.; Mauch, P.M.; et al. Radiation Therapy for Solitary Plasmacytoma and Multiple Myeloma: Guidelines From the International Lymphoma Radiation Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 794–808. [Google Scholar] [CrossRef]
- Elliott, J.; Looper, J.; Keyerleber, M.; Turek, M.; Blackwood, L.; Henry, J.; Gieger, T. Response and outcome following radiation therapy of macroscopic canine plasma cell tumours. Vet. Comp. Oncol. 2020, 18, 718–726. [Google Scholar] [CrossRef]
- Reising, A.J.; Donnelly, L.L.; Flesner, B.K.; Maitz, C.A.; Bryan, J.N. Solitary osseous plasmacytomas in dogs: 13 cases (2004–2019). J. Small. Anim. Pract. 2021, 62, 1114–1121. [Google Scholar] [CrossRef]
- Rusbridge, C.; Wheeler, S.J.; Lamb, C.R.; Page, R.L.; Carmichael, S.; Brearley, M.J.; Bjornson, A.P. Vertebral plasma cell tumors in 8 dogs. J. Vet. Intern. Med. 1999, 13, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Johannes, C.M. What you need to know about new cancer treatments for dogs. Today’s Vet. Pr. 2022, 12, 42–48. [Google Scholar]
- Saba, C.F.; Vickery, K.R.; Clifford, C.A.; Burgess, K.E.; Phillips, B.; Vail, D.M.; Wright, Z.M.; Morges, M.A.; Fan, T.M.; Thamm, D.H. Rabacfosadine for relapsed canine B-cell lymphoma: Efficacy and adverse event profiles of 2 different doses. Vet. Comp. Oncol. 2018, 16, E76–E82. [Google Scholar] [CrossRef]
- Weishaar, K.M.; Wright, Z.M.; Rosenberg, M.P.; Post, G.S.; McDaniel, J.A.; Clifford, C.A.; Phillips, B.S.; Bergman, P.J.; Randall, E.K.; Avery, A.C.; et al. Multicenter, randomized, double-blinded, placebo-controlled study of rabacfosadine in dogs with lymphoma. J. Vet. Intern. Med. 2022, 36, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, A.R.; Gardner, H.L.; Borgatti, A.; Wilson, H.; Vail, D.M.; Lachowicz, J.; Manley, C.; Turner, A.; Klein, M.K.; Waite, A.; et al. Phase II study of the oral selective inhibitor of nuclear export (SINE) KPT-335 (verdinexor) in dogs with lymphoma. BMC Vet. Res. 2018, 14, 250. [Google Scholar] [CrossRef]
- Tani, H.; Miyamoto, R.; Miyazaki, T.; Oniki, S.; Tamura, K.; Bonkobara, M. A feline case of multiple myeloma treated with bortezomib. BMC Vet. Res. 2022, 18, 384. [Google Scholar] [CrossRef]
- Sonneveld, P.; Dimopoulos, M.A.; Boccadoro, M.; Quach, H.; Ho, P.J.; Beksac, M.; Hulin, C.; Antonioli, E.; Leleu, X.; Mangiacavalli, S.; et al. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2024, 390, 301–313. [Google Scholar] [CrossRef]
- Kouroukis, T.C.; Baldassarre, F.G.; Haynes, A.E.; Imrie, K.; Reece, D.E.; Cheung, M.C. Bortezomib in multiple myeloma: Systematic review and clinical considerations. Curr. Oncol. 2014, 21, e573–e603. [Google Scholar] [CrossRef]
- Kapoor, P.; Ramakrishnan, V.; Rajkumar, S.V. Bortezomib combination therapy in multiple myeloma. Semin. Hematol. 2012, 49, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Cantadori, L.O.; Gaiolla, R.D.; Nunes-Nogueira, V.D.S. Effect of bortezomib on the treatment of multiple myeloma: A systematic review protocol. BMJ Open 2022, 12, e061808. [Google Scholar] [CrossRef]
- Mitsiades, C.S.; Mitsiades, N.S.; McMullan, C.J.; Poulaki, V.; Shringarpure, R.; Akiyama, M.; Hideshima, T.; Chauhan, D.; Joseph, M.; Libermann, T.A.; et al. Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell 2004, 5, 221–230. [Google Scholar] [CrossRef]
- Lind, J.; Czernilofsky, F.; Vallet, S.; Podar, K. Emerging protein kinase inhibitors for the treatment of multiple myeloma. Expert Opin. Emerg. Drugs 2019, 24, 133–152. [Google Scholar] [CrossRef]
- LeBlanc, A.K.; Atherton, M.; Bentley, R.T.; Boudreau, C.E.; Burton, J.H.; Curran, K.M.; Dow, S.; Giuffrida, M.A.; Kellihan, H.B.; Mason, N.J.; et al. Veterinary Cooperative Oncology Group-Common Terminology Criteria for Adverse Events (VCOG-CTCAE v2) following investigational therapy in dogs and cats. Vet. Comp. Oncol. 2021, 19, 311–352. [Google Scholar] [CrossRef] [PubMed]
- Alexanian, R.; Barlogie, B.; Tucker, S. VAD-based regimens as primary treatment for multiple myeloma. Am. J. Hematol. 1990, 33, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Akiyoshi, H.; Shimazaki, H.; Aoki, M.; Ohashi, F. VAD chemotherapy regimen in a young dog with multiple myeloma. Jpn. J. Vet. Med. Assoc. 2010, 63, 797–801. [Google Scholar] [CrossRef]
- Chen, B.; Cai, L.; Zhou, F. Management of acute spinal cord compression in multiple myeloma. Crit. Rev. Oncol. Hematol. 2021, 160, 103205. [Google Scholar] [CrossRef]
- Trivedi, R.J. Spinal cord compression as a consequence of spinal plasmacytoma in a patient with multiple myeloma: A case report. Clin. Pract. 2021, 11, 124–130. [Google Scholar] [CrossRef]
- Elhammali, A.; Amini, B.; Ludmir, E.B.; Gunther, J.R.; Milgrom, S.A.; Pinnix, C.C.; Andraos, T.; Yoder, A.; Weber, D.; Orlowski, R.; et al. New paradigm for radiation in multiple myeloma: Lower yet effective dose to avoid radiation toxicity. Haematologica 2020, 105, e355–e357. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Gilbo, P.; Meshrekey, R.; Ghaly, M. Local Radiation Therapy for Palliation in Patients With Multiple Myeloma of the Spine. Front. Oncol. 2019, 9, 601. [Google Scholar] [CrossRef]
- Vlodaver, E.M.; Keating, M.K.; Bidot, W.A.; Bruyette, D.S.; Rosenkrantz, W.S. Efficacy of verdinexor for the treatment of naive canine epitheliotropic cutaneous T-cell lymphoma: An open-label pilot study. Vet. Dermatol. 2024, 35, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Londhe, P.; Gutwillig, M.; London, C. Targeted Therapies in Veterinary Oncology. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 917–931. [Google Scholar] [CrossRef]
- Vogl, D.T.; Dingli, D.; Cornell, R.F.; Huff, C.A.; Jagannath, S.; Bhutani, D.; Zonder, J.; Baz, R.; Nooka, A.; Richter, J.; et al. Selective Inhibition of Nuclear Export With Oral Selinexor for Treatment of Relapsed or Refractory Multiple Myeloma. J. Clin. Oncol. 2018, 36, 859–866. [Google Scholar] [CrossRef]
- Gounder, M.M.; Zer, A.; Tap, W.D.; Salah, S.; Dickson, M.A.; Gupta, A.A.; Keohan, M.L.; Loong, H.H.; D’Angelo, S.P.; Baker, S.; et al. Phase IB Study of Selinexor, a First-in-Class Inhibitor of Nuclear Export, in Patients With Advanced Refractory Bone or Soft Tissue Sarcoma. J. Clin. Oncol. 2016, 34, 3166–3174. [Google Scholar] [CrossRef]
- Kuruvilla, J.; Savona, M.; Baz, R.; Mau-Sorensen, P.M.; Gabrail, N.; Garzon, R.; Stone, R.; Wang, M.; Savoie, L.; Martin, P.; et al. Selective inhibition of nuclear export with selinexor in patients with non-Hodgkin lymphoma. Blood 2017, 129, 3175–3183. [Google Scholar] [CrossRef]
- Karaszewski, K.; Jędrzejczak, W.W. Selinexor and Other Selective Inhibitors of Nuclear Export (SINEs)—A Novel Approach to Target Hematologic Malignancies and Solid Tumors. Drugs Drug Candidates 2023, 2, 459–476. [Google Scholar] [CrossRef]
- Saba, R.; Saleem, N.; Peace, D. Long-term survival consequent on the abscopal effect in a patient with multiple myeloma. BMJ Case Rep. 2016, 2016, bcr2016215237. [Google Scholar] [CrossRef]
- Kazandjian, D.; Dew, A.; Hill, E.; Ramirez, E.G.; Morrison, C.; Mena, E.; Lindenberg, L.; Yuan, C.; Maric, I.; Wang, H.W.; et al. Avelumab, a PD-L1 Inhibitor, in Combination with Hypofractionated Radiotherapy and the Abscopal Effect in Relapsed Refractory Multiple Myeloma. Oncologist 2021, 26, 288-e541. [Google Scholar] [CrossRef]
- Gandhi, U.H.; Senapedis, W.; Baloglu, E.; Unger, T.J.; Chari, A.; Vogl, D.; Cornell, R.F. Clinical Implications of Targeting XPO1-mediated Nuclear Export in Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2018, 18, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Kumar, S.; Lonial, S.; Mateos, M.V. Smoldering multiple myeloma current treatment algorithms. Blood Cancer J. 2022, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Patatsos, K.; Shekhar, T.M.; Hawkins, C.J. Pre-clinical evaluation of proteasome inhibitors for canine and human osteosarcoma. Vet. Comp. Oncol. 2018, 16, 544–553. [Google Scholar] [CrossRef]
- Prevedel, N.E.; Mee, M.W.; Wood, G.A.; Coomber, B.L. Effect of proteasome inhibitors on canine lymphoma cell response to CHOP chemotherapy in vitro. Vet. Comp. Oncol. 2024, 22, 96–105. [Google Scholar] [CrossRef]
- Kodera, Y.; Iguchi, T.; Kato, D.; Ikeda, N.; Shinada, M.; Aoki, S.; Soga, K.; Li, T.; Ohata, R.; Koseki, S.; et al. Anti-tumor effect of proteasome inhibitor on canine urothelial carcinoma. J. Vet. Med. Sci. 2024, 86, 961–965. [Google Scholar] [CrossRef]
- McCaughan, G.J.; Gandolfi, S.; Moore, J.J.; Richardson, P.G. Lenalidomide, bortezomib and dexamethasone induction therapy for the treatment of newly diagnosed multiple myeloma: A practical review. Br. J. Haematol. 2022, 199, 190–204. [Google Scholar] [CrossRef]
- Richardson, P.G.; Weller, E.; Lonial, S.; Jakubowiak, A.J.; Jagannath, S.; Raje, N.S.; Avigan, D.E.; Xie, W.; Ghobrial, I.M.; Schlossman, R.L.; et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 2010, 116, 679–686. [Google Scholar] [CrossRef]
- London, C.A.; Hannah, A.L.; Zadovoskaya, R.; Chien, M.B.; Kollias-Baker, C.; Rosenberg, M.; Downing, S.; Post, G.; Boucher, J.; Shenoy, N.; et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin. Cancer Res. 2003, 9, 2755–2768. [Google Scholar]
- Foskett, A.; Manley, C.; Naramore, R.; Gordon, I.K.; Stewart, B.M.; Khanna, C. Tolerability of oral sorafenib in pet dogs with a diagnosis of cancer. Vet. Med. Res. Rep. 2017, 8, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Pfarr, N.; Endris, V.; Mai, E.K.; Md Hanafiah, N.H.; Lehners, N.; Penzel, R.; Weichert, W.; Ho, A.D.; Schirmacher, P.; et al. Molecular signaling in multiple myeloma: Association of RAS/RAF mutations and MEK/ERK pathway activation. Oncogenesis 2017, 6, e337. [Google Scholar] [CrossRef]
- Mee, M.W.; Faulkner, S.; Wood, G.A.; Woods, J.P.; Bienzle, D.; Coomber, B.L. Longitudinal Study of Transcriptomic Changes Occurring over Six Weeks of CHOP Treatment in Canine Lymphoma Identifies Prognostic Subtypes. Vet. Sci. 2024, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Soh, P.X.Y.; Khatkar, M.S.; Williamson, P. Lymphoma in Border Collies: Genome-Wide Association and Pedigree Analysis. Vet. Sci. 2023, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Flynn, P.J.; Chng, W.J.J.; Lei, K.I.K.; Allred, J.; Laumann, K.; Webster, K.; Gertz, M.A.; Dispenzieri, A.; Greipp, P.R.; et al. A Phase II Trial of Sunitinib (SU11248) in Multiple Myeloma. Blood 2009, 114, 4954. [Google Scholar] [CrossRef]
- Wilhelm, S.; Chien, D.S. BAY 43-9006: Preclinical data. Curr. Pharm. Des. 2002, 8, 2255–2257. [Google Scholar] [CrossRef] [PubMed]
- Marconato, L.; Sabattini, S.; Marisi, G.; Rossi, F.; Leone, V.F.; Casadei-Gardini, A. Sorafenib for the Treatment of Unresectable Hepatocellular Carcinoma: Preliminary Toxicity and Activity Data in Dogs. Cancers 2020, 12, 1272. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, M.E.; Reilly, L.M.; Gerami, P.; Guitart, J. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann. Oncol. 2008, 19, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Al Hamed, R.; Bazarbachi, A.H.; Malard, F.; Harousseau, J.L.; Mohty, M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019, 9, 44. [Google Scholar] [CrossRef]
- Graves, S.S.; Storb, R. Evolution of haematopoietic cell transplantation for canine blood disorders and a platform for solid organ transplantation. Vet. Med. Sci. 2021, 7, 2156–2171. [Google Scholar] [CrossRef]
- Lupu, M.; Sullivan, E.W.; Westfall, T.E.; Little, M.T.; Weigler, B.J.; Moore, P.F.; Stroup, P.A.; Zellmer, E.; Kuhr, C.; Storb, R. Use of multigeneration-family molecular dog leukocyte antigen typing to select a hematopoietic cell transplant donor for a dog with T-cell lymphoma. J. Am. Vet. Med. Assoc. 2006, 228, 728–732. [Google Scholar] [CrossRef]
- Willcox, J.L.; Pruitt, A.; Suter, S.E. Autologous peripheral blood hematopoietic cell transplantation in dogs with B-cell lymphoma. J. Vet. Intern. Med. 2012, 26, 1155–1163. [Google Scholar] [CrossRef]
- Gareau, A.; Sekiguchi, T.; Warry, E.; Ripoll, A.Z.; Sullivan, E.; Westfall, T.; Chretin, J.; Fulton, L.M.; Harkey, M.; Storb, R.; et al. Allogeneic peripheral blood haematopoietic stem cell transplantation for the treatment of dogs with high-grade B-cell lymphoma. Vet. Comp. Oncol. 2022, 20, 862–870. [Google Scholar] [CrossRef]
- Warry, E.E.; Willcox, J.L.; Suter, S.E. Autologous peripheral blood hematopoietic cell transplantation in dogs with T-cell lymphoma. J. Vet. Intern. Med. 2014, 28, 529–537. [Google Scholar] [CrossRef]
- Benedict, W.; Suter, S.; Meritet, D. Postmortem pathologic findings in dogs that underwent total body irradiation and hematopoietic cell transplant: A case series of five dogs with B-cell multicentric lymphoma. Vet. Pathol. 2024, 61, 765–770. [Google Scholar] [CrossRef]
- Suter, S.E.; Hamilton, M.J.; Sullivan, E.W.; Venkataraman, G.M. Allogeneic hematopoietic cell transplantation in a dog with acute large granular lymphocytic leukemia. J. Am. Vet. Med. Assoc. 2015, 246, 994–997. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, N.W.; Moreau, P.; Plesner, T.; Palumbo, A.; Gay, F.; Laubach, J.P.; Malavasi, F.; Avet-Loiseau, H.; Mateos, M.V.; Sonneveld, P.; et al. Clinical efficacy and management of monoclonal antibodies targeting CD38 and SLAMF7 in multiple myeloma. Blood 2016, 127, 681–695. [Google Scholar] [CrossRef]
- van de Donk, N.W.C.J.; Zweegman, S. Monoclonal Antibodies in the Treatment of Multiple Myeloma. Hematol. Oncol. Clin. N. Am. 2024, 38, 337–360. [Google Scholar] [CrossRef]
- Caserta, E.; Chea, J.; Minnix, M.; Poku, E.K.; Viola, D.; Vonderfecht, S.; Yazaki, P.; Crow, D.; Khalife, J.; Sanchez, J.F.; et al. Copper 64–labeled daratumumab as a PET/CT imaging tracer for multiple myeloma. Blood 2018, 131, 741–745. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Sobol, N.B.; O’Donoghue, J.A.; Kirov, A.S.; Riedl, C.C.; Min, R.; Smith, E.; Carter, L.M.; Lyashchenko, S.K.; Lewis, J.S.; et al. CD38-targeted Immuno-PET of Multiple Myeloma: From Xenograft Models to First-in-Human Imaging. Radiology 2020, 295, 606–615. [Google Scholar] [CrossRef]
- Perez Rogers, A.; Estes, M. Hyperviscosity Syndrome; StatPearls: Treasure Island, FL, USA; p. 25.
- Lippi, I.; Perondi, F.; Ross, S.J.; Marchetti, V.; Lubas, G.; Guidi, G. Double filtration plasmapheresis in a dog with multiple myeloma and hyperviscosity syndrome. Open Vet. J. 2015, 5, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.R.; Harris, A.; Jeffries, C.; Avery, P.R.; Vickery, K. Retrospective evaluation of the use of the International Myeloma Working Group response criteria in dogs with secretory multiple myeloma. J. Vet. Intern. Med. 2021, 35, 442–450. [Google Scholar] [CrossRef] [PubMed]
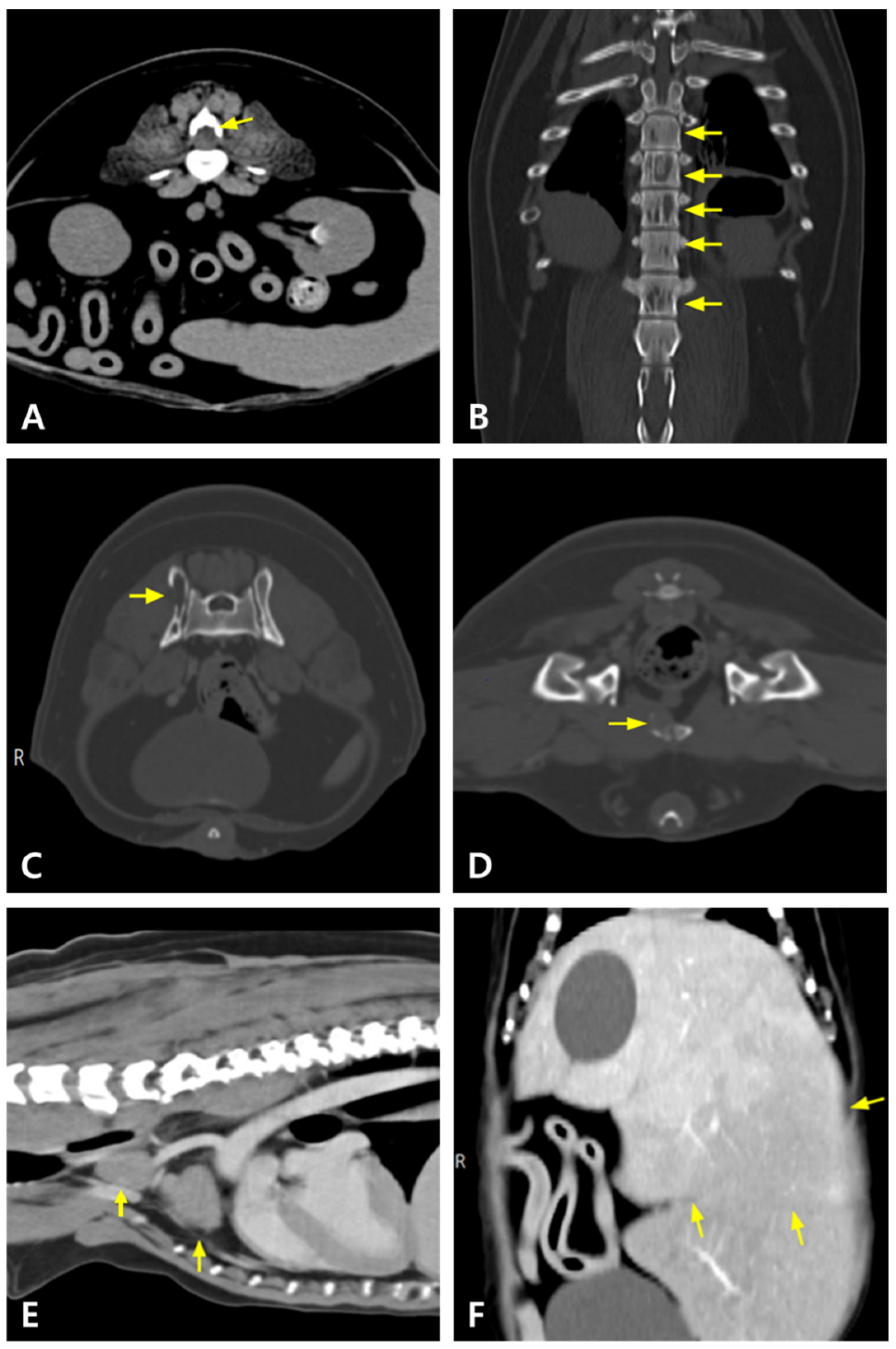

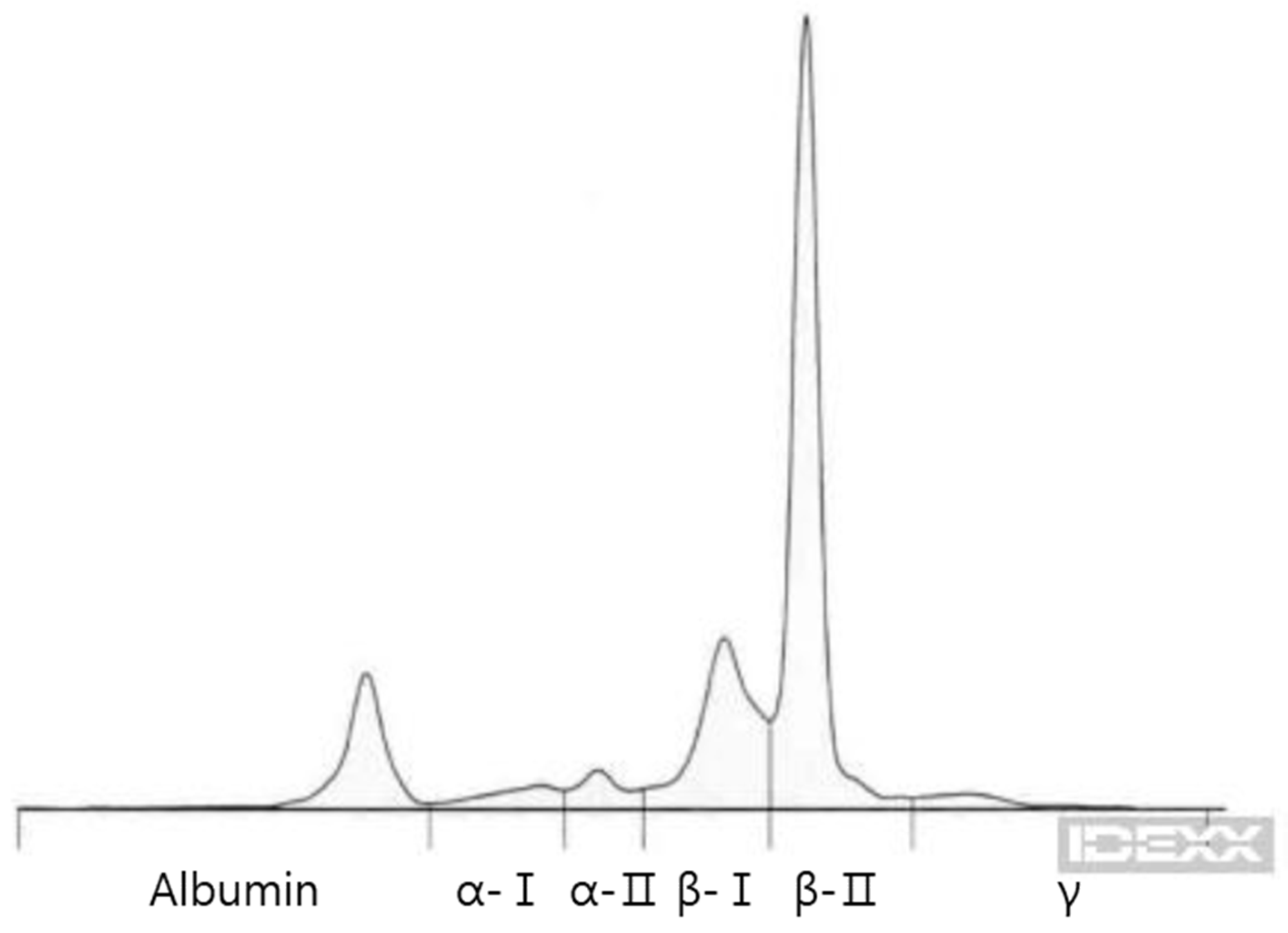
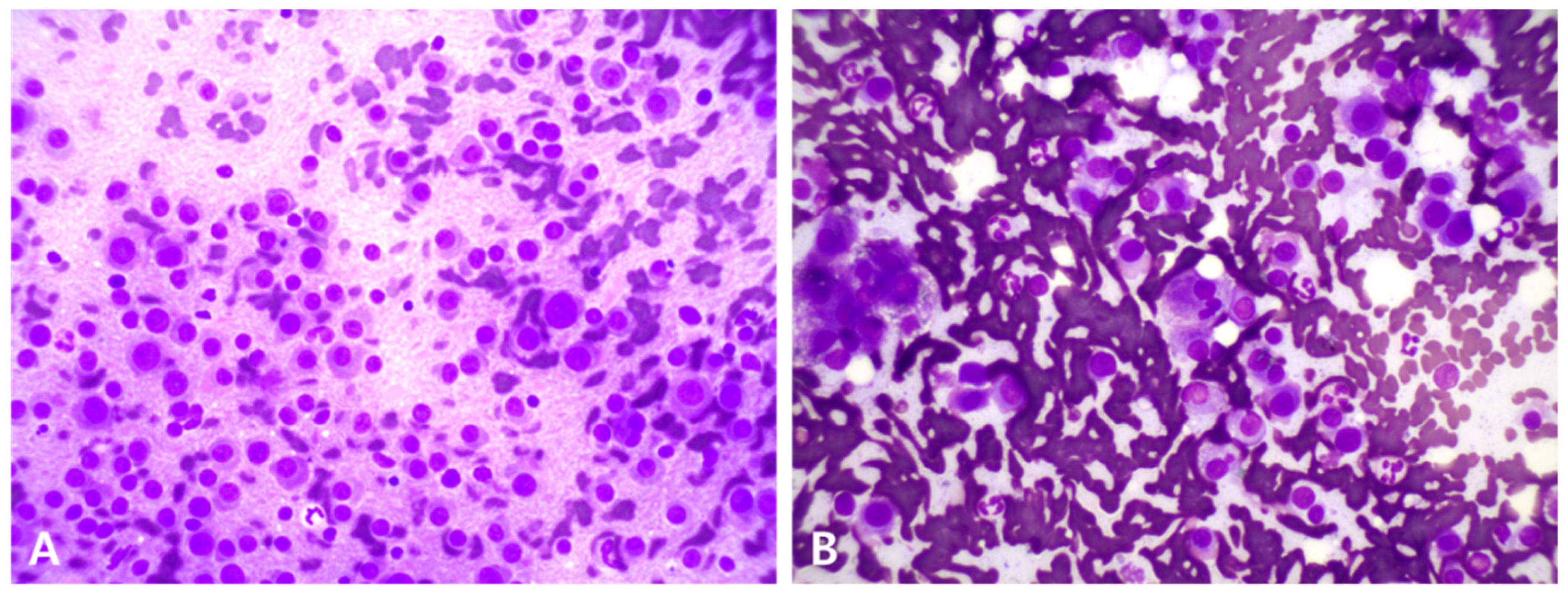


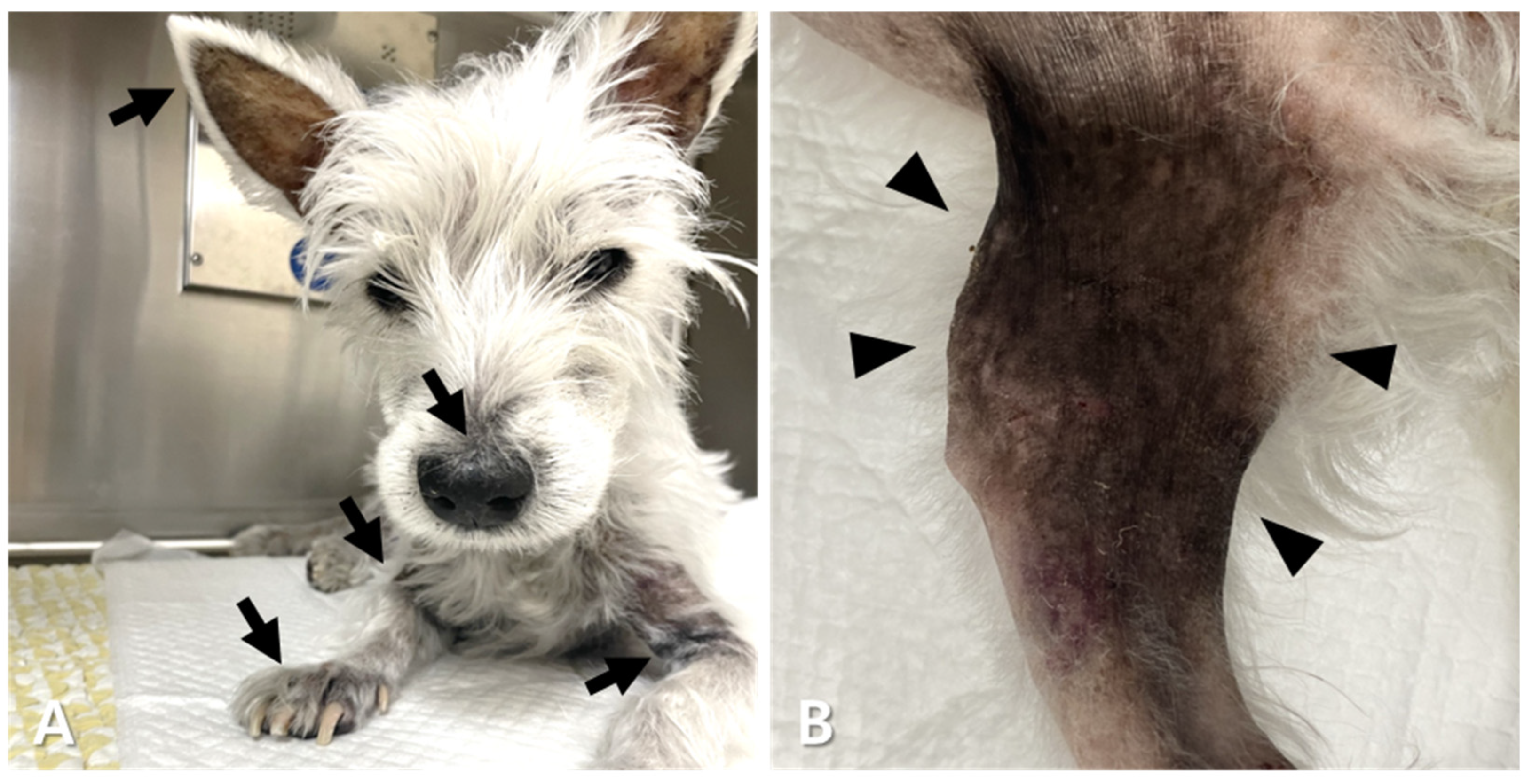


| Day 0 | Day 9 | Day 81 | Day 165 | Day 190 | Day 238 | Day 289 | Day 315 | Day 361 | Day 392 | Reference Range | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hematocrit (%) | 30.8 | 30.1 | 19 | 41.4 | 38.6 | 41.4 | 36.1 | 28.2 | 26.5 | 19.8 | 37.3–61.7 |
| White Blood Cells (k/μL) | 6.46 | 5.6 | 8.88 | 6.88 | 11.29 | 6.75 | 3.0 | 7.89 | 6.59 | 14.11 | 5.05–16.76 |
| Platelet (k/μL) | 197 | 215 | 54 | 193 | 84 | 90 | 55 | 121 | 26 | 22 | 148–484 |
| Total protein (g/dL) | 19.6 | 16.3 | 6.5 | 7.3 | 8.3 | 8.0 | 14.0 | 14.4 | 15.2 | 17.9 | 5.2–8.2 |
| Albumin (g/dL) | 2.2 | 2.4 | 3.0 | 3.3 | 3 | 3.3 | 2.6 | 4.5 | 3.6 | 3.6 | 2.3–4.0 |
| Globulin (g/dL) | 17.4 | 13.9 | 3.5 | 4 | 5.3 | 4.6 | 11.4 | 9.9 | 11.6 | 14.2 | 2.5–4.5 |
| ALT (U/L) | 540 | 309 | 14 | 38 | 41 | 59 | >1000 | - | 94 | 649 | 10–125 |
| ALP (U/L) | 277 | 495 | 534 | 280 | 563 | 264 | 836 | 807 | 544 | 436 | 23–212 |
| BUN (mg/dL) | 11 | 27 | 29.5 | 24 | 11 | 21 | - | 45 | 42 | 55 | 7–27 |
| Creatinine (mg/dL) | 1.0 | 1.8 | 0.7 | 1.4 | 0.6 | 0.8 | - | 1.7 | 2.3 | 2.4 | 0.51.8 |
| Calcium (μg/dL) | 12.9 | 12.2 | 10 | 9.9 | 9.8 | - | - | 11.7 | - | 12.3 | 7.9–12.0 |
| Phosphate (mg/mL) | 5.5 | 7.2 | 4.1 | 5.3 | 3.6 | 6.1 | - | 7.3 | 5.5 | 12.5 | 2.5–6.8 |
| Survival time after initial treatment for multiple myeloma | 398 days |
| Side effects of VAD | (VCOG-CTCAE v2) Grade 3 anemia, grade 2 thrombocytopenia, and grade 1 anorexia, vomiting, altered appetite, and lethargy |
| Side effects of verdinexor | None observed |
| Side effects of verdinexor + doxorubicin + melphalan + dexamethasone | (VCOG-CTCAE v2) Grade 2 anemia, grade 3 leukopenia, grade 4 thrombocytopenia, and grade 1 abdominal distention, anorexia, vomiting, diarrhea, and lethargy |
| Side effects of bortezomib + dexamethasone | (VCOG-CTCAE v2) Grade 2 thrombocytopenia and grade 1 diarrhea |
| Side effects of bortezomib + dexamethasone + melphalan | Grade 1 diarrhea and grade 1 keratitis |
| Side effects of bortezomib + dexamethasone + verdinexor | (VCOG-CTCAE v2) Grade 1 anemia and leukopenia, grade 2 thrombocytopenia, and grade 1 fever, anorexia, vomiting, diarrhea, and lethargy |
| Side effects of carfilzomib + dexamethasone | Not remarkable observation (clinical signs presumed to be tumor-related) |
| Side effects of ixazomib + dexamethasone | Not remarkable observation (anemia presumed to be tumor-related) |
| Side effects of bortezomib + sorafenib | (VCOG-CTCAE v2) Grade 2 anemia and thrombocytopenia, grade 2 alopecia and hyperpigmentation, grade 1 skin ulceration, grade 2 colitis, and grade 2 urinary tract infection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.; Jeong, H.; Sung, A.S.; Kwon, H.; Sohn, J.; Kim, J.-I.; Choi, M.-Y.; Huh, C.; Jung, D.-I. Refractory Multiple Myeloma in a West Highland White Terrier: Clinical Presentations and Therapeutic Interventions. Animals 2025, 15, 2405. https://doi.org/10.3390/ani15162405
Jang H, Jeong H, Sung AS, Kwon H, Sohn J, Kim J-I, Choi M-Y, Huh C, Jung D-I. Refractory Multiple Myeloma in a West Highland White Terrier: Clinical Presentations and Therapeutic Interventions. Animals. 2025; 15(16):2405. https://doi.org/10.3390/ani15162405
Chicago/Turabian StyleJang, Hyomi, Hyejin Jeong, A Sa Sung, Hyojun Kwon, Jiheui Sohn, Jong-In Kim, Moon-Yeong Choi, Chan Huh, and Dong-In Jung. 2025. "Refractory Multiple Myeloma in a West Highland White Terrier: Clinical Presentations and Therapeutic Interventions" Animals 15, no. 16: 2405. https://doi.org/10.3390/ani15162405
APA StyleJang, H., Jeong, H., Sung, A. S., Kwon, H., Sohn, J., Kim, J.-I., Choi, M.-Y., Huh, C., & Jung, D.-I. (2025). Refractory Multiple Myeloma in a West Highland White Terrier: Clinical Presentations and Therapeutic Interventions. Animals, 15(16), 2405. https://doi.org/10.3390/ani15162405






