Interactive Effects of Dietary Protein Levels and Magnetic Water Treatment on Water Quality, Growth Metrics, Carcass Composition, Redox Balance, Enzymatic Functions, and Immune Responses in Oreochromis niloticus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets
2.2. Experimental Fish, Treatments, and Environmental Conditions
2.3. Water Quality Parameters
2.4. Growth and Feed Utilization
2.5. Blood Serum Biochemical, Immunity, and Antioxidant Parameters
2.6. Statistical Analysis
3. Results
3.1. Water Quality
3.2. Growth Performance
3.3. Carcass Composition
3.4. Liver and Kidney Function Parameters
3.5. Blood Biochemical Parameters
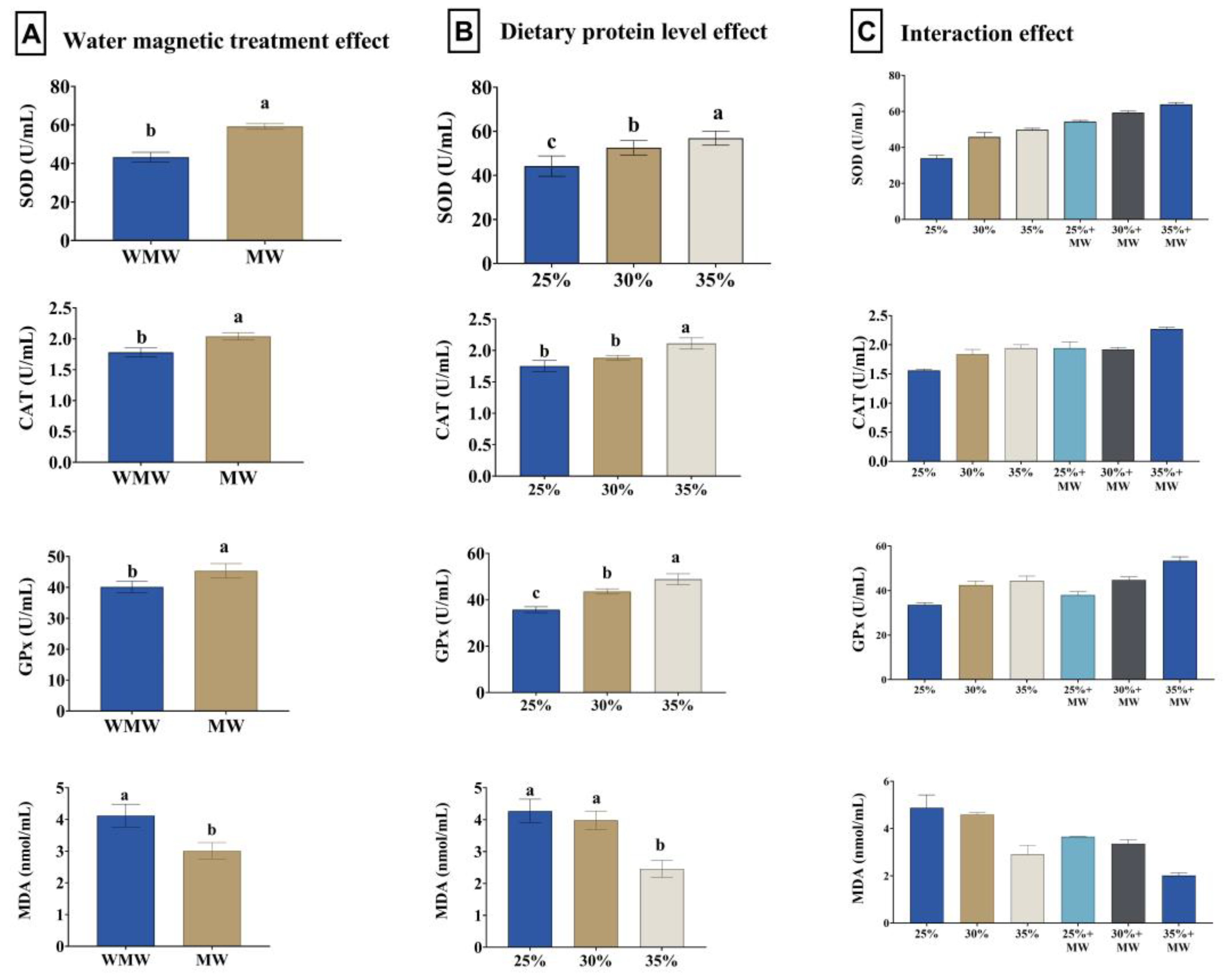
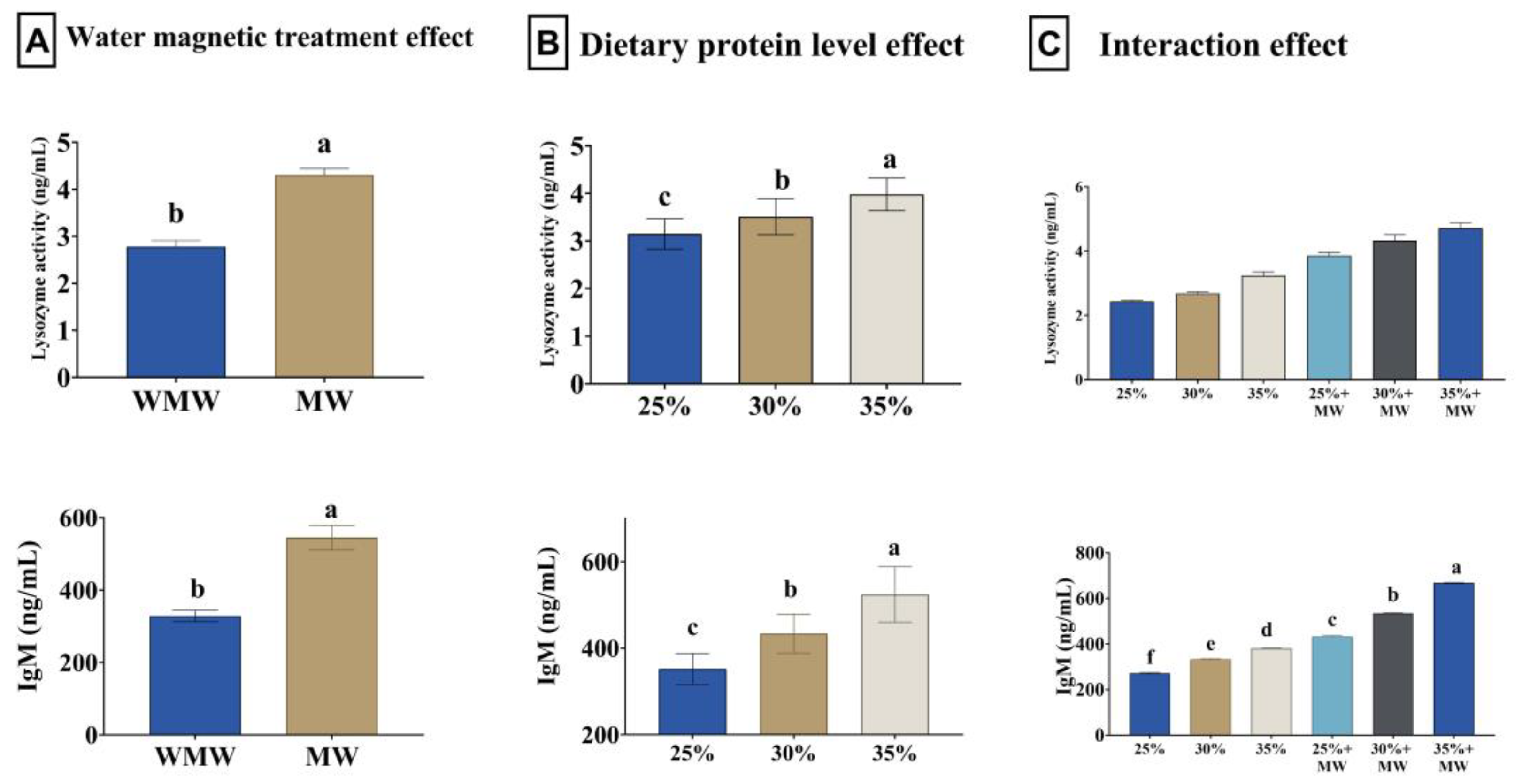
3.6. Antioxidant Status and Immune Response
3.7. Digestive Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. FAO Report: Global Fisheries and Aquaculture Production Reaches a New Record High. 2024. Available online: https://www.fao.org/newsroom/detail/fao-report-global-fisheries-and-aquaculture-production-reaches-a-new-record-high/en (accessed on 20 January 2025).
- Mounes, H.A.M.; Abd-El Azeem, Z.M.A.; Abd El-Bary, D.A.; Al-Sagheer, A.A.; Abd-Elhakim, Y.M.; Hassan, B.A.; Sadek, S.S.; Ahmed, K.M. Effect of substituting soybean meal in Oreochromis niloticus diets with pumpkin (Cucurbita maxima) seed cake on water quality, growth, antioxidant capacity, immunity, and carcass composition. Animals 2024, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Nader, M.M.; Salem, H.M.; El-Tahan, A.M.; Soliman, S.M.; Khafaga, A.F. Effect of environmental factors on growth performance of Nile tilapia (Oreochromis niloticus). Int. J. Biometeorol. 2022, 66, 2183–2194. [Google Scholar] [CrossRef]
- Wang, B.; Thompson, K.D.; Wangkahart, E.; Yamkasem, J.; Bondad-Reantaso, M.G.; Tattiyapong, P.; Jian, J.; Surachetpong, W. Strategies to enhance tilapia immunity to improve their health in aquaculture. Rev. Aquac. 2023, 15, 41–56. [Google Scholar] [CrossRef]
- Hamed, S.; El-Kassas, S.; Abo-Al-Ela, H.G.; Abdo, S.E.; Al Wakeel, R.A.; Abou-Ismail, U.A.; Mohamed, R.A. Interactive effects of water temperature and dietary protein on Nile tilapia: Growth, immunity, and physiological health. BMC Vet. Res. 2024, 20, 349. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food Agriculture Organization of the United Nations. 2025. Aquaculture Feed and Fertilizer Resources Information System. Available online: https://www.fao.org/fishery/affris/species-profiles/nile-tilapia/faqs/en/ (accessed on 20 May 2025).
- Abdel-Tawwab, M.; Ahmad, M.H.; Khattab, Y.A.E.; Shalaby, A.M.E. Effect of dietary protein level, initial body weight, and their interaction on the growth, feed utilization, and physiological alterations of Nile tilapia, Oreochromis niloticus (L.). Aquaculture 2010, 298, 267–274. [Google Scholar] [CrossRef]
- Liu, W.; Xu, C.; Li, Z.; Chen, L.; Wang, X.; Li, E. Reducing dietary protein content by increasing carbohydrates is more beneficial to the growth, antioxidative capacity, ion transport, and ammonia excretion of Nile tilapia (Oreochromis niloticus) under long-term alkalinity stress. Aquac. Nutr. 2023, 2023, 9775823. [Google Scholar] [CrossRef]
- Ahmed, N.; Abd El-Hamed, N. Impact of magnetic water treatment technology on water parameters, growth performance and blood parameters of the Nile tilapia (Oreochromis niloticus). Egypt. J. Aquat. Biol. Fish. 2020, 24, 645–655. [Google Scholar] [CrossRef]
- Wang, C.-T.; Pal, P.; Wang, X.-C. EM waves-based microbial fuel cells integrated to improve performance. Appl. Energy 2025, 377, 124412. [Google Scholar] [CrossRef]
- Pelesz, A.; Fojcik, M. Effect of high static electric field on germination and early stage of growth of Avena sativa and Raphanus sativus. J. Electrost. 2024, 130, 103939. [Google Scholar] [CrossRef]
- Helmy, H.S.; Elhay, Y.B.A.; Salem, A. Effect of magnetic water on the growth of the nile tilapia and lettuce plant in the aquaponic system. Egypt. J. Aquat. Biol. Fish. 2023, 27, 213–228. [Google Scholar] [CrossRef]
- Hassan, S.M.; Sulaiman, M.A.; Madlul, N.S.; Fadel, A.H.; Abdul Rahman, R. Influence of continuous magnetic field exposure on water properties and subsequent effects on the growth performance, plasma biochemistry, nutritive value and liver histopathology of Jade Perch Scortum barcoo in a recirculating system. Aquac. Res. 2019, 50, 1931–1941. [Google Scholar] [CrossRef]
- Hassan, S.M.; Sulaiman, M.A.; Rahman, R.A.; Kamaruddin, R. Effects of long term and continuous magnetic field exposure on the water properties, growth performance, plasma biochemistry and body composition of tilapia in a recirculating aquaculture system. Aquac. Eng. 2018, 83, 76–84. [Google Scholar] [CrossRef]
- Hassan, S.; Rahman, R.A. Effects of exposure to magnetic field on water properties and hatchability of Artemia salina. ARPN J. Agric. Biol. Sci. 2016, 11, 416–423. [Google Scholar]
- Tang, L.-S.; Qiu, C.-Z.; Zhang, H.-Y.; Ren, D.-L. Effects of 0.4 T, 3.0 T and 9.4 T static magnetic fields on development, behaviour and immune response in zebrafish (Danio rerio). NeuroImage 2023, 282, 120398. [Google Scholar] [CrossRef]
- Nofouzi, K.; Sheikhzadeh, N.; Mohamad-Zadeh Jassur, D.; Ashrafi-Helan, J. Influence of extremely low frequency electromagnetic fields on growth performance, innate immune response, biochemical parameters and disease resistance in rainbow trout, Oncorhynchus mykiss. Fish Physiol. Biochem. 2015, 41, 721–731. [Google Scholar] [CrossRef]
- Rosen, A.D. Studies on the effect of static magnetic fields on biological systems. Piers Online 2010, 6, 133–136. [Google Scholar] [CrossRef]
- Tang, J.; Zhao, W.; Chi, J.; Liu, G.; Yu, X.; Bian, L. Effects of magnetic treatment on growth and immune and digestive enzyme activity in the juvenile sea cucumber Apostichopus japonicus (Selenka). Aquaculture 2015, 435, 437–441. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Fish and Shrimp; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington DC, USA, 2017. [Google Scholar]
- Boyd, C.E. Bottom Soils, Sediment, and Pond Aquaculture; Springer: New York, NY, USA, 1995. [Google Scholar]
- Ghareghanipoora, M.; Akbary, P.; Akhlaghi, M.; Fereidouni, M. Non-specific immune responses and immune related genes expression of rainbow trout (Oncorhynchus mykiss, walbaum) fed Zataria multiflora boiss extract. Bull. Environ. Pharmacol. Life Sci. 2014, 3, 140–146. [Google Scholar]
- Tietz, N. Textbook of Clinical Chemistry; WB Saunders Co: Philadelphia, PA, USA, 1986. [Google Scholar]
- Shihabi, Z.K.; Bishop, C. Simplified turbidimetric assay for lipase activity. Clin. Chem. 1971, 17, 1150–1153. [Google Scholar] [CrossRef]
- Bernfeld, P. Enzymes of carbohydrate metabolism. Meth. Enzym. 1955, 1, 149–158. [Google Scholar]
- SAS. Statistical Analysis System (SAS), Proprietary Software Version 9.00; SAS Institute, Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Xin, M.; Zhao, Q.; Qiao, Y.; Ma, Y. Magnetized saline water drip irrigation alters soil water-salt infiltration and redistribution characteristics. Water 2024, 16, 2693. [Google Scholar] [CrossRef]
- Aziz, E.; Ahmed, B.A.; Ramadan, S.; Mahboub, H.D. Effects of magnetic water on productive performance, behaviour, and some physiological responses of Nile tilapia fish (Oreochromis niloticus) reared under normoxia and hypoxia conditions. Assiut Vet. Med. J. 2022, 68, 76–89. [Google Scholar] [CrossRef]
- Alkhazan, M.M.K.; Saddiq, A.A.N. The effect of magnetic field on the physical, chemical and microbiological properties of the lake water in Saudi Arabia. J. Evol. Biol. Res. 2010, 2, 7–14. [Google Scholar]
- El-Sayed, H.S.; Fadel, K.A.; El-Bermawi, N.; El-Greisy, Z.A.; Shaltout, O.E.; Abouelkheir, S.S.; Barakat, K.M. Effect of magnetized water on improving the growth performance, composition factors and microbiota of deteriorated sea bass larvae (Dicentrarchus labrax). Egypt. J. Aquat. Biol. Fish. 2022, 26, 937–953. [Google Scholar] [CrossRef]
- Alabdraba, W.; Albayati, M.; Radeef, A.Y.; Rejab, M.M. Influence of magnetic field on the efficiency of the coagulation process to remove turbidity from water. Int. Rev. Chem. Eng. 2013, 5, 293–298. [Google Scholar]
- Tyari, E.; Jamshidi, A.; Neisy, A. Magnetic water and its benefit in cattle breeding, pisciculture and poultry. Adv. Environ. Biol. 2014, 8, 1031–1036. [Google Scholar]
- Brizhik, L. Biological Effects of Pulsating Magnetic Fields: Role of Solitons. arXiv 2014. [Google Scholar] [CrossRef]
- Irhayyim, T.; Beliczky, G.; Havasi, M.; Bercsényi, M. Impacts of magnetic water treatment on water quality, feeding efficiency and growth performance of common carp in integrated recirculating aquaculture systems. J. Cent. Eur. Agric. 2020, 21, 246–255. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, M.; Lu, X.; Wen, H. Effects of dietary protein level on the gut microbiome and nutrient metabolism in tilapia (Oreochromis niloticus). Animals 2021, 11, 1024. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Geurden, I.; Figueiredo-Silva, A.C.; Kaushik, S.J.; Haidar, M.N.; Verreth, J.A.; Schrama, J.W. Control of voluntary feed intake in fish: A role for dietary oxygen demand in Nile tilapia (Oreochromis niloticus) fed diets with different macronutrient profiles. Br. J. Nutr. 2012, 108, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Santos, W.M.; Costa, L.S.; López-Olmeda, J.F.; Costa, N.C.S.; Santos, F.A.C.; Gamarano, P.G.; Silva, W.S.; Rosa, P.V.; Luz, R.K.; Ribeiro, P.A.P. Effects of dietary protein levels on activities of protease and expression of ingestion and protein digestion-related genes in Nile tilapia juveniles. Aquac. Res. 2020, 51, 2973–2984. [Google Scholar] [CrossRef]
- Kpundeh, M.D.; Qiang, J.; He, J.; Yang, H.; Xu, P. Effects of dietary protein levels on growth performance and haemato-immunological parameters of juvenile genetically improved farmed tilapia (GIFT), Oreochromis niloticus. Aquac. Int. 2015, 23, 1189–1201. [Google Scholar] [CrossRef]
- Ogunji, J.O.; Wirth, M. Effect of dietary protein content and sources on growth, food conversion and body composition of tilapia Oreochromis niloticus fingerling fed fish meal diet. J. Aquac. Trop. 2000, 15, 381–389. [Google Scholar]
- Islam, M.S.; Tanaka, M. Optimization of dietary protein requirement for pond-reared mahseer Tor putitora Hamilton (Cypriniformes: Cyprinidae). Aquac. Res. 2004, 35, 1270–1276. [Google Scholar] [CrossRef]
- Coutinho, F.; Peres, H.; Guerreiro, I.; Pousão-Ferreira, P.; Oliva-Teles, A. Dietary protein requirement of sharpsnout sea bream (Diplodus puntazzo, Cetti 1777) juveniles. Aquaculture 2012, 356, 391–397. [Google Scholar] [CrossRef]
- Daudpota, A.M.; Siddiqui, P.J.; Abbas, G.; Narejo, N.T.; Shah, S.S.A.; Khan, N.; Dastagir, G. Effect of dietary protein level on growth performance, protein utilization and body composition of Nile tilapia cultured in low salinity water. Int. J. Interdiscip. Multidiscip. Stud. 2014, 2, 135–147. [Google Scholar]
- Jia, R.; Gu, Z.; He, Q.; Du, J.; Cao, L.; Jeney, G.; Xu, P.; Yin, G. Anti-oxidative, anti-inflammatory and hepatoprotective effects of Radix Bupleuri extract against oxidative damage in tilapia (Oreochromis niloticus) via Nrf2 and TLRs signaling pathway. Fish Shellfish Immunol. 2019, 93, 395–405. [Google Scholar] [CrossRef]
- El-Shazly, S.A.; Alhejely, A.; Alghibiwi, H.K.; Dawoud, S.F.M.; Sharaf-Eldin, A.M.; Mostafa, A.A.; Zedan, A.M.G.; El-Sadawy, A.A.; El-Magd, M.A. Protective effect of magnetic water against AlCl(3)-induced hepatotoxicity in rats. Sci. Rep. 2024, 14, 24999. [Google Scholar] [CrossRef]
- Elmoslemany, A.M.; Ghamry, H.I.; Awad, A.A.; El-Kholy, R.I.; Almami, I.S.M.; Alyamani, N.M.; Zedan, A.M.G. Liver tissues oxidative status, epigenetic and molecular characteristics in rats administered magnetic and microwave treated water. Sci. Rep. 2023, 13, 4406. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-j.; Qiang, J.; Tao, Y.-f.; Ngoepe, T.K.; Bao, J.-w.; Chen, D.-j.; Xu, P. Physiological and gut microbiome changes associated with low dietary protein level in genetically improved farmed tilapia (GIFT, Oreochromis niloticus) determined by 16S rRNA sequence analysis. Microbiol. Open 2020, 9, e1000. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; QiQun, X.; YiRong, Y.; JingLin, Z.; Wei, X.; DaYu, L.; Ying, Z.Z. Effects of dietary protein level on growth performance, body composition, hematological indexes and hepatic non-specific immune indexes of juvenile Nile tilapia, Oreochromis niloticus. Chin. J. Anim. Nutr. 2012, 24, 2384–2392. [Google Scholar]
- Xiuju, P.; Xiaotong, L.; Bing, X.; Yaoyao, L.; Alejandro, S.; Hamza, B.; Zhongjian, C.; Wei, H. Gout therapeutics and drug delivery. J. Control. Release 2023, 362, 728–754. [Google Scholar] [CrossRef]
- Andrea, B.; Massimo, B.; Maria Giulia, B.; Letizia, P. Gender Influence on XOR Activities and Related Pathologies: A Narrative Review. Antioxidants 2024, 13, 211. [Google Scholar] [CrossRef]
- Ishtiyaq, A.; Imtiaz, A.; Nazir, A.D. Dietary valine improved growth, immunity, enzymatic activities and expression of TOR signaling cascade genes in rainbow trout, Oncorhynchus mykiss fingerlings. Sci. Rep. 2021, 11, 22089. [Google Scholar] [CrossRef]
- Lucas, J. What is Magnetism? Magnetic Fields and Magnetic Force; Live Science Contributor: New York, NY, USA, 2015. [Google Scholar]
- Lu, J.-F.; Mao, Y.-X.; Yang, Y.; Wang, Y.-C.; Shi, Y.-X.; Zhou, Q.-H. Influence of Magnetized Water on the Experimental Hyperlipemia and Atherosclerosis in Rabbits. Lab. Anim. Comp. Med. 2000, 20, 227. [Google Scholar]
- Eidl, A.E.-H.; Saiid, M.; Salama, R. Effect of protein levels on growth performance and economical evaluation of Nile tilapia (Oreochromis niloiicus). Egypt. J. Aquat. Biol. Fish. 2003, 7, 309–318. [Google Scholar] [CrossRef]
- Abd El-Ghany, W. Magnetized water as an alternative strategy to improve the poultry production system. Iran. J. Vet. Sci. Technol. 2022, 14, 1–10. [Google Scholar]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Y.; Huang, Z.; Long, Z.; Qin, H.; Lin, H.; Zhou, S.; Kong, L.; Ma, J.; Lin, Y.; et al. High soybean dietary supplementation with quercetin improves antioxidant capacity of spotted sea bass Lateolabrax maculatus. Aquac. Rep. 2024, 39, 102429. [Google Scholar] [CrossRef]
- Kuz’mina, V.V.; Ushakova, N.V.; Krylov, V.V. The effect of magnetic fields on the activity of proteinases and glycosidases in the intestine of the crucian carp Carassius carassius. Biol. Bull. 2015, 42, 61–66. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kang, M.-H. Effect of the magnetized water supplementation on blood glucose, lymphocyte DNA damage, antioxidant status, and lipid profiles in STZ-induced rats. Nutr. Res. Pract. 2013, 7, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Xiao, L.; Feng, K.; Li, W.; Liao, C.; Zhang, T.; Liu, J. Effect of dietary protein levels on the growth, enzyme activity, and immunological status of Culter mongolicus fingerlings. PLoS ONE 2022, 17, e0263507. [Google Scholar] [CrossRef] [PubMed]
- Jahan-Mihan, A.; Luhovyy, B.L.; El Khoury, D.; Anderson, G.H. Dietary proteins as determinants of metabolic and physiologic functions of the gastrointestinal tract. Nutrients 2011, 3, 574–603. [Google Scholar] [CrossRef] [PubMed]

| Crude Protein Levels | |||
|---|---|---|---|
| 25% | 30% | 35% | |
| Ingredients | |||
| Fish meal 65% | 10 | 10 | 10 |
| Soybean meal 46% | 25 | 37 | 50 |
| Yellow corn | 51 | 39 | 26 |
| Rice bran | 7 | 7 | 7 |
| Corn gluten | 4 | 4 | 4 |
| Fish oil | 1 | 1 | 1 |
| Vegetable oil | 1 | 1 | 1 |
| Premix 1 | 0.5 | 0.5 | 0.5 |
| Salt | 0.5 | 0.5 | 0.5 |
| Proximate composition | |||
| Crude protein (N × 6.25) | 25.46 | 30.06 | 35.04 |
| Crude lipids | 6.49 | 6.51 | 6.54 |
| Crude fiber | 2.23 | 2.16 | 2.07 |
| Ash | 6.89 | 7.28 | 7.51 |
| Magnetic Treatment | Dietary Crude Protein | Temperature (°C) | Dissolved Oxygen (mg/L) | pH | Ammonia (NH3, mg/L) | Nitrite (NO2−, mg/L) | Nitrate (NO3−, mg/L) |
|---|---|---|---|---|---|---|---|
| Individual treatment means | |||||||
| Without | 25% | 28.67 ± 0.63 | 5.61 ± 0.36 | 7.16 ± 0.10 | 0.17 ± 0.03 | 0.08 ± 0.01 | 0.007 ± 0.001 |
| 30% | 28.71 ± 0.67 | 5.67 ± 0.26 | 7.07 ± 0.05 | 0.18 ± 0.03 | 0.08 ± 0.01 | 0.007 ± 0.001 | |
| 35% | 28.73 ± 0.63 | 5.65 ± 0.19 | 6.99 ± 0.09 | 0.17 ± 0.04 | 0.10 ± 0.01 | 0.008 ± 0.001 | |
| With | 25% | 28.88 ± 0.49 | 8.61 ± 0.46 | 7.60 ± 0.09 | 0.08 ± 0.01 | 0.02 ± 0.01 | 0.001 ± 0.0003 |
| 30% | 28.74 ± 0.54 | 8.91 ± 0.50 | 7.49 ± 0.09 | 0.08 ± 0.01 | 0.04 ± 0.01 | 0.002 ± 0.001 | |
| 35% | 28.56 ± 0.62 | 8.98 ± 0.53 | 7.39 ± 0.08 | 0.09 ± 0.01 | 0.05 ± 0.02 | 0.003 ± 0.001 | |
| Water magnetic treatment effect | |||||||
| Without | 28.70 ± 0.35 | 5.64 ± 0.15 | 7.07 ± 0.05 | 0.17 ± 0.02 | 0.09 ± 0.01 | 0.007 ± 0.001 | |
| With | 28.73 ± 0.30 | 8.83 ± 0.27 | 7.49 ± 0.05 | 0.08 ± 0.01 | 0.03 ± 0.01 | 0.002 ± 0.0003 | |
| Dietary crude protein effect | |||||||
| 25% | 28.78 ± 0.38 | 7.11 ± 0.53 | 7.38 ± 0.09 | 0.12 ± 0.02 | 0.05 ± 0.01 | 0.004 ± 0.001 | |
| 30% | 28.73 ± 0.41 | 7.29 ± 0.56 | 7.28 ± 0.08 | 0.13 ± 0.02 | 0.06 ± 0.01 | 0.005 ± 0.001 | |
| 35% | 28.64 ± 0.42 | 7.32 ± 0.57 | 7.19 ± 0.08 | 0.13 ± 0.02 | 0.07 ± 0.01 | 0.005 ± 0.001 | |
| Two-way ANOVA: p-values | |||||||
| Interaction | 0.950 | 0.912 | 0.987 | 0.867 | 0.788 | 0.948 | |
| Water magnetic treatment | 0.955 | ˂0.001 | ˂0.001 | ˂0.001 | ˂0.001 | ˂0.001 | |
| Dietary crude protein | 0.975 | 0.854 | 0.092 | 0.968 | 0.270 | 0.134 | |
| Magnetic Treatment | Dietary Crude Protein | IW (g/fish) | FW (g/fish) | ADG (g/day) | Feed Intake (g/fish) | SGR (%/day) | FCR | SR (%) |
|---|---|---|---|---|---|---|---|---|
| Individual treatment means | ||||||||
| Without | 25% | 4.13 ± 0.001 | 14.65 ± 0.45 | 0.150 ± 0.006 | 21.79 ± 0.55 | 1.81 ± 0.04 | 2.07 ± 0.04 | 93.33 ± 3.33 b |
| 30% | 4.15 ± 0.01 | 15.87 ± 0.43 | 0.167 ± 0.006 | 19.80 ± 0.89 | 1.92 ± 0.04 | 1.69 ± 0.02 | 100 ± 0.00 a | |
| 35% | 4.13 ± 0.02 | 17.25 ± 0.36 | 0.187 ± 0.005 | 21.62 ± 0.38 | 2.04 ± 0.03 | 1.65 ± 0.04 | 100 ± 0.00 a | |
| With | 25% | 4.12 ± 0.01 | 15.10 ± 0.37 | 0.157 ± 0.005 | 21.62 ± 0.26 | 1.86 ± 0.04 | 1.97 ± 0.05 | 100 ± 0.00 a |
| 30% | 4.12 ± 0.01 | 17.66 ± 0.16 | 0.193 ± 0.002 | 19.66 ± 0.32 | 2.08 ± 0.01 | 1.45 ± 0.04 | 100 ± 0.00 a | |
| 35% | 4.13 ± 0.002 | 18.10 ± 0.34 | 0.200 ± 0.005 | 19.63 ± 0.57 | 2.11 ± 0.03 | 1.41 ± 0.08 | 100 ± 0.00 a | |
| Water magnetic treatment effect | ||||||||
| Without | 4.14 ± 0.01 | 15.92 ± 0.43 | 0.168 ± 0.006 | 22.18 ± 0.45 | 21.07 ± 0.04 | 1.80 ± 0.07 | 97.78 ± 1.47 | |
| With | 4.12 ± 0.004 | 16.96 ± 0.49 | 0.183 ± 0.007 | 18.97 ± 0.39 | 20.30 ± 0.04 | 1.61 ± 0.09 | 100 ± 0.00 | |
| Dietary protein level effect | ||||||||
| 25% | 4.12 ± 0.01 | 14.88 ± 0.28 c | 0.154 ± 0.004 c | 21.71 ± 0.27 a | 1.83 ± 0.03 c | 2.02 ± 0.04 a | 96.67 ± 2.11 b | |
| 30% | 4.14 ± 0.01 | 16.77 ± 0.45 b | 0.180 ± 0.006 b | 19.73 ± 0.42 b | 2.00 ± 0.04 b | 1.57 ± 0.06 b | 100 ± 0.00 a | |
| 35% | 4.13 ± 0.01 | 17.68 ± 0.29 a | 0.193 ± 0.004 a | 20.62 ± 0.54 ab | 2.08 ± 0.02 a | 1.53 ± 0.07 b | 100 ± 0.00 a | |
| Two-way ANOVA: p-values | ||||||||
| Interaction | 0.233 | 0.210 | 0.198 | 0.185 | 0.204 | 0.287 | 0.047 | |
| Water magnetic treatment | 0.098 | 0.005 | 0.004 | 0.107 | 0.004 | ˂0.001 | 0.069 | |
| Dietary protein level | 0.466 | ˂0.001 | ˂0.001 | 0.011 | ˂0.001 | ˂0.001 | 0.047 | |
| Magnetic Treatment | Dietary Crude Protein | Moisture | Crude Lipids | Ash | Crude Protein |
|---|---|---|---|---|---|
| Individual treatment means | |||||
| Without | 25% | 77.11 ± 0.57 | 4.00 ± 0.14 | 4.10 ± 0.14 b | 13.91 ± 0.28 |
| 30% | 76.35 ± 0.37 | 3.30 ± 0.04 | 4.31 ± 0.09 b | 15.06 ± 0.28 | |
| 35% | 74.55 ± 0.32 | 3.33 ± 0.03 | 4.73 ± 0.08 a | 16.41 ± 0.28 | |
| With | 25% | 76.14 ± 0.54 | 4.00 ± 0.04 | 4.79 ± 0.09 a | 14.51 ± 0.35 |
| 30% | 75.12 ± 0.25 | 3.20 ± 0.04 | 4.71 ± 0.06 a | 16.01 ± 0.08 | |
| 35% | 74.96 ± 0.54 | 3.20 ± 0.04 | 4.19 ± 0.14 b | 16.75 ± 0.30 | |
| Water magnetic treatment effect | |||||
| Without | 76.00 ± 0.41 | 3.54 ± 0.12 | 4.38 ± 0.11 | 15.13 ± 0.39 | |
| With | 75.41 ± 0.25 | 3.47 ± 0.14 | 4.56 ± 0.11 | 15.76 ± 0.36 | |
| Dietary crude protein effect | |||||
| 25% | 76.63 ± 0.41 a | 4.00 ± 0.07 a | 4.45 ± 0.17 | 14.21 ± 0.24 c | |
| 30% | 75.12 ± 0.25 ab | 3.25 ± 0.03 b | 4.51 ± 0.10 | 15.54 ± 0.25 b | |
| 35% | 74.76 ± 0.30 b | 3.27 ± 0.04 b | 4.46 ± 0.14 | 16.58 ± 0.20 a | |
| Two-way ANOVA: p-values | |||||
| Interaction | 0.190 | 0.581 | ˂0.001 | 0.558 | |
| Water magnetic treatment | 0.129 | 0.190 | 0.058 | 0.016 | |
| Dietary crude protein | 0.005 | ˂0.001 | 0.834 | ˂0.001 | |
| Magnetic Treatment | Dietary Crude Protein | AST (IU/L) | ALT (IU/L) | ALP (IU/L) | Creat. (mg/dL) | UA (mg/dL) | Urea (mg/dL) |
|---|---|---|---|---|---|---|---|
| Individual treatment means | |||||||
| Without | 25% | 43.46 ± 1.87 | 25.79 ± 0.70 | 39.11 ± 0.73 | 0.21 ± 0.02 | 1.62 ± 0.11 | 6.27 a ± 0.19 |
| 30% | 36.89 ± 1.37 | 22.33 ± 0.92 | 39.49 ± 1.45 | 0.16 ± 0.02 | 1.73 ± 0.08 | 5.84 a ± 0.38 | |
| 35% | 37.02 ± 0.41 | 22.72 ± 0.40 | 34.68 ± 0.46 | 0.14 ± 0.02 | 1.99 ± 0.11 | 4.67 b ± 0.09 | |
| With | 25% | 37.76 ± 0.25 | 19.71 ± 0.37 | 29.50 ± 0.65 | 0.13 ± 0.02 | 1.52 ± 0.10 | 4.24 bc ± 0.14 |
| 30% | 32.59 ± 0.54 | 16.38 ± 0.56 | 28.53 ± 0.54 | 0.17 ± 0.03 | 1.63 ± 0.05 | 3.94 c ± 0.13 | |
| 35% | 31.74 ± 0.43 | 15.86 ± 0.43 | 26.53 ± 0.58 | 0.11 ± 0.02 | 1.96 ± 0.06 | 3.80 c ± 0.17 | |
| Water magnetic treatment effect | |||||||
| Without | 39.12 ± 1.28 | 23.61 ± 0.65 | 37.76 ± 0.91 | 0.17 ± 0.01 | 1.78 ± 0.07 | 5.59 ± 0.27 | |
| With | 34.03 ± 0.96 | 17.32 ± 0.65 | 28.19 ± 0.53 | 0.14 ± 0.01 | 1.70 ± 0.08 | 3.99 ± 0.10 | |
| Dietary crude protein effect | |||||||
| 25% | 40.61 a ± 1.53 | 22.75 a ± 1.40 | 34.31 a ± 2.19 | 0.17 ± 0.02 | 1.57 b ± 0.07 | 5.26 a ± 0.47 | |
| 30% | 34.74 b ± 1.17 | 19.36 b ± 1.42 | 34.01 a ± 2.55 | 0.17 ± 0.02 | 1.68 b ± 0.03 | 4.89 a ± 0.46 | |
| 35% | 34.38 b ± 1.21 | 19.29 b ± 1.52 | 30.61 b ± 1.85 | 0.13 ± 0.01 | 1.98 a ± 0.06 | 4.24 b ± 0.21 | |
| Two-way ANOVA: p-values | |||||||
| Interaction | 0.779 | 0.718 | 0.257 | 0.132 | 0.889 | 0.030 | |
| Water magnetic treatment | <0.001 | <0.001 | <0.001 | 0.070 | 0.280 | <0.001 | |
| Dietary crude protein | <0.001 | <0.001 | 0.001 | 0.095 | 0.001 | 0.001 | |
| Magnetic Treatment | Dietary Crude Protein | TP (g/dL) | ALB (g/dL) | GLO (g/dL) | ALB/GLO | Glucose mg/dL | Cholesterol mg/dL | Triglycerides mg/dL |
|---|---|---|---|---|---|---|---|---|
| Individual treatment means | ||||||||
| Without | 25% | 1.65 c ± 0.04 | 0.82 ± 0.05 | 0.83 d ± 0.02 | 0.99 ± 0.08 | 72.27 ± 0.54 | 144.50 ± 0.71 | 168.23 a ± 1.45 |
| 30% | 1.74 c ± 0.05 | 0.81 ± 0.05 | 0.90 d ± 0.03 | 0.90 ± 0.09 | 78.69 ± 0.49 | 146.33 ± 2.11 | 170.57 a ± 0.38 | |
| 35% | 2.33 b ± 0.08 | 1.11 ± 0.10 | 1.22 c ± 0.03 | 0.91 ± 0.09 | 76.23 ± 0.71 | 160.18 ± 3.92 | 169.23 a ± 1.42 | |
| With | 25% | 2.48 b ± 0.09 | 1.21 ± 0.10 | 1.27 c ± 0.01 | 0.95 ± 0.08 | 62.25 ± 1.75 | 130.75 ± 1.44 | 139.72 d ± 1.41 |
| 30% | 2.94 a ± 0.10 | 1.35 ± 0.07 | 1.56 b ± 0.04 | 0.86 ± 0.03 | 64.84 ± 1.23 | 140.63 ± 2.03 | 145.63 c ± 1.41 | |
| 35% | 3.12 a ± 0.08 | 1.46 ± 0.03 | 1.66 a ± 0.05 | 0.88 ± 0.02 | 65.94 ± 1.01 | 155.25 ± 0.64 | 159.25 b ± 1.42 | |
| Water magnetic treatment effect | ||||||||
| Without | 1.91 ± 0.11 | 0.91 ± 0.06 | 0.98 ± 0.06 | 0.94 ± 0.05 | 75.73 ± 0.98 | 150.34 ± 2.80 | 169.35 ± 0.69 | |
| With | 2.85 ± 0.11 | 1.34 ± 0.05 | 1.49 ± 0.06 | 0.90 ± 0.03 | 64.34 ± 0.88 | 142.21 ± 3.63 | 148.20 ± 2.98 | |
| Dietary crude protein effect | ||||||||
| 25% | 2.07 c ± 0.19 | 1.02 ± 0.10 | 1.05 c ± 0.10 | 0.97 ± 0.05 | 67.26 ± 2.39 | 137.63 ± 3.16 | 153.98 c ± 6.44 | |
| 30% | 2.34 b ± 0.27 | 1.08 ± 0.13 | 1.23 b ± 0.15 | 0.88 ± 0.04 | 71.77 ± 3.15 | 143.48 ± 1.83 | 158.10 b ± 5.61 | |
| 35% | 2.73 a ± 0.18 | 1.29 ± 0.09 | 1.44 a ± 0.10 | 0.88 ± 0.02 | 71.09 ± 2.37 | 157.72 ± 2.09 | 164.24 a ± 2.41 | |
| Two-way ANOVA: p-values | ||||||||
| Interaction | 0.033 | 0.386 | 0.006 | 0.995 | 0.169 | 0.111 | <0.001 | |
| Water magnetic treatment | <0.001 | <0.001 | <0.001 | 0.526 | <0.001 | 0.001 | <0.001 | |
| Dietary crude protein | <0.001 | 0.006 | <0.001 | 0.426 | 0.002 | <0.001 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-El Azeem, Z.M.A.; Ahmed, K.M.; Abdelhay, R.A.; Mounes, H.A.M.; Al-Sagheer, A.A.; Abd El-Ghaffar, H.A.; Abd-Elhakim, Y.M.; Hassan, B.A.; Abd El-Bary, D.A. Interactive Effects of Dietary Protein Levels and Magnetic Water Treatment on Water Quality, Growth Metrics, Carcass Composition, Redox Balance, Enzymatic Functions, and Immune Responses in Oreochromis niloticus. Animals 2025, 15, 2388. https://doi.org/10.3390/ani15162388
Abd-El Azeem ZMA, Ahmed KM, Abdelhay RA, Mounes HAM, Al-Sagheer AA, Abd El-Ghaffar HA, Abd-Elhakim YM, Hassan BA, Abd El-Bary DA. Interactive Effects of Dietary Protein Levels and Magnetic Water Treatment on Water Quality, Growth Metrics, Carcass Composition, Redox Balance, Enzymatic Functions, and Immune Responses in Oreochromis niloticus. Animals. 2025; 15(16):2388. https://doi.org/10.3390/ani15162388
Chicago/Turabian StyleAbd-El Azeem, Zeinab M. A., Kareem M. Ahmed, Reham A. Abdelhay, Hossam A. M. Mounes, Adham A. Al-Sagheer, Haytham A. Abd El-Ghaffar, Yasmina M. Abd-Elhakim, Bayan A. Hassan, and Dena A. Abd El-Bary. 2025. "Interactive Effects of Dietary Protein Levels and Magnetic Water Treatment on Water Quality, Growth Metrics, Carcass Composition, Redox Balance, Enzymatic Functions, and Immune Responses in Oreochromis niloticus" Animals 15, no. 16: 2388. https://doi.org/10.3390/ani15162388
APA StyleAbd-El Azeem, Z. M. A., Ahmed, K. M., Abdelhay, R. A., Mounes, H. A. M., Al-Sagheer, A. A., Abd El-Ghaffar, H. A., Abd-Elhakim, Y. M., Hassan, B. A., & Abd El-Bary, D. A. (2025). Interactive Effects of Dietary Protein Levels and Magnetic Water Treatment on Water Quality, Growth Metrics, Carcass Composition, Redox Balance, Enzymatic Functions, and Immune Responses in Oreochromis niloticus. Animals, 15(16), 2388. https://doi.org/10.3390/ani15162388








