Whole-Genome Resequencing Analysis of Athletic Traits in Grassland-Thoroughbred
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Library Construction and Sequencing
2.3. Data Quality Control
2.4. Variant Detection
2.5. Principal Component Analysis
2.6. Whole-Genome Selection Signal Analysis
2.7. Detection and Annotation of Candidate Genes
2.8. Functional Enrichment Analysis
3. Results
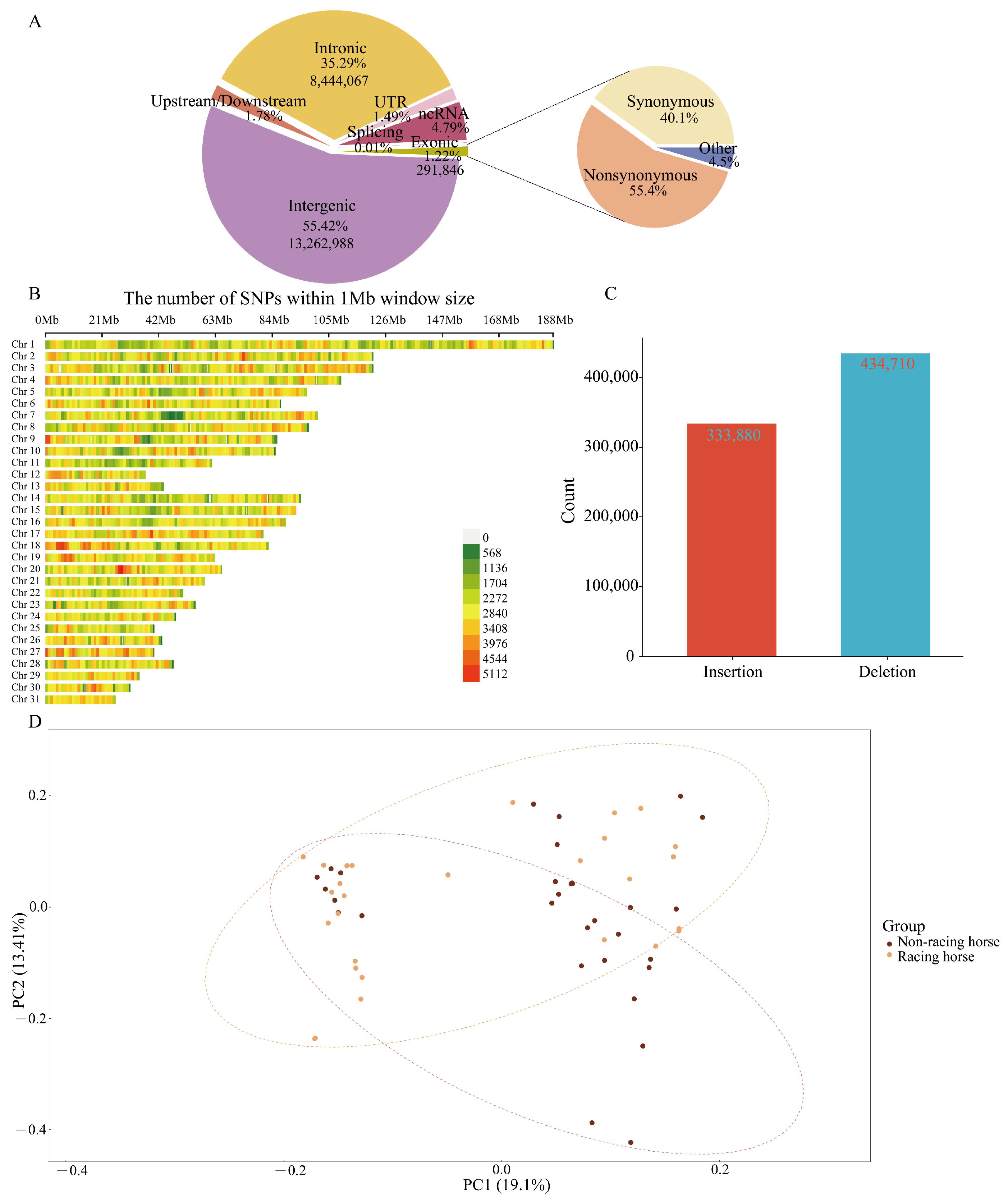
3.1. Sequencing and Detection of SNPs and Indels
3.2. Population Structure Analysis
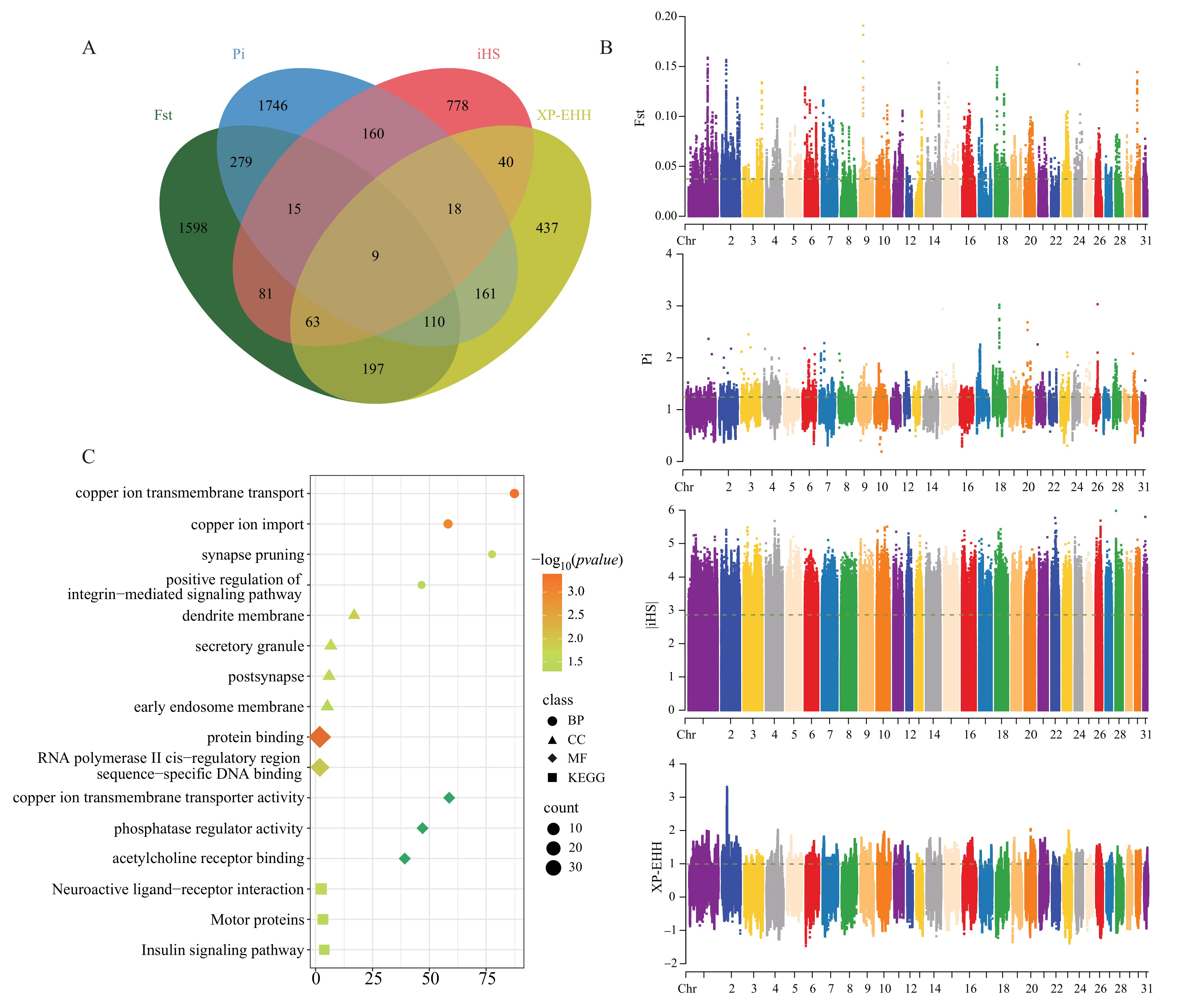
3.3. Genome-Wide Selective Signature Detection
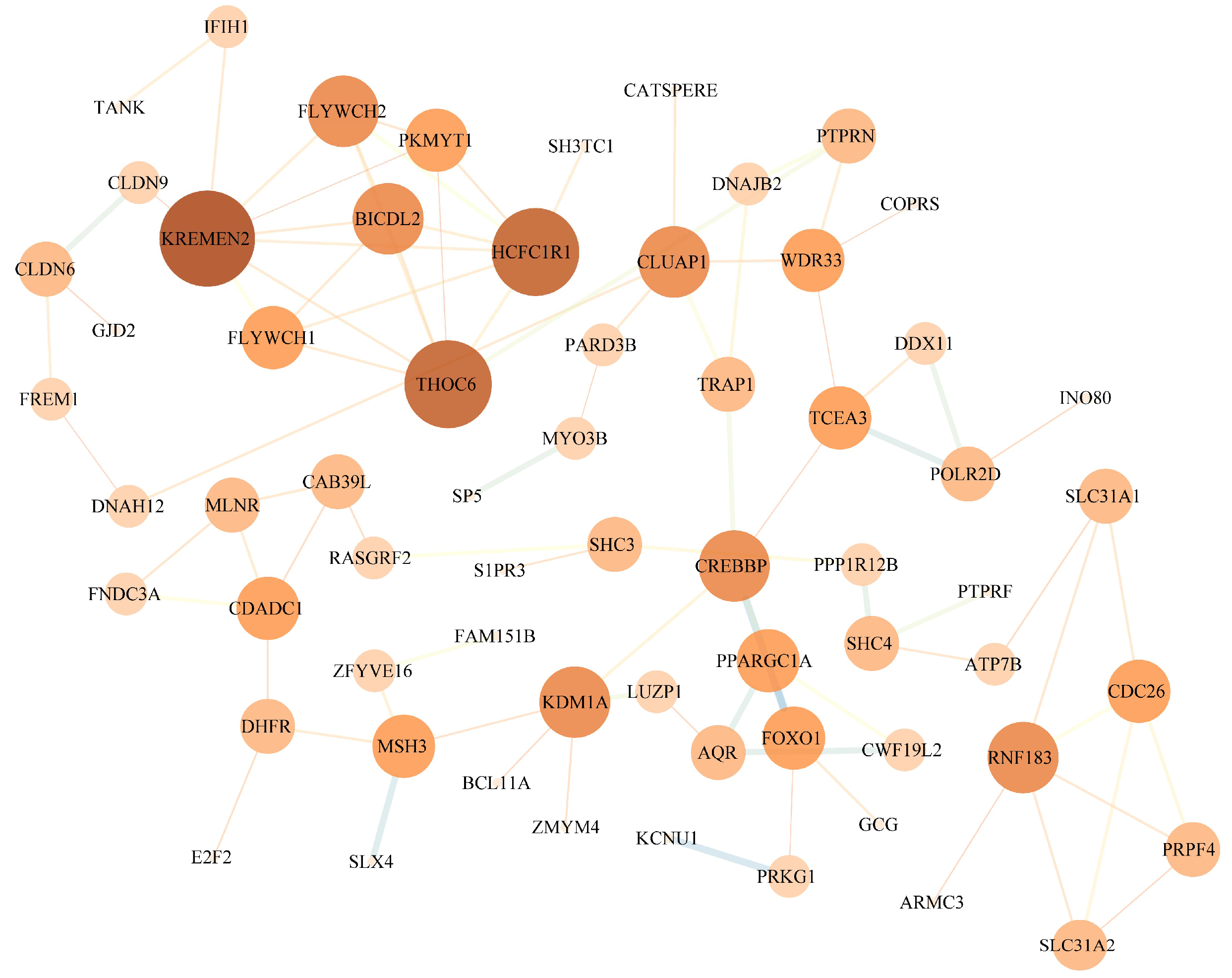
3.4. Candidate Gene Enrichment Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DuBois, C.; Nakonechny, L.; Derisoud, E.; Merkies, K. Examining Canadian Equine Industry Participants’ Perceptions of Horses and Their Welfare. Animals 2018, 8, 201. [Google Scholar] [CrossRef]
- Authorities IFoHR. IFHRA Annual Report 2019; International Federation of Horse Racing Authorities: Boulogne, France, 2019. [Google Scholar]
- Chien, P.M.; Council for Australasian Tourism and Hospitality Education. CAUTHE 2014: Tourism and Hospitality in the Contemporary World: Trends, Changes and Complexity; The University of Queensland: Brisbane, Australia, 2014. [Google Scholar]
- Worthington, A.C. National exuberance: A note on the Melbourne Cup effect in Australian stock returns. Econ. Pap. A J. Appl. Econ. Policy 2007, 26, 170–179. [Google Scholar] [CrossRef]
- Narayan, P.K.; Smyth, R. The race that stops a nation: The demand for the Melbourne Cup. Econ. Rec. 2004, 80, 193–207. [Google Scholar] [CrossRef]
- Cassidy, R. The Sport of Kings: Kinship, Class and Thoroughbred Breeding in Newmarket; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Kay, J.; Vamplew, W. Encyclopedia of British horse Racing; Routledge: London, UK, 2012. [Google Scholar]
- Davis, M. When Things Get Dark: A Mongolian Winter’s Tale; Macmillan: London, UK, 2010. [Google Scholar]
- Qi, B. Xilin Gol League Animal Husbandry Chronicle; Inner Mongolia People’s Publishing House: Hohhot, China, 2002. [Google Scholar]
- Sackton, T.B. Studying Natural Selection in the Era of Ubiquitous Genomes. Trends Genet. 2020, 36, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Orlando, L.; Ginolhac, A.; Zhang, G.; Froese, D.; Albrechtsen, A.; Stiller, M.; Schubert, M.; Cappellini, E.; Petersen, B.; Moltke, I. Recalibrating Equus evolution using the genome sequence of an early Middle Pleistocene horse. Nature 2013, 499, 74–78. [Google Scholar] [CrossRef]
- Otey, C.A.; Rachlin, A.; Moza, M.; Arneman, D.; Carpen, O. The palladin/myotilin/myopalladin family of actin-associated scaffolds. Int. Rev. Cytol. 2005, 246, 31–58. [Google Scholar]
- Hill, E.W.; McGivney, B.A.; Gu, J.; Whiston, R.; Machugh, D.E. A genome-wide SNP-association study confirms a sequence variant (g.66493737C>T) in the equine myostatin (MSTN) gene as the most powerful predictor of optimum racing distance for Thoroughbred racehorses. BMC Genom. 2010, 11, 552. [Google Scholar] [CrossRef]
- Negro Rama, S.; Valera, M.; Membrillo, A.; Gómez, M.D.; Solé, M.; Menendez-Buxadera, A.; Anaya, G.; Molina, A. Quantitative analysis of short- and long-distance racing performance in young and adult horses and association analysis with functional candidate genes in Spanish Trotter horses. J. Anim. Breed. Genet. 2016, 133, 347–356. [Google Scholar] [CrossRef]
- Jäderkvist Fegraeus, K.; Velie, B.D.; Axelsson, J.; Ang, R.; Hamilton, N.A.; Andersson, L.; Meadows, J.R.S.; Lindgren, G. A potential regulatory region near the EDN3 gene may control both harness racing performance and coat color variation in horses. Physiol. Rep. 2018, 6, e13700. [Google Scholar] [CrossRef]
- Thomas, J.C.; Khoury, R.; Neeley, C.K.; Akroush, A.M.; Davies, E.C. A fast CTAB method of human DNA isolation for polymerase chain reaction applications. Biochem. Educ. 1997, 25, 233–235. [Google Scholar] [CrossRef]
- Mazuet, C.; Legeay, C.; Sautereau, J.; Bouchier, C.; Criscuolo, A.; Bouvet, P.; Trehard, H.; Jourdan Da Silva, N.; Popoff, M. Characterization of Clostridium Baratii Type F Strains Responsible for an Outbreak of Botulism Linked to Beef Meat Consumption in France. PLoS Curr. 2017, 9. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Wingett, S.W.; Andrews, S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Res 2018, 7, 1338. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Foll, M.; Gaggiotti, O. A genome-scan method to identify selected loci appropriate for both dominant and codominant markers: A Bayesian perspective. Genetics 2008, 180, 977–993. [Google Scholar] [CrossRef] [PubMed]
- Wright, S. The genetical structure of populations. Ann. Eugen. 1950, 15, 323–354. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, X.; Qanbari, S.; Weigend, S.; Zhang, Q.; Simianer, H. Properties of different selection signature statistics and a new strategy for combining them. Heredity 2015, 115, 426–436. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C. Estimating F-statistics for the analysis of population structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef]
- Sabeti, P.C.; Schaffner, S.F.; Fry, B.; Lohmueller, J.; Varilly, P.; Shamovsky, O.; Palma, A.; Mikkelsen, T.S.; Altshuler, D.; Lander, E.S. Positive natural selection in the human lineage. Science 2006, 312, 1614–1620. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.R.; Lv, F.H.; He, S.G.; Tian, S.L.; Peng, W.F.; Sun, Y.W.; Zhao, Y.X.; Tu, X.L.; Zhang, M.; et al. Whole-Genome Sequencing of Native Sheep Provides Insights into Rapid Adaptations to Extreme Environments. Mol. Biol. Evol. 2016, 33, 2576–2592. [Google Scholar] [CrossRef]
- Qanbari, S.; Pausch, H.; Jansen, S.; Somel, M.; Strom, T.M.; Fries, R.; Nielsen, R.; Simianer, H. Classic selective sweeps revealed by massive sequencing in cattle. PLoS Genet. 2014, 10, e1004148. [Google Scholar] [CrossRef]
- Randhawa, I.A.; Khatkar, M.S.; Thomson, P.C.; Raadsma, H.W. A meta-assembly of selection signatures in cattle. PLoS ONE 2016, 11, e0153013. [Google Scholar] [CrossRef]
- Voight, B.F.; Kudaravalli, S.; Wen, X.; Pritchard, J.K. A map of recent positive selection in the human genome. PLoS Biol. 2006, 4, e72. [Google Scholar]
- Browning, S.R.; Browning, B.L. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 2007, 81, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, L.; Yang, L.; Zhao, G.; Li, J.; Liu, D.; Li, Y. Discovery of genomic characteristics and selection signatures in southern Chinese local cattle. Front. Genet. 2020, 11, 533052. [Google Scholar] [CrossRef] [PubMed]
- Szpiech, Z.A.; Hernandez, R.D. Selscan: An efficient multithreaded program to perform EHH-based scans for positive selection. Mol. Biol. Evol. 2014, 31, 2824–2827. [Google Scholar] [CrossRef]
- Dennis, G.; Sherman, B.T., Jr.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, P3. [Google Scholar] [CrossRef]
- Choi, J.W.; Liao, X.; Stothard, P.; Chung, W.H.; Jeon, H.J.; Miller, S.P.; Choi, S.Y.; Lee, J.K.; Yang, B.; Lee, K.T.; et al. Whole-genome analyses of Korean native and Holstein cattle breeds by massively parallel sequencing. PLoS ONE 2014, 9, e101127. [Google Scholar] [CrossRef]
- Choi, J.W.; Liao, X.; Park, S.; Jeon, H.J.; Chung, W.H.; Stothard, P.; Park, Y.S.; Lee, J.K.; Lee, K.T.; Kim, S.H.; et al. Massively parallel sequencing of Chikso (Korean brindle cattle) to discover genome-wide SNPs and InDels. Mol. Cells 2013, 36, 203–211. [Google Scholar] [CrossRef]
- Hill, E.W.; Gu, J.; McGivney, B.A.; MacHugh, D.E. Targets of selection in the Thoroughbred genome contain exercise-relevant gene SNPs associated with elite racecourse performance. Anim. Genet. 2010, 41 (Suppl. S2), 56–63. [Google Scholar] [CrossRef]
- Han, H.; McGivney, B.A.; Allen, L.; Bai, D.; Corduff, L.R.; Davaakhuu, G.; Davaasambuu, J.; Dorjgotov, D.; Hall, T.J.; Hemmings, A.J.; et al. Common protein-coding variants influence the racing phenotype in galloping racehorse breeds. Commun. Biol. 2022, 5, 1320. [Google Scholar] [CrossRef]
- Santos, W.B.; Pereira, C.B.; Maiorano, A.M.; Arce, C.D.S.; Baldassini, W.A.; Pereira, G.L.; Chardulo, L.A.L.; Neto, O.R.M.; Oliveira, H.N.; Curi, R.A. Genomic inbreeding estimation, runs of homozygosity, and heterozygosity-enriched regions uncover signals of selection in the Quarter Horse racing line. J. Anim. Breed. Genet. 2023, 140, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; McGivney, B.A.; Farries, G.; Katz, L.M.; MacHugh, D.E.; Randhawa, I.A.S.; Hill, E.W. Selection in Australian Thoroughbred horses acts on a locus associated with early two-year old speed. PLoS ONE 2020, 15, e0227212. [Google Scholar] [CrossRef] [PubMed]
- Eivers, S.S.; McGivney, B.A.; Fonseca, R.G.; MacHugh, D.E.; Menson, K.; Park, S.D.; Rivero, J.L.; Taylor, C.T.; Katz, L.M.; Hill, E.W. Alterations in oxidative gene expression in equine skeletal muscle following exercise and training. Physiol. Genom. 2010, 40, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef]
- Lira, V.A.; Benton, C.R.; Yan, Z.; Bonen, A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E145–E161. [Google Scholar] [CrossRef]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef]
- Kupr, B.; Handschin, C. Complex Coordination of Cell Plasticity by a PGC-1α-controlled Transcriptional Network in Skeletal Muscle. Front. Physiol. 2015, 6, 325. [Google Scholar] [CrossRef]
- Wright, D.C.; Han, D.H.; Garcia-Roves, P.M.; Geiger, P.C.; Jones, T.E.; Holloszy, J.O. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J. Biol. Chem. 2007, 282, 194–199. [Google Scholar] [CrossRef]
- Ikeda, S.; Kizaki, T.; Haga, S.; Ohno, H.; Takemasa, T. Acute exercise induces biphasic increase in respiratory mRNA in skeletal muscle. Biochem. Biophys. Res. Commun. 2008, 368, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Popov, D.V.; Lysenko, E.A.; Makhnovskii, P.A.; Kurochkina, N.S.; Vinogradova, O.L. Regulation of PPARGC1A gene expression in trained and untrained human skeletal muscle. Physiol. Rep. 2017, 5, e13339. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Hussien, R.; Oommen, S.; Gohil, K.; Brooks, G.A. Lactate sensitive transcription factor network in L6 cells: Activation of MCT1 and mitochondrial biogenesis. Faseb. J. 2007, 21, 2602–2612. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, Y.; Takeda, K.; Tamura, Y.; Hatta, H. Lactate administration increases mRNA expression of PGC-1α and UCP3 in mouse skeletal muscle. Appl. Physiol. Nutr. Metab. 2016, 41, 695–698. [Google Scholar] [CrossRef]
- Zhang, S.L.; Lu, W.S.; Yan, L.; Wu, M.C.; Xu, M.T.; Chen, L.H.; Cheng, H. Association between peroxisome proliferator-activated receptor-gamma coactivator-1alpha gene polymorphisms type 2 diabetes in southern Chinese population: Role of altered interaction with myocyte enhancer factor, 2.C. Chin. Med. J. 2007, 120, 1878–1885. [Google Scholar] [CrossRef]
- Lucia, A.; Gómez-Gallego, F.; Barroso, I.; Rabadán, M.; Bandrés, F.; San Juan, A.F.; Chicharro, J.L.; Ekelund, U.; Brage, S.; Earnest, C.P.; et al. PPARGC1A genotype (Gly482Ser) predicts exceptional endurance capacity in European men. J. Appl. Physiol. 2005, 99, 344–348. [Google Scholar] [CrossRef]
- Eynon, N.; Meckel, Y.; Sagiv, M.; Yamin, C.; Amir, R.; Sagiv, M.; Goldhammer, E.; Duarte, J.A.; Oliveira, J. Do PPARGC1A and PPARalpha polymorphisms influence sprint or endurance phenotypes? Scand. J. Med. Sci. Sports 2010, 20, e145–e150. [Google Scholar] [CrossRef]
- Gineviciene, V.; Jakaitiene, A.; Aksenov, M.O.; Aksenova, A.V.; Druzhevskaya, A.M.; Astratenkova, I.V.; Egorova, E.S.; Gabdrakhmanova, L.J.; Tubelis, L.; Kucinskas, V.; et al. Association analysis of ACE, ACTN3 and PPARGC1A gene polymorphisms in two cohorts of European strength and power athletes. Biol. Sport. 2016, 33, 199–206. [Google Scholar] [CrossRef]
- Jin, H.-J.; Hwang, I.-W.; Kim, K.-C.; Cho, H.-I.; Park, T.-H.; Shin, Y.-A.; Lee, H.-S.; Hwang, J.-H.; Kim, A.-R.; Lee, K.-H. Is there a relationship between PPARD T294C/PPARGC1A Gly482Ser variations and physical endurance performance in the Korean population? Genes. Genom. 2016, 38, 389–395. [Google Scholar] [CrossRef]
- Maciejewska, A.; Sawczuk, M.; Cieszczyk, P.; Mozhayskaya, I.A.; Ahmetov, I.I. The PPARGC1Agene Gly482Ser in Polish Russian athletes. J. Sports Sci. 2012, 30, 101–113. [Google Scholar] [CrossRef]
- Cheng, Z. FoxOtranscription factors in mitochondrial homeostasis. Biochem. J. 2022, 479, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z. The FoxO–autophagy axis in health and disease. Trends Endocrinol. Metab. 2019, 30, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z. FOXO1: Mute for a tuned metabolism? Trends Endocrinol. Metab. 2015, 26, 402–403. [Google Scholar] [CrossRef] [PubMed]
- Sedding, D.G. FoxO transcription factors in oxidative stress response and ageing--a new fork on the way to longevity? Biol. Chem. 2008, 389, 279–283. [Google Scholar] [CrossRef]
- Essers, M.A.; de Vries-Smits, L.M.; Barker, N.; Polderman, P.E.; Burgering, B.M.; Korswagen, H.C. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science 2005, 308, 1181–1184. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Candau, R.B.; Bernardi, H. FoxO transcription factors: Their roles in the maintenance of skeletal muscle homeostasis. Cell. Mol. Life Sci. 2014, 71, 1657–1671. [Google Scholar] [CrossRef]
- Weeks, K.L.; Tham, Y.K.; Yildiz, S.G.; Alexander, Y.; Donner, D.G.; Kiriazis, H.; Harmawan, C.A.; Hsu, A.; Bernardo, B.C.; Matsumoto, A.; et al. FOXO1 is required for physiological cardiac hypertrophy induced by exercise but not by constitutively active, P.I.3.K. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1470–H1485. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Bernardi, H.; Py, G.; Candau, R.B. Autophagy is essential to support skeletal muscle plasticity in response to endurance exercise. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2014, 307, R956–R969. [Google Scholar] [CrossRef]
- Koltai, E.; Bori, Z.; Osvath, P.; Ihasz, F.; Peter, S.; Toth, G.; Degens, H.; Rittweger, J.; Boldogh, I.; Radak, Z. Master athletes have higher miR-7, SIRT3 and SOD2 expression in skeletal muscle than age-matched sedentary controls. Redox Biol 2018, 19, 46–51. [Google Scholar] [CrossRef]
- Eivers, S.S.; McGivney, B.A.; Gu, J.; MacHugh, D.E.; Katz, L.M.; Hill, E.W. PGC-1α encoded by the PPARGC1A gene regulates oxidative energy metabolism in equine skeletal muscle during exercise. Anim. Genet. 2012, 43, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Marín-García, J. Cardiomyopathies: A Comparative Analysis of Phenotypes and Genotypes; Elsevier: Amsterdam, The Netherlands, 2014; pp. 363–426. [Google Scholar][Green Version]
- Sabharwal, R.; Chapleau, M.W. Autonomic, locomotor and cardiac abnormalities in a mouse model of muscular dystrophy: Targeting the renin–angiotensin system. Exp. Physiol. 2014, 99, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Palma-Flores, C.; Cano-Martínez, L.J.; Fernández-Valverde, F.; Torres-Pérez, I.; de Los Santos, S.; Hernández-Hernández, J.M.; Hernández-Herrera, A.F.; García, S.; Canto, P.; Zentella-Dehesa, A. Differential histological features myogenic protein levels in distinct muscles of d-sarcoglycan null muscular dystrophy mouse model. J. Mol. Histol. 2023, 54, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.A.; Kelly, S.M.; LoPresti, P.P.; Heydemann, A.; Earley, J.U.; Ferguson, E.L.; Wolf, M.J.; McNally, E.M. SMAD signaling drives heart and muscle dysfunction in a Drosophila model of muscular dystrophy. Hum. Mol. Genet. 2011, 20, 894–904. [Google Scholar] [CrossRef]
- Bround, M.J.; Havens, J.R.; York, A.J.; Sargent, M.A.; Karch, J.; Molkentin, J.D. ANT-dependent MPTP underlies necrotic myofiber death in muscular dystrophy. Sci. Adv. 2023, 9, eadi2767. [Google Scholar] [CrossRef]
- Bessho, C.; Yamada, S.; Tanida, T.; Tanaka, M. FoxP2 protein decreases at a specific region in the chick midbrain after hatching. Neurosci. Lett. 2023, 800, 137119. [Google Scholar] [CrossRef]
- Fisher, S.E.; Scharff, C. FOXP2 as a molecular window into speech and language. Trends Genet. 2009, 25, 166–177. [Google Scholar] [CrossRef]
- Rodríguez-Urgellés, E.; Casas-Torremocha, D.; Sancho-Balsells, A.; Ballasch, I.; García-García, E.; Miquel-Rio, L.; Manasanch, A.; Del Castillo, I.; Chen, W.; Pupak, A.; et al. Thalamic Foxp2 regulates output connectivity and sensory-motor impairments in a model of Huntington’s Disease. Cell Mol. Life Sci. 2023, 80, 367. [Google Scholar] [CrossRef]
- Feil, R.; Hofmann, F.; Kleppisch, T. Function of cGMP-dependent protein kinases in the nervous system. Rev. Neurosci. 2005, 16, 23–41. [Google Scholar] [CrossRef]
- Gao, S.; Yao, W.; Zhou, R.; Pei, Z. Exercise training affects calcium ion transport by downregulating the CACNA2D1 protein to reduce hypertension-induced myocardial injury in mice. iScience 2024, 27, 109351. [Google Scholar] [CrossRef]
- Woodhead, J.S.T.; Merry, T.L. Mitochondrial-derived peptides and exercise. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 130011. [Google Scholar] [CrossRef]
- Many, G.M.; Sanford, J.A.; Sagendorf, T.J.; Hou, Z.; Nigro, P.; Whytock, K.L.; Amar, D.; Caputo, T.; Gay, N.R.; Gaul, D.A.; et al. Sexual dimorphism and the multi-omic response to exercise training in rat subcutaneous white adipose tissue. Nat. Metab. 2024, 6, 963–979. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Li, X.; Yin, Y.; Wu, Z.; Liu, C.; Tekwe, C.D.; Wu, G. Regulatory roles for L-arginine in reducing white adipose tissue. Front. Biosci. 2012, 17, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.M.; Zanatta, A.; Knebel, L.A.; Schuck, P.F.; Tonin, A.M.; Ferreira Gda, C.; Amaral, A.U.; Dutra Filho, C.S.; Wannmacher, C.M.; Wajner, M. Experimental evidence that ornithine and homocitrulline disrupt energy metabolism in brain of young rats. Brain Res. 2009, 1291, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Choi, D.H.; Cheon, M.W.; Kim, J.G.; Kim, J.S.; Shin, M.G.; Kim, H.R.; Youn, D. Changes in Mitochondria-Related Gene Expression upon Acupuncture at LR3 in the D-Galactosamine-Induced Liver Damage Rat Model. Evid. Based Complement. Alternat. Med. 2022, 2022, 3294273. [Google Scholar] [CrossRef]
- Kay, L.; Nicolay, K.; Wieringa, B.; Saks, V.; Wallimann, T. Direct evidence for the control of mitochondrial respiration by mitochondrial creatine kinase in oxidative muscle cells in situ. J. Biol. Chem. 2000, 275, 6937–6944. [Google Scholar] [CrossRef]
- Touron, J.; Perrault, H.; Maisonnave, L.; Patrac, V.; Walrand, S.; Malpuech-Brugere, C.; Pereira, B.; Burelle, Y.; Costes, F.; Richard, R. Effects of exercise-induced metabolic and mechanical loading on skeletal muscle mitochondrial function in male rats. J. Appl. Physiol. 2022, 133, 611–621. [Google Scholar] [CrossRef]
- Kang, S.; Kang, B.H. Structure Function Inhibitors of the Mitochondrial Chaperone TRAP1. J. Med. Chem. 2022, 65, 16155–16172. [Google Scholar] [CrossRef]
- Lisanti, S.; Tavecchio, M.; Chae, Y.C.; Liu, Q.; Brice, A.K.; Thakur, M.L.; Languino, L.R.; Altieri, D.C. Deletion of the mitochondrial chaperone TRAP-1 uncovers global reprogramming of metabolic networks. Cell Rep. 2014, 8, 671–677. [Google Scholar] [CrossRef]
- Schmidt, J.F.; Andersen, T.R.; Andersen, L.J.; Randers, M.B.; Hornstrup, T.; Hansen, P.R.; Bangsbo, J.; Krustrup, P. Cardiovascular function is better in veteran football players than age-matched untrained elderly healthy men Scand. J. Med. Sci. Sports 2015, 25, 61–69. [Google Scholar]
- Mancini, A.; Vitucci, D.; Randers, M.B.; Schmidt, J.F.; Hagman, M.; Andersen, T.R.; Imperlini, E.; Mandola, A.; Orrù, S.; Krustrup, P.; et al. Lifelong Football Training: Effects on Autophagy and Healthy Longevity Promotion. Front. Physiol. 2019, 10, 132. [Google Scholar] [CrossRef]
- Mills, R.E.; Pittard, W.S.; Mullaney, J.M.; Farooq, U.; Creasy, T.H.; Mahurkar, A.A.; Kemeza, D.M.; Strassler, D.S.; Ponting, C.P.; Webber, C. Natural genetic variation caused by small insertions and deletions in the human genome. Genome Res. 2011, 21, 830–839. [Google Scholar] [CrossRef]
- Chen, X.; Nie, S.; Hu, L.; Fang, Y.; Cui, W.; Xu, H.; Zhao, C.; Zhu, B.F. Forensic efficacy evaluation and genetic structure exploration of the Yunnan Miao group by a multiplex InDel panel. Electrophoresis 2022, 43, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, C.; Xiao, C.; Chen, X.; Han, X.; Yi, S.; Huang, D. Mutation analysis of 28 autosomal short tandem repeats in the Chinese Han population. Mol. Biol. Rep. 2021, 48, 5363–5369. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chuang, T.-J.; Liao, B.-Y.; Chen, F.-C. Scanning for the signatures of positive selection for human-specific insertions and deletions. Genome Biol. Evol. 2009, 1, 415–419. [Google Scholar] [CrossRef]
- Boschiero, C.; Moreira, G.C.M.; Gheyas, A.A.; Godoy, T.F.; Gasparin, G.; Mariani, P.; Paduan, M.; Cesar, A.S.M.; Ledur, M.C.; Coutinho, L.L. Genome-wide characterization of genetic variants and putative regions under selection in meat and egg-type chicken lines. BMC Genom. 2018, 19, 83. [Google Scholar] [CrossRef] [PubMed]
- Rooney, M.F.; Hill, E.W.; Kelly, V.P.; Porter, R.K. The “speed gene” effect of myostatin arises in Thoroughbred horses due to a promoter proximal SINE insertion. PLoS ONE 2018, 13, e0205664. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.L.; Valberg, S.J.; Mickelson, J.R.; McCue, M.E. Haplotype diversity in the equine myostatin gene with focus on variants associated with race distance propensity and muscle fiber type proportions. Anim. Genet. 2014, 45, 827–835. [Google Scholar] [CrossRef]
- Rieder, S.; Taourit, S.; Mariat, D.; Langlois, B.; Guérin, G. Mutations in the agouti (ASIP), the extension (MC1R), and the brown (TYRP1) loci and their association to coat color phenotypes in horses (Equus caballus). Mamm. Genome 2001, 12, 450–455. [Google Scholar] [CrossRef]
- Biglari, S.; Afousi, A.G.; Mafi, F.; Shabkhiz, F. High-intensity interval training-induced hypertrophy in gastrocnemius muscle via improved IGF-I/Akt/FoxO and myostatin/Smad signaling pathways in rats. Physiol. Int. 2020, 107, 220–230. [Google Scholar] [CrossRef]
- Korge, P. Factors limiting adenosine triphosphatase function during high intensity exercise. Thermodynamic and regulatory considerations. Sports Med. 1995, 20, 215–225. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Gong, W.; Bou, T.; Shi, L.; Lin, Y.; Shi, X.; Li, Z.; Wu, H.; Dugarjaviin, M.; Bai, D. Whole-Genome Resequencing Analysis of Athletic Traits in Grassland-Thoroughbred. Animals 2025, 15, 2323. https://doi.org/10.3390/ani15152323
Ding W, Gong W, Bou T, Shi L, Lin Y, Shi X, Li Z, Wu H, Dugarjaviin M, Bai D. Whole-Genome Resequencing Analysis of Athletic Traits in Grassland-Thoroughbred. Animals. 2025; 15(15):2323. https://doi.org/10.3390/ani15152323
Chicago/Turabian StyleDing, Wenqi, Wendian Gong, Tugeqin Bou, Lin Shi, Yanan Lin, Xiaoyuan Shi, Zheng Li, Huize Wu, Manglai Dugarjaviin, and Dongyi Bai. 2025. "Whole-Genome Resequencing Analysis of Athletic Traits in Grassland-Thoroughbred" Animals 15, no. 15: 2323. https://doi.org/10.3390/ani15152323
APA StyleDing, W., Gong, W., Bou, T., Shi, L., Lin, Y., Shi, X., Li, Z., Wu, H., Dugarjaviin, M., & Bai, D. (2025). Whole-Genome Resequencing Analysis of Athletic Traits in Grassland-Thoroughbred. Animals, 15(15), 2323. https://doi.org/10.3390/ani15152323




