The Use of Humic Substances as an Additive to Feed Mixtures in Pheasant Breeding
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Laboratory Analysis
2.3. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Examination of Pheasant Droppings
3.3. Digestive Organs Characteristics
3.4. Short-Chain Fatty Acids and pH in the Contents of the Cecum
3.5. Serum Biochemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuźniacka, J.; Adamski, M.; Bernacki, Z. Effect of age and sex of pheasants (Phasianus colchicus L.) on selected physical properties and chemical composition of meat. Ann. Anim. Sci. 2007, 7, 45–53. [Google Scholar]
- Rojas, M.; González, I.; De la Cruz, S.; Hernández, P.E.; García, T.; Martín, R. Application of species-specific polymerase chain reaction assays to verify the labeling of quail (Coturnix coturnix), pheasant (Phasianus colchicus) and ostrich (Struthio camelus) in pet foods. Anim. Feed Sci. Technol. 2011, 169, 128–133. [Google Scholar] [CrossRef]
- Bird Life International. Birds in the European Union: A Status Assessment; Bird Life International: Wageningen, The Netherlands, 2004; pp. 1–50. [Google Scholar]
- European Commission. Regulation (EC) No 1831/2003 of the European parliament and of the council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Union. 2003, 268, 29–43. [Google Scholar]
- Aiken, G.R.; McKnight, D.M.; Wershaw, R.L.; MacCarthy, P. An Introduction to Humic Substance in Soil, Sediment, and Water, Humic Substances in Soil, Sediment, and Water, Ed.; Wiley: New York, NY, USA, 1985; pp. 1–13. [Google Scholar]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; Wiley-Inter-Science: New York, NY, USA, 1994; pp. 34–41. [Google Scholar]
- EMEA. Committee for Veterinary Medicinal Products. Humic Acids and Their Sodium Salts, Summary Report; European Agency for the Evaluation of Medicinal Products: Amsterdam, The Netherlands, 1999; Available online: https://www.ema.europa.eu/en/documents/mrl-report/humic-acids-and-their-sodium-salts-summary-report-committee-veterinary-medicinal-products_en.pdf (accessed on 28 August 2010).
- Ji, F.; McGlone, J.J.; Kim, S.W. Effects of dietary humic substances on pig growth performance, carcass characteristics, and ammonia emission. J. Anim. Sci. 2006, 84, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- Trckova, M.; Lorencova, A.; Babak, V.; Neca, J.; Ciganek, M. The effect of leonardite and lignite on the health of weaned piglets. Res. Vet. Sci. 2018, 119, 134–142. [Google Scholar] [CrossRef]
- Abdl Razek Mohmed Mohmed, S.; Elsebai, A.; Elghalid, O.A.; Abd El-Hady, A.M. Productive performance, lipid profile and caecum microbial counts of growing rabbits treated with humic acid. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1233–1241. [Google Scholar] [CrossRef]
- Lacková, Z.; Zigo, F.; Farkašová, Z.; Ondrašovičová, S. The effect of humic substances as an organic supplement on the fattening performance, quality of meat, and selected biochemical parameters of rabbits. Life 2022, 12, 1016. [Google Scholar] [CrossRef]
- Hassan, A.A.; Salem, A.Z.M.; Elghandour, M.M.Y.; Hafsa, S.A.; Reddy, P.R.K.; Atia, S.E.S.; Vidu, L. Humic substances isolated from clay soil may improve the ruminal fermentation, milk yield, and fatty acid profile: A novel approach in dairy cows. Anim. Feed Sci. Technol. 2020, 268, 114601. [Google Scholar] [CrossRef]
- Degirmencioglu, T. Using humic acid in diets for dairy goats. Anim. Sci. Pap. Rep. 2014, 32, 25–32. [Google Scholar]
- Šamudovská, A.; Demeterová, M. Effect of diet supplemented with natural humic compounds and sodium humate on performance and selected metabolic variables in broiler chickens. Acta Vet. Brno 2010, 79, 385–393. [Google Scholar] [CrossRef]
- Ozturk, E.; Ocak, N.; Turan, A.; Erener, G.; Altop, A.; Cankaya, S. Performance, carcass, gastrointestinal tract and meat quality traits, and selected blood parameters of broilers fed diets supplemented with humic substances. J. Sci. Food Agric. 2012, 92, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Rehman, A.; Saeed, M.; Abd El-Hack, M.E.; Arain, M.A.; Haseebarshad, M.; Zakria, H.M.; Abbasi, I.H. Impacts of dietary humic acid supplementation on growth performance, some blood metabolites and carcass traits of broiler chicks. Indian J. Anim. Sci. 2016, 86, 1073–1078. [Google Scholar] [CrossRef]
- Arpasova, H.; Kacaniova, M.; Pistova, V.; Galik, B.; Fik, M.; Hleba, L. Effect of probiotics and humic acid on egg production and quality parameters of laying hens eggs. Sci. Pap. Anim. Sci. Biotechnol. 2016, 49, 1–9. [Google Scholar]
- Taklimi, S.M.S.M.; Ghahri, H.; Isakan, M.A. Influence of different levels of humic acid and esterified glucomannan on growth performance and intestinal morphology of broiler chickens. Agric. Sci. 2012, 3, 663–668. [Google Scholar] [CrossRef]
- Mudroňová, D.; Karaffová, V.; Pešulová, T.; Koščová, J.; Cingeľová Maruščáková, I.; Bartkovský, M.; Marcinčáková, D.; Ševčíková, Z.; Marcinčák, S. The effect of humic substances on gut microbiota and immune response of broilers. Food Agr. Immunol. 2020, 31, 137–149. [Google Scholar] [CrossRef]
- Domínguez-Negrete, A.; Gómez-Rosales, S.; Angeles, M.d.L.; López-Hernández, L.H.; Reis de Souza, T.C.; Latorre-Cárdenas, J.D.; Téllez-Isaias, G. Addition of different levels of humic substances extracted from worm compost in broiler feeds. Animals 2021, 11, 3199. [Google Scholar] [CrossRef]
- Maguey-González, J.A.; Nava-Ramírez, M.d.J.; Gómez-Rosales, S.; Ángeles, M.d.L.; Solís-Cruz, B.; Hernández-Patlán, D.; Merino-Guzmán, R.; Hernandez-Velasco, X.; Hernández-Ramírez, J.O.; Loeza, I.; et al. Evaluation of the efficacy of humic acids to counteract the toxic effects of aflatoxin B1 in turkey poults. Front. Vet. Sci. 2023, 10, 1276754. [Google Scholar] [CrossRef]
- Gálik, B.; Hrnčár, C.; Gašparovič, M.; Rolinec, M.; Hanušovský, O.; Juráček, M.; Šimko, M.; Zábranský, L.; Kovacik, A. The effect of humic substances on the meat quality in the fattening of farm pheasants (Phasianus colchicus). Agriculture 2023, 13, 295. [Google Scholar] [CrossRef]
- Kovacik, A.; Gasparovic, M.; Tvrda, E.; Tokarova, K.; Kovacikova, E.; Rolinec, M.; Rumanova, L.; Capcarova, M.; Galik, B. Effects of humic acid diet on the serum biochemistry and oxidative status markers in pheasants. Vet. Med.-Czech. 2020, 65, 258–268. [Google Scholar] [CrossRef]
- Haluzina, L.I.; Harashchuk, M.I. Morphological characteristics of the pheasant blood modulated with the exposure to natural adaptogen. Theor. Appl. Vet. Med. 2023, 11, 29–33. [Google Scholar] [CrossRef]
- European Commission. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 33–79. [Google Scholar]
- European Commission. Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union. 2009, 54, 1–130. [Google Scholar]
- Kocabağli, N.; Alp, M.; Acar, N.; Kahraman, R. The effects of dietary humate supplementation on broiler growth and carcass yield. Poult. Sci. 2002, 81, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Avci, M.; Denek, N.; Kaplan, O. Effects of humic acid at different levels on growth performance, carcass yields and some biochemical parameters of quails. J. Anim. Vet. Adv. 2007, 6, 1–4. [Google Scholar]
- Abdel-Mageed, M.A.A. Effect of dietary humic substances supplementation on performance and immunity of Japanese quail. Egypt. Poult. Sci. J. 2012, 32, 645–660. [Google Scholar]
- El-Kelawy, M.I.; ELnaggar, A.S.; Abd El-khalek, E. The influence of supplementing broiler chickens with humic acid or biochar as natural growth promoters on their productive performance, nutrient digestibility, and physiological performance. Egypt. Poult. Sci. J. 2024, 44, 123–142. [Google Scholar] [CrossRef]
- ELnaggar, A.S.; El-Kelawy, M.I. Effect of humic acid supplementation on productive performance, blood constituents, immune response and carcass characteristics of Sasso chicken. Egyptian J. Anim. Prod. 2018, 55, 75–84. [Google Scholar] [CrossRef]
- Lala, A.O.; Okwelum, N.; Oso, A.O.; Ajao, A.O.; Adegbenjo, A.A. Response of broiler chickens to varying dosage of humic acid in drinking water. J. Anim. Prod. Res. 2017, 29, 288–294. [Google Scholar]
- Mao, Y. Modulation of the growth performance, meat composition, oxidative status, and immunity of broilers by dietary fulvic acids. Poult. Sci. 2019, 98, 4509–4513. [Google Scholar] [CrossRef]
- Liu, L.; Yang, N.; Chen, Y.; Xu, Z.; Zhang, Q.; Miao, X.; Zhao, Y.; Hu, G.; Liu, L.; Song, Z.; et al. Effects of fulvic acid on broiler performance, blood biochemistry, and intestinal microflora. Poult. Sci. 2024, 103, 103273. [Google Scholar] [CrossRef]
- Arif, M.; Rehman, A.; Abd El-Hack, M.E.; Saeed, M.; Khan, F.; Akhtar, M.; Swelum, A.A.; Saadeldin, I.M.; Alowaimer, A.N. Growth, carcass traits, cecal microbial counts, and blood chemistry of meat-type quail fed diets supplemented with humic acid and black cumin seeds. Asian-Australas. J. Anim. Sci. 2018, 31, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Elnesr, S.S.; Abdel-Razik, A.R.H.; Elwan, H.A.M. Impact of humate substances and Bacillus subtilis PB6 on thyroid activity and histomorphometry, iron profile and blood haematology of quail. J. Anim. Physiol. Anim. Nutr. 2022, 106, 110–117. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, O.M.; Abdallah, A.G.; Abdel-Latif, K.O. The influence of biological feed additives on broiler performance. Int. J. Poult. Sci. 2008, 7, 862–871. [Google Scholar] [CrossRef]
- Maguey-Gonzalez, J.A.; Michel, M.A.; Baxter, M.F.; Tellez, G., Jr.; Moore, P.A., Jr.; Solis-Cruz, B.; Hernández-Patlan, D.; Merino-Guzman, R.; Hernandez-Velasco, X.; Latorre, J.D.; et al. Effect of humic acids on intestinal viscosity, leaky gut and ammonia excretion in a 24 hr feed restriction model to induce intestinal permeability in broiler chickens. Anim. Sci. J. 2018, 89, 1002–1010. [Google Scholar] [CrossRef]
- Grashorn, M.A.; Gruzauskas, R.; Dauksiene, A.; Raceviciute-Stupeliene, A.; Zdunczyk, Z.; Juskiewicz, J.; Bliznikas, S.; Svirmickas, G.J.; Slausgalvis, V. Influence of organic acids supplement to the diet on functioning of the digestive system in laying hens. Arch. Geflügelk. 2013, 77, 155–159. [Google Scholar] [CrossRef]
- Hreško Šamudovská, A.; Bujňák, L.; Zigo, F. Carcass characteristic, caecal metabolites and dropping quality in broiler chickens fed diets containing a humic substances. Asian J. Agric. Food Sci. 2022, 10, 133–138. [Google Scholar] [CrossRef]
- Biggs, P.; Parsons, C.M. The effects of whole grains on nutrient digestibilities, growth performance, and cecal short-chain fatty acid concentrations in young chicks fed ground corn-soybean meal diets. Poult. Sci. 2009, 88, 1893–1905. [Google Scholar] [CrossRef]
- Lan, R.X.; Li, S.Q.; Zhao, Z.; An, L.L. Sodium butyrate as an effective feed additive to improve growth performance and gastrointestinal development in broilers. Vet. Med. Sci. 2020, 6, 491–499. [Google Scholar] [CrossRef]
- Abu Hafsa, S.H.; Hassan, A.A.; Sabek, A.; Elghandour, M.M.M.Y.; Barbabosa-Pliego, A.; Alqaisi, O.; Salem, A.Z.M. Extracted and characterized humic substances as feed supplement in rabbit feeding: Effects on performance, blood metabolites and caecal fermentation activity. Waste Biomass Valor. 2021, 12, 5471–5479. [Google Scholar] [CrossRef]
- Liao, X.; Shao, Y.; Sun, G.; Yang, Y.; Zhang, L.; Guo, Y.; Luo, X.; Lu, L. The relationship among gut microbiota, short-chain fatty acids, and intestinal morphology of growing and healthy broilers. Poult. Sci. 2020, 99, 5883–5895. [Google Scholar] [CrossRef]
- Shermer, C.L.; Maciorowski, K.G.; Bailey, C.A.; Byers, F.M.; Ricke, S.C. Caecal metabolites and microbial populations in chickens consuming diets containing a mined humate compound. J. Sci. Food Agric. 1998, 77, 479–486. [Google Scholar] [CrossRef]
- Hreško Šamudovská, A.; Karaffová, V.; Marcin, A.; Mihok, T.; Harčárová, M.; Koščová, J.; Hreško, S.; Bujňák, L. Effect of humic substances, Limosilactobacillus fermentum and their combinations on growth performance, fermentation activity of the microbial population and mucosal immunity in the digestive tract of turkeys. Ital. J. Anim. Sci. 2025, 24, 1323–1335. [Google Scholar] [CrossRef]
- Kaya, C.A.; Tuncer, S.D. The effects of humates on fattening performance, carcass quality and some blood parameters of broilers. J. Anim. Vet. Adv. 2009, 8, 281–284. [Google Scholar]
- Lala, A.O.; Okwelum, N.; Bello, K.O.; Famakinde, N.A.; Alamu, M.O. Comparative study between ISA Brown and Fulani Ecotype chickens supplemented with humic acid. Slovak J. Anim. Sci. 2016, 49, 68–75. [Google Scholar]
- Mohammadsadeghi, F.; Afsharmanesh, M.; Ebrahimnejad, H. The substitution of humic material complex with mineral premix in diet and interaction of that with probiotic on performance, intestinal morphology and microflora of chickens. Livest. Sci. 2019, 228, 1–4. [Google Scholar] [CrossRef]
- Jaďuttová, I.; Marcinčáková, D.; Bartkovský, M.; Semjon, B.; Harčárová, M.; Nagyová, A.; Váczi, P.; Marcinčák, S. The effect of dietary humic substances on the fattening performance, carcass yield, blood biochemistry parameters and bone mineral profile of broiler chickens. Acta Vet. Brno 2019, 88, 307–313. [Google Scholar] [CrossRef]
- Hreško Šamudovská, A.; Bujňák, L.; Marcin, A.; Mihok, T.; Harčárová, M.; Zábranský, L.; Naď, P. Influence of humic substances on growth performance and blood serum parameters in fattening turkeys. Pol. J. Vet. Sci. 2024, 27, 165–172. [Google Scholar] [CrossRef]
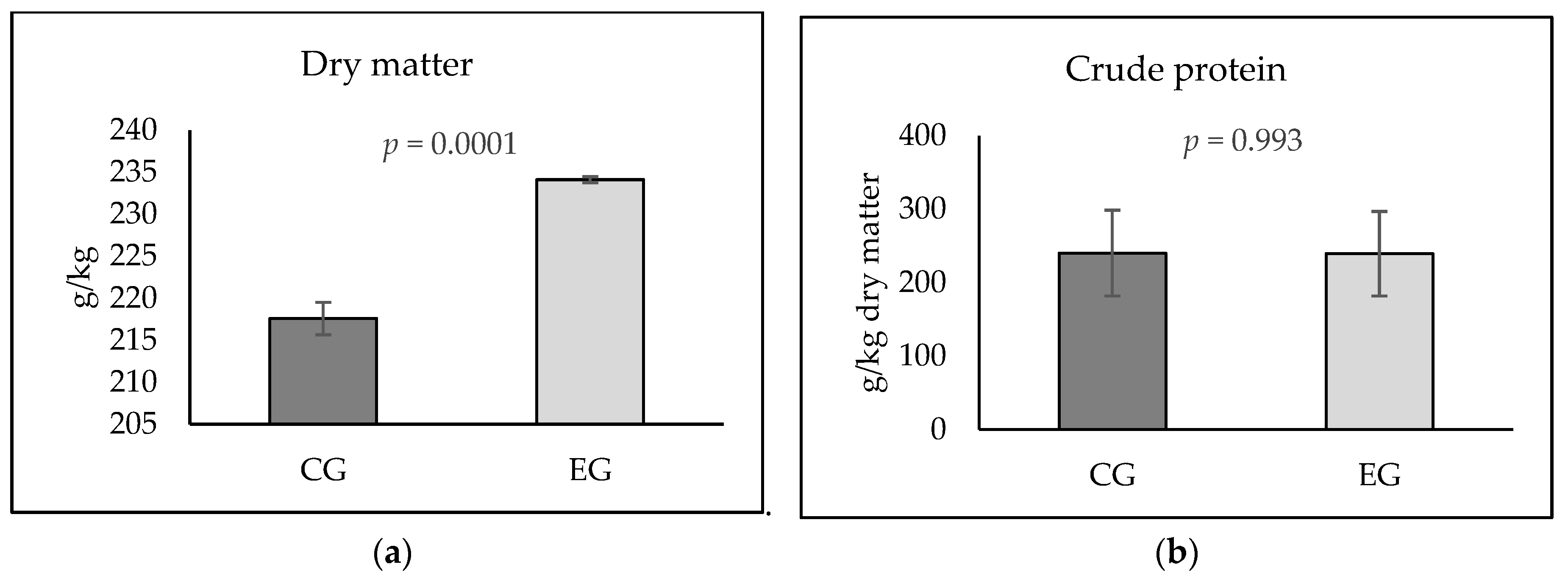
| Starter Feed Mixtures (1–28 Days) | Grower Feed Mixtures (29–49 Days) | |||
|---|---|---|---|---|
| CG | EG | CG | EG | |
| Dry matter (g/kg) | 889.0 | 890.8 | 889.8 | 887.5 |
| Crude protein (g/kg DM) | 274.7 | 265.4 | 245.0 | 242.1 |
| Ether extract (g/kg DM) | 23.4 | 24.8 | 21.9 | 24.3 |
| Crude fiber (g/kg DM) | 51.0 | 49.1 | 45.0 | 45.4 |
| Ash (g/kg DM) | 91.5 | 92.5 | 82.5 | 81.7 |
| ME (MJ/kg DM) | 12.0 | 12.0 | 12.1 | 12.1 |
| CG | EG | SEM | p-Value | |
|---|---|---|---|---|
| 1st day | 18.16 | 18.80 | 0.182 | 0.074 |
| 28th day | 199.13 | 196.44 | 1.379 | 0.361 |
| 49th day | 403.71 b | 415.24 a | 2.298 | 0.003 |
| CG | EG | SEM | p-Value | |
|---|---|---|---|---|
| Average daily weight gain (g/pc) | ||||
| 1–28 days | 6.46 | 6.34 | 0.050 | 0.258 |
| 29–49 days | 9.74 b | 10.42 a | 0.149 | 0.011 |
| 1–49 days | 7.87 b | 8.09 a | 0.045 | 0.004 |
| Average daily feed intake (g/pc/day) | ||||
| 1–28 days | 13.25 | 14.38 | 0.337 | 0.097 |
| 29–49 days | 35.05 | 35.71 | 0.312 | 0.318 |
| 1–49 days | 22.60 | 23.52 | 0.253 | 0.062 |
| Feed conversion ratio (kg/kg) | ||||
| 1–28 days | 2.05 b | 2.27 a | 0.057 | 0.049 |
| 29–49 days | 3.60 a | 3.43 b | 0.044 | 0.049 |
| 1–49 days | 2.87 | 2.91 | 0.028 | 0.564 |
| CG | EG | SEM | p-Value | |
|---|---|---|---|---|
| Proventriculus | 0.54 | 0.56 | 0.014 | 0.495 |
| Gizzard | 2.42 b | 2.71 a | 0.059 | 0.013 |
| Duodenum | 0.87 | 0.96 | 0.031 | 0.180 |
| Jejunum | 1.31 b | 1.54 a | 0.052 | 0.028 |
| Ileum | 1.13 | 1.22 | 0.051 | 0.400 |
| Small intestine | 3.32 b | 3.72 a | 0.088 | 0.022 |
| Pancreas | 0.34 | 0.33 | 0.012 | 0.728 |
| Liver | 2.36 | 2.43 | 0.052 | 0.533 |
| CG | EG | SEM | p-Value | |
|---|---|---|---|---|
| the relative length (cm/100 g body weight) | ||||
| Duodenum | 3.66 b | 4.16 a | 0.095 | 0.008 |
| Jejunum | 8.92 | 9.22 | 0.206 | 0.478 |
| Ileum | 9.11 | 9.32 | 0.217 | 0.638 |
| Small intestine | 21.69 | 22.70 | 0.414 | 0.237 |
| % of the length of the small intestine | ||||
| Duodenum | 16.94 b | 18.35 a | 0.358 | 0.046 |
| Jejunum | 41.10 | 40.64 | 0.555 | 0.690 |
| Ileum | 41.96 | 41.01 | 0.501 | 0.356 |
| CG | EG | SEM | p-Value | |
|---|---|---|---|---|
| Acetic acid | 64.17 | 70.33 | 3.520 | 0.398 |
| Propionic acid | 32.11 | 34.69 | 2.402 | 0.610 |
| Butyric acid | 18.75 | 19.80 | 1.815 | 0.784 |
| Total SCFAs | 115.02 | 124.83 | 6.466 | 0.467 |
| Cecal pH | 6.07 | 6.07 | 0.108 | 0.988 |
| CG | EG | SEM | p-Value | |
|---|---|---|---|---|
| Total protein (g/L) | 35.74 | 33.27 | 0.677 | 0.061 |
| Albumin (g/L) | 15.30 | 13.93 | 0.395 | 0.128 |
| Globulin (g/L) | 20.45 | 19.34 | 0.421 | 0.146 |
| Urea (mmol/L) | 0.90 | 0.77 | 0.058 | 0.258 |
| Uric acid (μmol/L) | 455.73 | 387.80 | 21.878 | 0.124 |
| Glucose (mmol/L) | 19.18 | 20.06 | 0.290 | 0.124 |
| Total lipids (g/L) | 3.55 | 3.18 | 0.143 | 0.197 |
| Cholesterol (mmol/L) | 3.04 | 2.95 | 0.134 | 0.787 |
| Triacylglycerols (mmol/L) | 1.02 | 1.12 | 0.025 | 0.063 |
| ALP (μkat/L) | 33.50 | 31.13 | 1.055 | 0.255 |
| AST (μkat/L) | 3.40 | 3.30 | 0.201 | 0.778 |
| Calcium (mmol/L) | 2.38 a | 2.23 b | 0.037 | 0.038 |
| Phosphorus (mmol/L) | 2.21 | 2.06 | 0.056 | 0.180 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hreško Šamudovská, A.; Hreško, S.; Maskaľová, I.; Tvrdá, A.; Bujňák, L. The Use of Humic Substances as an Additive to Feed Mixtures in Pheasant Breeding. Animals 2025, 15, 2321. https://doi.org/10.3390/ani15152321
Hreško Šamudovská A, Hreško S, Maskaľová I, Tvrdá A, Bujňák L. The Use of Humic Substances as an Additive to Feed Mixtures in Pheasant Breeding. Animals. 2025; 15(15):2321. https://doi.org/10.3390/ani15152321
Chicago/Turabian StyleHreško Šamudovská, Alena, Stanislav Hreško, Iveta Maskaľová, Alica Tvrdá, and Lukáš Bujňák. 2025. "The Use of Humic Substances as an Additive to Feed Mixtures in Pheasant Breeding" Animals 15, no. 15: 2321. https://doi.org/10.3390/ani15152321
APA StyleHreško Šamudovská, A., Hreško, S., Maskaľová, I., Tvrdá, A., & Bujňák, L. (2025). The Use of Humic Substances as an Additive to Feed Mixtures in Pheasant Breeding. Animals, 15(15), 2321. https://doi.org/10.3390/ani15152321






