Effects of Rumen-Protected Chromium-Nicotinic Acid on Lactation Performance, Nutrient Digestion, Ruminal Fermentation, Serum Biochemical Parameters, and Antioxidant in Lactating Water Buffaloes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Design
2.2. Sampling and Chemical Analysis
2.3. Statistical Analysis
3. Results
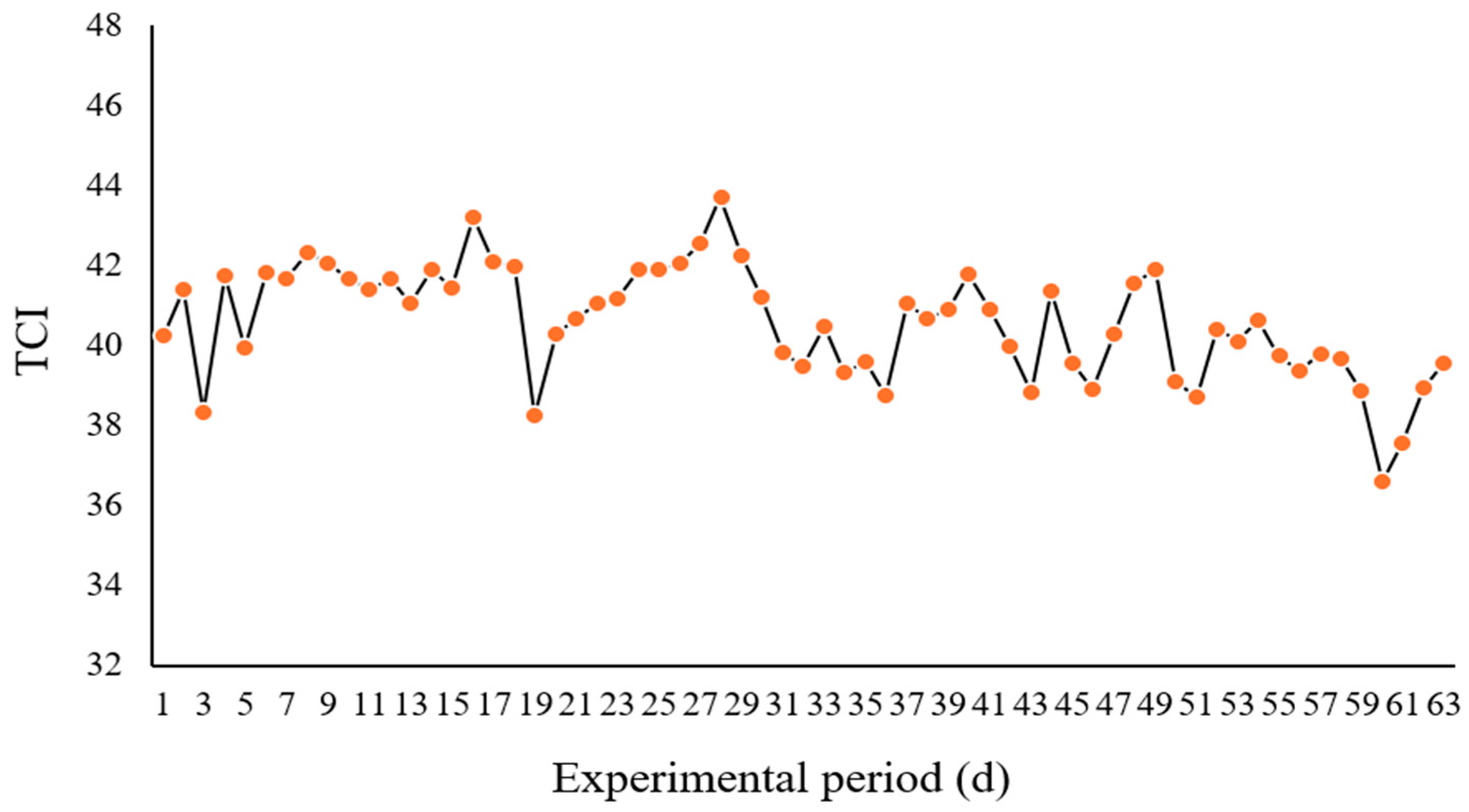
3.1. Measurement of Thermal Comfort Index
3.2. Lactation Performance
3.3. Nutrient Digestion
3.4. Ruminal Fermentation
3.5. Serum Biochemical Parameters
3.6. Serum Antioxidant
4. Discussion
4.1. Measurement of Thermal Comfort Index
4.2. Lactation Performance
4.3. Nutrient Digestion
4.4. Ruminal Fermentation
4.5. Serum Biochemical Parameters
4.6. Serum Antioxidant
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Salam, M.H.A.; El-Shibiny, S. A comprehensive review on the composition and properties of buffalo milk. Dairy Sci. Technol. 2011, 91, 663–699. [Google Scholar] [CrossRef]
- El Debaky, H.A.; Kutchy, N.A.; Ul-Husna, A.; Indriastuti, R.; Akhter, S.; Purwantara, B.; Memili, E. Review: Potential of water buffalo in world agriculture: Challenges and opportunities. Appl. Anim. Sci. 2019, 35, 255–268. [Google Scholar] [CrossRef]
- Becskei, Z.; Savi, M.; Irkovi, D.; Raeta, M.; Puvaa, N.; Paji, M.; Orevi, S.; Paska, S. Assessment of Water Buffalo Milk and Traditional Milk Products in a Sustainable Production System. Sustainability 2020, 12, 6616. [Google Scholar] [CrossRef]
- Ahmad, S.; Gaucher, I.; Rousseau, F.; Beaucher, E.; Piot, M.; Grongnet, J.F.O.; Gaucheron, F. Effects of acidification on physico-chemical characteristics of buffalo milk: A comparison with cow’s milk. Food Chem. 2008, 106, 11–17. [Google Scholar] [CrossRef]
- Sheehan, W.J.; Phipatanakul, W. Tolerance to water buffalo milk in a child with cow milk allergy. Am. Coll. Allergy Asthma Immunol. 2009, 102, 349. [Google Scholar] [CrossRef]
- Li, M.; Liang, X.; Tang, Z.; Hassan, F.U.; Li, L.; Guo, Y.; Peng, K.; Liang, X.; Yang, C. Thermal Comfort Index for Lactating Water Buffaloes under Hot and Humid Climate. Animals 2021, 11, 2067. [Google Scholar] [CrossRef]
- Alessandro, N. Buffalo Production and Research. Ital. J. Anim. Sci. 2010, 5, 203. [Google Scholar] [CrossRef]
- Michelizzi, V.N.; Dodson, M.V.; Pan, Z.; Amaral, M.E.J.; Michal, J.J.; Mclean, D.J.; Womack, J.E.; Jiang, Z. Water Buffalo Genome Science Comes of Age. Int. J. Biol. Sci. 2010, 6, 333–349. [Google Scholar] [CrossRef]
- Nagarcenkar, R.; Sethi, R.K. Association of adaptive traits with performance traits in buffaloes. Indian J. Anim. Sci. 1981, 51, 1121–1123. [Google Scholar]
- Silva, J.A.R.d.; Araújo, A.A.d.; Lourenço, J.d.B., Jr.; Santos, N.d.F.A.d.; Viana, R.B.; Garcia, A.R.; Rondina, D.; Grise, M.M. Hormonal changes in female buffaloes under shading in tropical climate of Eastern Amazon, Brazil. Rev. Bras. Zootec. 2014, 43, 44–48. [Google Scholar] [CrossRef][Green Version]
- Petrocchi Jasinski, F.; Evangelista, C.; Basiricò, L.; Bernabucci, U. Responses of Dairy Buffalo to Heat Stress Conditions and Mitigation Strategies: A Review. Animals 2023, 13, 126. [Google Scholar] [CrossRef]
- Marai, I.F.M.; Haeeb, A.A.M. Buffalo’s biological functions as affected by heat stress—A review. Livest. Sci. 2010, 127, 89–109. [Google Scholar] [CrossRef]
- Yari, M.; Nikkhah, A.; Alikhani, M.; Khorvash, M.; Rahmani, H.; Ghorbani, G.R. Physiological calf responses to increased chromium supply in summer. J. Dairy Sci. 2010, 93, 4111–4120. [Google Scholar] [CrossRef]
- Vidhyalakshmi, M.; Mahato, D.; Dangi, S.S.; Maurya, V.P.; Singh, G. Effect of Supplementation of Chromium Picolinate on Skin Surface Temperature in Buffaloes during Heat Stress. Int. J. Livest. Res. 2017, 8, 277–1964. [Google Scholar] [CrossRef]
- Kargar, S.; Mousavi, F.; Karimi-Dehkordi, S.; Ghaffari, M.H. Growth performance, feeding behavior, health status, and blood metabolites of environmentally heat-loaded Holstein dairy calves fed diets supplemented with chromium. J. Dairy Sci. 2018, 101, 9876–9887. [Google Scholar] [CrossRef]
- Wo, Y.; Ma, F.; Shan, Q.; Gao, D.; Jin, Y.; Sun, P. Plasma metabolic profiling reveals that chromium yeast alleviates the negative effects of heat stress in mid-lactation dairy cows. Anim. Nutr. 2023, 13, 401–410. [Google Scholar] [CrossRef]
- Mousavi, F.; Karimi-Dehkordi, S.; Kargar, S.; Ghaffari, M.H. Effect of chromium supplementation on growth performance, meal pattern, metabolic and antioxidant status and insulin sensitivity of summer-exposed weaned dairy calves. Anim. Int. J. Anim. Biosci. 2019, 13, 968–974. [Google Scholar] [CrossRef]
- Bin-Jumah, M.; Abd El-Hack, M.E.; Abdelnour, S.A.; Hendy, Y.A.; Ghanem, H.A.; Alsafy, S.A.; Khafaga, A.F.; Noreldin, A.E.; Shaheen, H.; Samak, D.; et al. Potential use of chromium to combat thermal stress in animals: A review. Sci. Total Environ. 2020, 707, 135996. [Google Scholar] [CrossRef]
- Zhang, F.J.; Weng, X.G.; Wang, J.F.; Zhou, D.; Zhu, Y.H. Effects of temperature-humidity index and chromium supplementation on antioxidant capacity, heat shock protein 72, and cytokine responses of lactating cows. J. Anim. Sci. 2014, 92, 3026. [Google Scholar] [CrossRef]
- Deka, R.S.; Mani, V.; Kumar, M.; Shiwajirao, Z.S.; Kaur, H. Chromium Supplements in the Feed for Lactating Murrah Buffaloes (Bubalus bubalis): Influence on Nutrient Utilization, Lactation Performance, and Metabolic Responses. Biol. Trace Elem. Res. 2015, 168, 362–371. [Google Scholar] [CrossRef]
- Kumar, M.; Kaur, H.; Tyagi, A.; Mani, V.; Deka, R.S.; Chandra, G.; Sharma, V.K. Assessment of chromium content of feedstuffs, their estimated requirement, and effects of dietary chromium supplementation on nutrient utilization, growth performance, and mineral balance in summer-exposed buffalo calves (Bubalus bubalis). Biol. Trace Elem. Res. 2013, 155, 29–37. [Google Scholar] [CrossRef]
- Kegley, E.B.; Spears, J.W.; Eisemann, J.H. Performance and glucose metabolism in calves fed a chromium-nicotinic acid complex or chromium chloride. J. Dairy Sci. 1997, 80, 1744–1750. [Google Scholar] [CrossRef]
- Chen, H.; Zhen, J.; Wu, Z.; Li, X.; Liu, S.; Tang, Z.; Sun, Z. Grape seed extract and chromium nicotinate reduce impacts of heat stress in Simmental × Qinchuan steers. Anim. Prod. Sci. 2019, 59, 1868–1879. [Google Scholar] [CrossRef]
- Li, Z.F.; Duan, L.Y.; Fu, T.; Liu, B.; Gao, T.Y. Effect of Supplement Chromium Nicotinate and Chromium Yeast on Heat-stressed Dairy Cows in Dry Lactating Period. Acta Agric. Univ. Jiangxiensis 2010, 6, 1230–1235. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, Z.L.; Dong, G.Z.; Wei, X.L.; Song, D.J. Dietary calcium soaps of fatty acids and chromium nicotinate affect lactation performance, physiological and serum biochemical indices of dairy cows under heat stress. Chin. J. Anim. Nutr. 2012, 24, 145–151. [Google Scholar]
- Costanzo, A.D.; Spain, J.N.; Spiers, D.E. Supplementation of Nicotinic Acid for Lactating Holstein Cows Under Heat Stress Conditions. J. Dairy Sci. 1997, 80, 1200–1206. [Google Scholar] [CrossRef]
- Bradford, B.J.; Mamedova, L.K. Effects of encapsulated niacin on metabolism and production of periparturient holstein cows. Kans. Agric. Exp. Stn. Res. Rep. 2009, 41–45. [Google Scholar] [CrossRef]
- Wang, Z.; Niu, K.; Rushdi, H.E.; Zhang, M.; Fu, T.; Gao, T.; Yang, L.; Liu, S.; Lin, F. Heat Stress Induces Shifts in the Rumen Bacteria and Metabolome of Buffalo. Animals 2022, 12, 1300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.D.; Wang, C.; Du, H.S.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, J.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L. Effects of sodium selenite and coated sodium selenite on lactation performance, total tract nutrient digestion and rumen fermentation in Holstein dairy cows. Animal 2020, 14, 2091–2099. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Guo, G.; Huo, W.J.; Ma, L.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L.; Wang, H. Effects of rumen-protected folic acid on ruminal fermentation, microbial enzyme activity, cellulolytic bacteria and urinary excretion of purine derivatives in growing beef steers. Anim. Feed Sci. Technol. 2016, 221, 185–194. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International: Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Maryland, MD, USA, 1995; Volume 1. [Google Scholar]
- Keulen, J.V.; Young, B.A. Evaluation of Acid-Insoluble Ash as a Natural Marker in Ruminant Digestibility Studies. J. Anim. Sci. 1977, 44, 282–287. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Salomonsson, A.C.; Theander, O.; Westerlund, E. Chemical characterization of some Swedish cereal whole meal and bran fractions. Swed. J. Agric. Res. 1984, 14, 111–117. [Google Scholar]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Sklan, D.; Ashkenazi, R.; Braun, A.; Devorin, A.; Tabori, K. Fatty acids, calcium soaps of fatty acids, and cottonseeds fed to high yielding cows. J. Dairy Sci. 1992, 75, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Bryan, M.A.; Socha, M.T.; Tomlinson, D.J. Supplementing Intensively Grazed Late-Gestation and Early-Lactation Dairy Cattle with Chromium. J. Dairy Sci. 2004, 87, 4269–4277. [Google Scholar] [CrossRef]
- Subiyatno, A.; Mowat, D.N.; Yang, W.Z. Metabolite and hormonal responses to glucose or propionate infusions in periparturient dairy cows supplemented with chromium. J. Dairy Sci. 1996, 79, 1436–1445. [Google Scholar] [CrossRef]
- Pechová, A.; Podhorsk, A.; Lokajová, E.; Pavlata, L.; Illek, J. Metabolic Effects of Chromium Supplementation in Dairy Cows in the Peripartal Period. Acta Vet. Brno 2002, 71, 9–18. [Google Scholar] [CrossRef]
- Hayirli, A.; Bremmer, D.R.; Bertics, S.J. Effect of chromium supplementation on production and metabolic parameters in periparturient dairy cows. J. Dairy Sci. 2001, 84, 1218–1230. [Google Scholar] [CrossRef]
- McNamara, J.P.; Valdez, F. Adipose tissue metabolism and production responses to calcium propionate and chromium propionate. J. Dairy Sci. 2005, 88, 2498–2507. [Google Scholar] [CrossRef] [PubMed]
- Lashkari, S.; Habibian, M.; Jensen, S.K. A Review on the Role of Chromium Supplementation in Ruminant Nutrition-Effects on Productive Performance, Blood Metabolites, Antioxidant Status, and Immunocompetence. Biol. Trace Elem. Res. 2018, 186, 305–321. [Google Scholar] [CrossRef]
- Wu, Z.; Peng, W.; Liu, J.; Xu, G.; Wang, D. Effect of chromium methionine supplementation on lactation performance, hepatic respiratory rate and anti-oxidative capacity in early-lactating dairy cows. Animal 2021, 15, 100326. [Google Scholar] [CrossRef]
- Yang, W.Z.; Mowat, D.N.; Subiyatno, A.; Liptrap, R.M. Effects of chromium supplementation on early lactation performance of Holstein cows. Can. J. Anim. Sci. 1996, 76, 221–230. [Google Scholar] [CrossRef]
- Soltan, M.A. Effect of dietary chromium supplementation on productive and reproductive performance of early lactating dairy cows under heat stress. J. Anim. Physiol. Anim. Nutr. 2010, 94, 264–272. [Google Scholar] [CrossRef]
- Vargas-Rodriguez, C.F.; Yuan, K.; Titgemeyer, E.C.; Mamedova, L.K.; Griswold, K.E.; Bradford, B.J. Effects of supplemental chromium propionate and rumen-protected amino acids on productivity, diet digestibility, and energy balance of peak-lactation dairy cattle. J. Dairy Sci. 2014, 97, 3815–3821. [Google Scholar] [CrossRef] [PubMed]
- Yasui, T.; Mcart, J.A.; Ryan, C.M.; Gilbert, R.O.; Nydam, D.V.; Valdez, F.; Griswold, K.E.; Overton, T.R. Effects of chromium propionate supplementation during the periparturient period and early lactation on metabolism, performance, and cytological endometritis in dairy cows. J. Dairy Sci. 2014, 97, 6400–6410. [Google Scholar] [CrossRef]
- Qi, Z.; Gao, J.; Zhao, C.; Zhang, Y.; Liu, Y.; Wang, X.; Li, H. PSXVII-30 Effects of dietary supplementation of yeast chromium and dihydropyridine on serum biochemical indices and HSP70 mRNA expression of lactating dairy cows in summer. J. Anim. Sci. 2018, 96 (Suppl. S3), 448–449. [Google Scholar] [CrossRef]
- Owens, F.N.; Bergen, W.G. Nitrogen metabolism of ruminant animals: Historical perspective, current understanding and future implications. J. Anim. Sci. 1983, 57, 498–518. [Google Scholar]
- Tillman, A.D.; Sidhu, K.S. Nitrogen metabolism in ruminants: Rate of ruminal ammonia production and nitrogen utilization by ruminants--a review. J. Anim. Sci. 1969, 28, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Shil, K.; Pal, S. Metabolic and morphological disorientations in the liver and skeletal muscle of mice exposed to hexavalent chromium. Comp. Clin. Pathol. 2019, 28, 1729–1741. [Google Scholar] [CrossRef]
- Yang, C.M.J. Response of Forage Fiber Degradation by Ruminal Microorganisms to Branched-Chain Volatile Fatty Acids, Amino Acids, and Dipeptides. J. Dairy Sci. 2002, 85, 1183–1190. [Google Scholar] [CrossRef]
- Shan, Q.; Ma, F.; Huang, Q.; Wo, Y.; Sun, P. Chromium yeast promotes milk protein synthesis by regulating ruminal microbiota and amino acid metabolites in heat-stressed dairy cows. Anim. Nutr. 2025, 20, 120–130. [Google Scholar] [CrossRef]
- Zhao, C.; Shen, B.; Huang, Y.; Kong, Y.; Tan, P.; Zhou, Y.; Yang, J.; Xu, C.; Wang, J. Effects of Chromium Propionate and Calcium Propionate on Lactation Performance and Rumen Microbiota in Postpartum Heat-Stressed Holstein Dairy Cows. Microorganisms 2023, 11, 1625. [Google Scholar] [CrossRef]
- Cai, F.; Lin, M.; Jin, W.; Chen, C.; Liu, G. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxvalerate) from volatile fatty acids by Cupriavidus necator. J. Basic Microbiol. 2023, 63, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Abreu, L.R.D. Factors Affecting the Biosynthesis of Branched-Chain Fatty Acids in Milk Fat; University of Wisconsin: Madison, WI, USA, 1993. [Google Scholar]
- Chang, X.; Mowat, D.N. Supplemental chromium for stressed and growing feeder calves. J. Anim. Sci. 1992, 70, 559. [Google Scholar] [CrossRef]
- Mallard, B.A.; Borgs, P.; Ireland, M.J.; Mcbride, B.W.; Brown, D.B.; Irwin, J.A. Immunomodulatory effects of chromium (III) in ruminants: A review of potential health benefits and effects on production and milk quality. J. Trace Elem. Exp. Med. 1999, 12, 131–140. [Google Scholar] [CrossRef]
- Fatma, U.; Guclu, B.K.L.; Tayfur, B.; Mustafa, U.; Ahmet, S. The Effect of Chromium Supplementation on Body Weight, Serum Glucose, Proteins, Lipids, Minerals and Ovarian Follicular Activity in Working Horses. J. Anim. Vet. Adv. 2012, 7, 771–776. [Google Scholar]
- Bagchi, D.; Stohs, S.J.; Downs, B.W.; Bagchi, M.; Preuss, H.G. Cytotoxicity and oxidative mechanisms of different forms of chromium. Toxicology 2002, 180, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.K.; Wakabayashi; Nobunao; Greenlaw; Jennifer, L.; Yamamoto; Masayuki; Kensler; Thomas, W. Antioxidants Enhance Mammalian Proteasome Expression through the Keap1-Nrf2 Signaling Pathway. Mol. Cell. Biol. 2003, 23, 8786–8794. [Google Scholar] [CrossRef]
- Puertollano, M.A.; Puertollano, E.; de Cienfuegos, G.; de Pablo, M.A. Dietary antioxidants: Immunity and host defense. Curr. Top. Med. Chem. 2011, 11, 1752–1766. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Dai, X.; Xing, C.; Zhang, C.; Cao, H.; Guo, X.; Liu, P.; Yang, F.; Zhuang, Y.; Hu, G. Hexavalent-Chromium-Induced Disruption of Mitochondrial Dynamics and Apoptosis in the Liver via the AMPK-PGC-1α Pathway in Ducks. Int. J. Mol. Sci. 2023, 24, 17241. [Google Scholar] [CrossRef] [PubMed]
| Item | Content |
|---|---|
| Ingredient, % of DM 1 | |
| Corn silage | 41.4 |
| Elephant grass | 25.9 |
| Brewers’ spent grain | 20.0 |
| Molasses residue | 5.0 |
| Soybean Hulls | 5.0 |
| Wheat bran | 2.5 |
| Calcium phosphate | 0.10 |
| Salt | 0.05 |
| Premix 2 | 0.05 |
| Chemical composition, % of DM | |
| Crude protein | 14.8 |
| Ether extract | 6.7 |
| Neutral detergent fiber | 44.3 |
| Acid detergent fiber | 22.8 |
| Calcium | 0.68 |
| Phosphorus | 0.42 |
| Treatments 1 | p-Values 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | Control | RPCNA2 | RPCNA4 | RPCNA6 | SEM | Treatment | Linear | Quadratic | Cubic |
| Dry matter intake, kg/d | 9.45 | 9.55 | 9.45 | 9.35 | 0.05 | 0.395 | 0.265 | 0.201 | 0.712 |
| Milk yield, kg/d | 5.78 | 5.94 | 5.80 | 6.05 | 0.09 | 0.871 | 0.699 | 0.836 | 0.428 |
| 4% FCM 3 yield, kg/d | 7.25 | 7.30 | 7.73 | 7.26 | 0.13 | 0.664 | 0.742 | 0.516 | 0.378 |
| Milk composition content | |||||||||
| Fat, % | 5.73 | 5.53 | 6.24 | 5.43 | 0.23 | 0.514 | 0.942 | 0.346 | 0.087 |
| Protein, % | 4.17 | 4.13 | 4.03 | 4.23 | 0.09 | 0.514 | 0.818 | 0.373 | 0.290 |
| Lactose, % | 4.61 | 4.28 | 4.34 | 4.06 | 0.10 | 0.701 | 0.283 | 0.936 | 0.662 |
| Total solids, % | 14.5 | 13.9 | 14.6 | 13.7 | 0.21 | 0.573 | 0.621 | 0.767 | 0.074 |
| Somatic cell count, 104 cell/mL | 17.5 | 15.2 | 15.5 | 16.3 | 0.92 | 0.882 | 0.678 | 0.631 | 0.783 |
| Milk urea nitrogen, mg/dL | 22.0 | 18.1 | 20.5 | 22.0 | 0.89 | 0.281 | 0.757 | 0.289 | 0.242 |
| Treatments 1 | p-Values 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | Control | RPCNA2 | RPCNA4 | RPCNA6 | SEM | Treatment | Linear | Quadratic | Cubic |
| Dry matter, % | 64.0 | 64.8 | 65.6 | 65.3 | 0.69 | 0.799 | 0.193 | 0.688 | 0.871 |
| Organic matter, % | 64.8 | 65.1 | 65.3 | 65.0 | 0.61 | 0.994 | 0.826 | 0.849 | 0.972 |
| Crude protein, % | 74.8 | 75.0 | 74.6 | 75.6 | 0.41 | 0.655 | 0.549 | 0.575 | 0.318 |
| Ether extract, % | 61.4 | 62.2 | 61.5 | 63.4 | 0.72 | 0.811 | 0.504 | 0.797 | 0.504 |
| Starch, % | 93.8 | 93.6 | 93.5 | 93.7 | 0.33 | 0.996 | 0.846 | 0.840 | 0.957 |
| Neutral detergent fiber, % | 56.1 | 57.9 | 58.1 | 56.1 | 0.48 | 0.645 | 0.987 | 0.425 | 0.910 |
| Acid detergent fiber, % | 34.1 | 34.8 | 34.1 | 34.4 | 0.30 | 0.987 | 0.953 | 0.915 | 0.796 |
| Treatments 1 | p-Values 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | Control | RPCNA2 | RPCNA4 | RPCNA6 | SEM | Treatment | Linear | Quadratic | Cubic |
| pH | 6.65 | 6.76 | 6.85 | 6.77 | 0.04 | 0.255 | 0.127 | 0.259 | 0.691 |
| Ammonia N, mmol/L | 15.1 a | 16.2 a | 7.8 b | 9.6 b | 0.79 | <0.001 | 0.998 | 0.023 | 0.894 |
| Volatile fatty acid, % | |||||||||
| Acetate | 62.8 | 62.8 | 66.1 | 63.3 | 0.42 | 0.156 | 0.288 | 0.307 | 0.170 |
| Propionate | 23.4 | 23.2 | 20.8 | 22.9 | 0.43 | 0.154 | 0.421 | 0.123 | 0.191 |
| Butyrate | 9.65 | 9.05 | 9.32 | 9.47 | 0.15 | 0.886 | 0.896 | 0.668 | 0.543 |
| Isobutyrate | 1.07 bc | 1.47 a | 0.74 c | 1.13 ab | 0.08 | 0.003 | 0.079 | 0.958 | 0.001 |
| Valerate | 1.40 | 1.57 | 1.52 | 1.42 | 0.03 | 0.475 | 0.955 | <0.001 | 0.840 |
| Isovalerate | 1.78 | 1.90 | 1.53 | 1.77 | 0.04 | 0.501 | 0.437 | 0.735 | 0.413 |
| Acetate/Propionate | 2.69 | 2.71 | 3.20 | 2.79 | 0.07 | 0.159 | 0.320 | 0.102 | 0.175 |
| Total volatile fatty acid, mmol/L | 90.1 | 90.3 | 86.9 | 90.5 | 0.92 | 0.516 | 0.837 | 0.216 | 0.368 |
| Treatments 1 | p-Values 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | Control | RPCNA2 | RPCNA4 | RPCNA6 | SEM | Treatment | Linear | Quadratic | Cubic |
| Total protein, g/L | 78.4 a | 64.7 c | 71.8 b | 69.2 bc | 0.83 | 0.013 | 0.088 | 0.108 | 0.070 |
| Albumin, g/L | 48.5 b | 55.8 a | 44.7 b | 46.2 b | 0.92 | 0.023 | 0.008 | 0.443 | 0.029 |
| Blood urea nitrogen, mM | 3.49 | 3.96 | 3.96 | 4.57 | 0.20 | 0.511 | 0.209 | 0.922 | 0.381 |
| Triglyceride, mM | 0.35 | 0.31 | 0.32 | 0.37 | 0.02 | 0.439 | 0.680 | 0.240 | 0.959 |
| Total cholesterol, mM | 2.69 | 2.57 | 2.45 | 3.07 | 0.18 | 0.133 | 0.202 | 0.197 | 0.322 |
| Aspartate aminotransferase, U/L | 104 | 110 | 116 | 114 | 8.52 | 0.879 | 0.428 | 0.689 | 0.907 |
| Alanine aminotransferase, U/L | 35.3 | 31.6 | 39.1 | 38.9 | 0.48 | 0.491 | 0.372 | 0.459 | 0.448 |
| Treatments 1 | p-Values 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | Control | RPCNA2 | RPCNA4 | RPCNA6 | SEM | Treatment | Linear | Quadratic | Cubic |
| Catalase, U/mL | 5.94 a | 5.31 ab | 3.27 c | 4.83 b | 0.03 | <0.001 | 0.028 | 0.006 | 0.015 |
| Superoxide dismutase, U/mL | 10.2 | 13.2 | 14.2 | 13.0 | 0.61 | 0.058 | 0.001 | 0.217 | 0.955 |
| Glutathione peroxidase, U/mL | 346 b | 361 b | 430 a | 365 b | 11.1 | 0.018 | 0.134 | 0.025 | 0.139 |
| Malondialdehyde, nmol/mL | 4.10 | 3.13 | 2.97 | 2.12 | 0.12 | 0.291 | 0.042 | 0.942 | 0.693 |
| Total antioxidant capacity, mmol/L | 0.49 b | 0.68 a | 0.75 a | 0.77 a | 0.02 | 0.013 | 0.015 | 0.177 | 0.846 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Zhao, R.; Zhang, S.; Yan, H.; Sun, J.; Zhao, Y.; Huo, W.; Liu, Q.; Wang, C.; Chen, L.; et al. Effects of Rumen-Protected Chromium-Nicotinic Acid on Lactation Performance, Nutrient Digestion, Ruminal Fermentation, Serum Biochemical Parameters, and Antioxidant in Lactating Water Buffaloes. Animals 2025, 15, 2394. https://doi.org/10.3390/ani15162394
Lin Y, Zhao R, Zhang S, Yan H, Sun J, Zhao Y, Huo W, Liu Q, Wang C, Chen L, et al. Effects of Rumen-Protected Chromium-Nicotinic Acid on Lactation Performance, Nutrient Digestion, Ruminal Fermentation, Serum Biochemical Parameters, and Antioxidant in Lactating Water Buffaloes. Animals. 2025; 15(16):2394. https://doi.org/10.3390/ani15162394
Chicago/Turabian StyleLin, Yitong, Rong Zhao, Shiyue Zhang, Haichao Yan, Jiajin Sun, Yuqi Zhao, Wenjie Huo, Qiang Liu, Cong Wang, Lei Chen, and et al. 2025. "Effects of Rumen-Protected Chromium-Nicotinic Acid on Lactation Performance, Nutrient Digestion, Ruminal Fermentation, Serum Biochemical Parameters, and Antioxidant in Lactating Water Buffaloes" Animals 15, no. 16: 2394. https://doi.org/10.3390/ani15162394
APA StyleLin, Y., Zhao, R., Zhang, S., Yan, H., Sun, J., Zhao, Y., Huo, W., Liu, Q., Wang, C., Chen, L., & Guo, G. (2025). Effects of Rumen-Protected Chromium-Nicotinic Acid on Lactation Performance, Nutrient Digestion, Ruminal Fermentation, Serum Biochemical Parameters, and Antioxidant in Lactating Water Buffaloes. Animals, 15(16), 2394. https://doi.org/10.3390/ani15162394





