The Role of Sensor Technologies in Estrus Detection in Beef Cattle: A Review of Current Applications
Simple Summary
Abstract
1. Introduction
2. Literature Review Methodology
3. Estrous Cycle Physiology
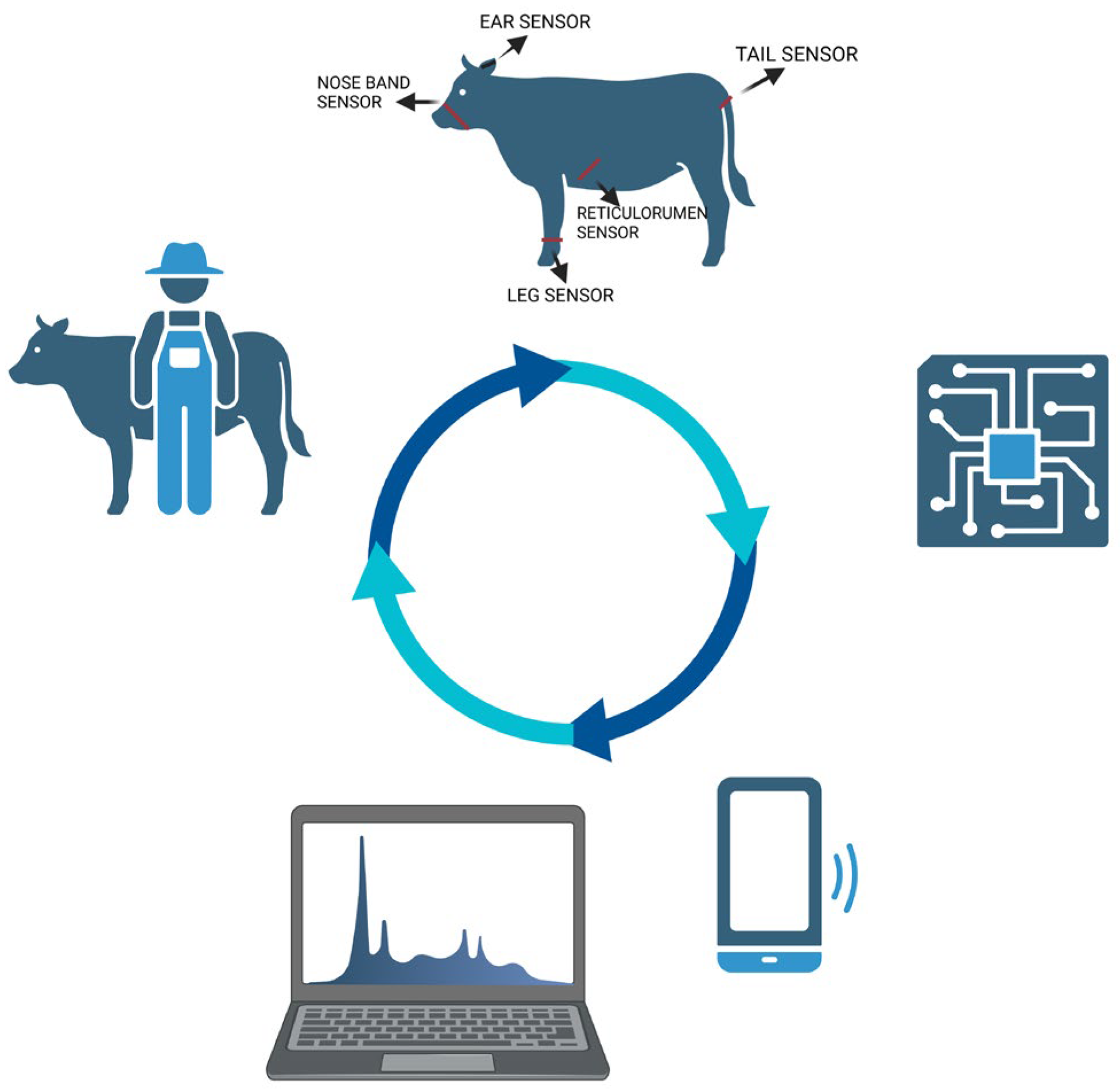
4. Sensor-Based Estrous Detection
4.1. Pedometer and Accelerometers
4.2. Limination of Sensor Use and Animal Welfare Challenges
5. Infrared Thermography
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitnall, T.; Pitts, N. Global trends in meat consumption. Agric. Commod. 2019, 9, 96–99. [Google Scholar] [CrossRef]
- Mottet, A.; De Haan, C.; Falcucci, A.; Tempio, G.; Opio, C.; Gerber, P. Livestock: On our plates or eating at our table? A new analysis of the feed/food debate. Glob. Food Secur. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Holroyd, R.G.; McGowan, M.R. Reproductive management of beef cattle. Beef Cattle Prod. Trade 2014, 291, 57–73. [Google Scholar]
- Severino Lendechy, V.H.; Montiel Palacios, F.; Ahuja Aguirre, C.C.; Gómez de Lucio, H.; Piñeiro Vázquez, A.T.; Chay Canul, A.J. Effect of restricted suckling and feed supplementation on postpartum follicular development and ovarian activity in beef cows. Rev. Bio Cienc. 2020, 7, e732. [Google Scholar] [CrossRef]
- Taher, J.K.; Hussain, S.O. Study of estrogen, progesterone and glucose level in cows during overt and silent estrus. Thi-Qar Univ. J. Agric. Res. 2022, 11, 79–83. [Google Scholar] [CrossRef]
- Bo, G.A.; Cutais, L.; Peres, L.C.; Pincinato, D.; Maraña, D.; Baruselli, P.S. Technologies for fixed–time artificial insemination and their influence on reproductive performance of Bos indicus cattle. Biosci. Proc. 2019, 6, 223–236. [Google Scholar] [CrossRef]
- Vicentini, R.R.; Montanholi, Y.R.; Veroneze, R.; Oliveira, A.P.; Lima, M.L.P.; Ujita, A.; El Faro, L. Infrared thermography reveals surface body temperature changes during proestrus and estrus reproductive phases in Gyr heifers (Bos taurus indicus). J. Therm. Biol. 2020, 92, 102662. [Google Scholar] [CrossRef]
- Bova, T.L.; Chiavaccini, L.; Cline, G.F.; Hart, C.G.; Matheny, K.; Muth, A.M.; Voelz, B.E.; Kesler, D.; Memili, E. Environmental stressors influencing hormones and systems physiology in cattle. Reprod. Biol. Endocrinol. 2014, 12, 58. [Google Scholar] [CrossRef]
- Moorey, S.E.; Read, C.C.; Perry, G.A.; Pohler, K.G.; McLean, M.K.; Ciernia, L.A.; Ketchum, J.N.; Smith, M.F.; Patterson, D.J. Physiology of the estrus cycle. In Proceedings of the Applied Reproductive Strategies in Beef Cattle, North Platte, NE, USA, 20–21 August 2019. [Google Scholar]
- Van Eerdenburg, F.; Loeffler, H.; Van Vliet, J.H. Detection of oestrus in dairy cows: A new approach to an old problem. Vet. Q. 1996, 18, 52–54. [Google Scholar] [CrossRef]
- Maatje, K.; Loeffler, S.H.; Engel, B. Predicting optimal time of insemination in cows that show visual signs of estrus by estimating onset of estrus with pedometers. J. Dairy Sci. 1997, 80, 1098–1105. [Google Scholar] [CrossRef]
- Wangler, A.; Meyer, A.; Rehbock, F.; Sanftleben, P. Wie effizient ist die Aktivitätsmessung als ein Hilfsmittel in der Brunsterkennung bei Milchrindern. Züchtungskunde 2005, 77, 110–127. [Google Scholar] [CrossRef]
- Kerbrat, S.; Disenhaus, C. A proposition for an updated behavioural characterisation of the oestrus period in dairy cows. Appl. Anim. Behav. Sci. 2004, 87, 223–238. [Google Scholar] [CrossRef]
- Shine, P.; Murphy, M.D. Over 20 Years of Machine Learning Applications on Dairy Farms: A Comprehensive Mapping Study. Sensors 2022, 22, 52. [Google Scholar] [CrossRef]
- Cushman, R.A.; Kaps, M.; Snider, A.P.; Crouse, M.S.; Woodbury, B.L.; Keel, B.N.; McCarthy, K.L. Relationship of length of the estrous cycle to antral follicle number in crossbred beef heifers. Transl. Anim. Sci. 2024, 8, txae074. [Google Scholar] [CrossRef]
- Cooper–Prado, M.J.; Long, N.M.; Wright, E.C.; Goad, C.L.; Wettemann, R.P. Relationship of ruminal temperature with parturition and estrus of beef cows. J. Anim. Sci. 2011, 89, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kwon, W.; Ha, J.; Moon, J.; Yi, J. Increased accuracy of estrus prediction using ruminoreticular biocapsule sensors in Hanwoo (Bos taurus coreanae) cows. J. Anim. Sci. Technol. 2023, 65, 759. [Google Scholar] [CrossRef] [PubMed]
- Haadem, C.S.; Holmøy, I.H.; Nødtvedt, A.; Martin, A.D. Time of insemination in relation to pregnancy rates in beef cattle after oestrus detection with automated activity monitoring system. Acta Vet. Scand. 2023, 65, 20. [Google Scholar] [CrossRef] [PubMed]
- Hojo, T.; Sakatani, M.; Takenouchi, N. Efficiency of a pedometer device for detecting estrus in standing heat and silent heat in Japanese Black cattle. Anim. Sci. J. 2018, 89, 1067–1072. [Google Scholar] [CrossRef]
- Michelena, A.; Fontenla-Romero, O.; Calvo-Rolle, J.L. A review and future trends of precision livestock over dairy and beef cow cattle with artificial intelligence. Log. J. IGPL 2024, 33, jzae111. [Google Scholar] [CrossRef]
- Yoshioka, H.; Ito, M.; Tanimoto, Y. Effectiveness of a real–time radiotelemetric pedometer for estrus detection and insemination in Japanese Black cows. J. Reprod. Dev. 2010, 56, 351–355. [Google Scholar] [CrossRef]
- Richeson, J.T.; Lawrence, T.E.; White, B.J. Using advanced technologies to quantify beef cattle behavior. Transl. Anim. Sci. 2018, 2, 223–229. [Google Scholar] [CrossRef]
- Robért, B.D.; White, B.J.; Renter, D.G.; Larson, R.L. Determination of lying behavior patterns in healthy beef cattle by use of wireless accelerometers. Am. J. Vet. Res. 2011, 72, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Fromm, K.; Heinicke, J.; Ammon, C.; Amon, T.; Hoffmann, G. Performance of a UHF RFID Detection System to Assess Activity Levels and Lying Behaviour in Fattening Bulls. AgriEngineering 2024, 6, 1886–1897. [Google Scholar] [CrossRef]
- Jobirov, F.; Yuejie, Z.; Kibona, C.A. Evaluating profitability of beef cattle farming and its determinants among smallholder beef cattle farmers in the Baljovan District of Khatlon region, Tajikistan. PLoS ONE 2022, 17, e0274391. [Google Scholar] [CrossRef]
- Willy, B.T.; Beyene, A.B.; Amare, D.M. The determinants of beef cattle market participation on beef cattle producers’ welfare: A case study of West Shewa Zone, Oromia Region, Ethiopia. Adv. Agric. 2023, 2023, 8822032. [Google Scholar] [CrossRef]
- Pezeshki, A.; Stordeur, P.; Wallemacq, H.; Schynts, F.; Stevens, M.; Boutet, P.; Peelman, L.J.; De Spiegeleer, B.; Duchateau, L.; Bureau, F. Variation of inflammatory dynamics and mediators in primiparous cows after intramammary challenge with Escherichia coli. Vet. Res. 2011, 42, 15. [Google Scholar] [CrossRef]
- Uskenov, R.; Issabekova, S.; Mukhanbetkaliyeva, A.; Akibekov, O.; Zhagipar, F. Digital technologies in dairy cattle breeding to improve the reproductive function of cows and heifers: A case study in Northern Kazakhstan. Vet. World 2024, 17, 2385–2397. [Google Scholar] [CrossRef]
- Hockey, C.D.; Morton, J.M.; Norman, S.T.; McGowan, M.R. Evaluation of a neck mounted 2-hourly activity meter system for detecting cows about to ovulate in two paddock-based Australian dairy herds. Reprod. Domest. Anim. 2010, 45, 107–117. [Google Scholar] [CrossRef]
- Neethirajan, S. The role of sensors, big data and machine learning in modern animal farming. Sens. Bio-Sens. Res. 2020, 29, 100367. [Google Scholar] [CrossRef]
- Johnston, D.J.; Dayman, M.; Grant, T.P.; Hubbard, K.; Goodwin, K.; Doughty, A.K.; Cook, J.D. Remote Sensor Collars Measure Age at Puberty in Tropical Beef Heifers in Northern Australia; Association for the Advancement of Animal Breeding and Genetics (AAABG): Armidale, NSW, Australia, 2023; pp. 246–249. [Google Scholar]
- Ferreira, C.; Todorovic, M.; Sugrue, P.; Teixeira, S.; Galvin, P. Review: Emerging sensors and instrumentation systems for bovine health monitoring. Animal 2025, 19, 101527. [Google Scholar] [CrossRef]
- Cutress, D. The Future of Technologies for Cattle Fertility and Calf Health; Farming Connect: Aberystwyth, UK, 2020; pp. 1–7. [Google Scholar]
- Wrenn, T.R.; Bitman, J.; Sykes, J.F. Body temperature variations in dairy cattle during the estrous cycle and pregnancy. J. Dairy Sci. 1958, 41, 1071–1076. [Google Scholar] [CrossRef]
- Naylor, J.M.; Streeter, R.M.; Torgerson, P. Factors affecting rectal temperature measurement using commonly available digital thermometers. Res. Vet. Sci. 2012, 92, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Tuyttens, F.A.M.; Molento, C.F.M.; Benaissa, S. Twelve Threats of Precision Livestock Farming (PLF) for Animal Welfare. Front. Vet. Sci. 2022, 9, 889623. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Koundal, D. Cattle health monitoring system using wireless sensor network: A survey from innovation perspective. IET Wirel. Sens. Syst. 2018, 8, 143–151. [Google Scholar] [CrossRef]
- Musa, P.; Sugeru, H.; Wibowo, E.P. Wireless Sensor Networks for Precision Agriculture: A Review of NPK Sensor Implementations. Sensors 2024, 24, 51. [Google Scholar] [CrossRef]
- Carneiro, M.W.; Brancato, L.; Wylleman, B.; van Zwol, E.; Conings, L.; Vueghs, P.; Gorbaslieva, I.; Van den Bossche, J.; Rudenko, O.; Janicot, M. Safety evaluation of long-term temperature controlled whole-body thermal treatment in female Aachen minipig. Int. J. Hyperth. 2021, 38, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Caola, G.; Refinetti, R. Daily and estrous rhythmicity of body temperature in domestic cattle. BMC Physiol. 2003, 3, 7. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Wang, C.; Zhang, Y.; Zong, Z.; Wang, H.; Su, L.; Du, Y. A non-contact cow estrus monitoring method based on the thermal infrared images of cows. Agriculture 2023, 13, 385. [Google Scholar] [CrossRef]
- Talukder, S.; Kerrisk, K.L.; Ingenhoff, L.; Thomson, P.C.; Garcia, S.C.; Celi, P. Infrared technology for estrus detection and as a predictor of time of ovulation in dairy cows in a pasture–based system. Theriogenology 2014, 81, 925–935. [Google Scholar] [CrossRef]
- Riaz, U.; Idris, M.; Ahmed, M.; Ali, F.; Farooq, U.; Yang, L. The Potential of Infrared Thermography for Early Pregnancy Diagnosis in Nili-Ravi Buffaloes. Animals 2024, 14, 1966. [Google Scholar] [CrossRef]
- Marquez, H.P.; Ambrose, D.J.; Schaefer, A.L.; Cook, N.J.; Bench, C.J. Evaluation of infrared thermography combined with behavioral biometrics for estrus detection in naturally cycling dairy cows. Animal 2021, 15, 100205. [Google Scholar] [CrossRef]
- Skliarov, P.; Fedorenko, S.; Naumenko, S.; Onyshchenko, O.; Bilyi, D.; Mylostyvyi, R.; Vakulyk, V.; Kuraksina, L. Infrared thermography as a method of diagnosing reproductive pathologies in animals. Multidiscip. Rev. 2023, 6, 2023007. [Google Scholar] [CrossRef]
- Burfeind, O.; Von Keyserlingk, M.; Weary, D.M.; Veira, D.M.; Heuwieser, W. Repeatability of measures of rectal temperature in dairy cows. J. Dairy Sci. 2010, 93, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Lunstra, D.D.; Coulter, G.H. Scrotal thermography as a tool for predicting semen quality and natural–mating fertility in young beef bulls. Beef Res. Program Prog. Rep. 1993, 4, 85–89. [Google Scholar]
- Wang, Y.; Kang, X.; Chu, M.; Liu, G. Deep learning-based automatic dairy cow ocular surface temperature detection from thermal images. Comput. Electron. Agric. 2022, 202, 107429. [Google Scholar] [CrossRef]
- Church, J.S.; Hegadoren, P.R.; Paetkau, M.J.; Miller, C.C.; Regev-Shoshani, G.; Schaefer, A.L.; Schwartzkopf-Genswein, K.S. Influence of environmental factors on infrared eye temperature measurements in cattle. Res. Vet. Sci. 2014, 96, 220–226. [Google Scholar] [CrossRef]
- Talukder, S.; Thomson, P.C.; Kerrisk, K.L.; Clark, C.; Celi, P. Evaluation of infrared thermography body temperature and collar–mounted accelerometer and acoustic technology for predicting time of ovulation of cows in a pasture-based system. Theriogenology 2015, 83, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Radigonda, V.L.; Pereira, G.R.; Da Cruz Favaro, P.; Barca Júnior, F.A.; Borges, M.H.F.; Galdioli, V.H.G.; Júnior, C.K. Infrared thermography relationship between the temperature of the vulvar skin, ovarian activity, and pregnancy rates in Braford cows. Trop. Anim. Health Prod. 2017, 49, 1787–1791. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, R.; Inoue, S.; Yorozui, Y.; Ichikawa, R.; Yamada, N.; Higashi, S.; Matsuyama, S.; Tsukamura, H.; Ohkura, S.; Uenoyama, Y. Capturing temperature changes on the ocular surface along with estrus and ovulation using infrared thermography in Japanese Black cows. J. Reprod. Dev. 2024, 70, 49–54. [Google Scholar] [CrossRef] [PubMed]
- De Ruediger, F.R.; Yamada, P.H.; Barbosa, L.G.B.; Chacur, M.G.M.; Ferreira, J.C.P.; de Carvalho, N.A.T.; Soriano, G.A.M.; Codognoto, V.M.; Oba, E. Effect of estrous cycle phase on vulvar, orbital area and muzzle surface temperatures as determined using digital infrared thermography in buffalo. Anim. Reprod. Sci. 2018, 197, 154–161. [Google Scholar] [CrossRef]
- George, W.D.; Godfrey, R.W.; Ketring, R.C.; Vinson, M.C.; Willard, S.T. Relationship among eye and muzzle temperatures measured using digital infrared thermal imaging and vaginal and rectal temperatures in hair sheep and cattle. J. Anim. Sci. 2014, 92, 4949–4955. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Park, G.; Na, Y.; Kwon, H.; Lee, S. PSII–16 Thermal sensor-based multiple object tracking system for estrus detection in Korean native beef cattle. J. Anim. Sci. 2019, 97, 238. [Google Scholar] [CrossRef]
- Casas-Alvarado, A.; Mota-Rojas, D.; Hernández-Ávalos, I.; Mora-Medina, P.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Reyes-Sotelo, B.; Martínez-Burnes, J. Advances in infrared thermography: Surgical aspects, vascular changes, and pain monitoring in veterinary medicine. J. Therm. Biol. 2020, 92, 102664. [Google Scholar] [CrossRef]
- Racewicz, P.; Sobek, J.; Majewski, M.; Różańska-Zawieja, J. The use of thermal imaging measurements in dairy cow herds. Rocz. Nauk. Pol. Tow. Zootech. 2018, 14, 55–69. [Google Scholar] [CrossRef]
- Riaz, U.; Idris, M.; Ahmed, M.; Ali, F.; Yang, L. Infrared Thermography as a Potential Non-Invasive Tool for Estrus Detection in Cattle and Buffaloes. Animals 2023, 13, 1425. [Google Scholar] [CrossRef] [PubMed]

| Type of Sensor | Product Name, Company, Country | Sensitivity | Application | Parameters Measured | References |
|---|---|---|---|---|---|
| Accelerometer, Ear tag | HeatTime Pro+ (Allflex, Madison, WI, USA) | 85–95% | Activity-based estrus detection | Physical activity, rest patterns | [16] |
| Accelerometer, Ear tag | CowManager (Agis, Harmelen, The Netherlands) | 85–95% | Activity-based estrus detection | Physical activity, rumination | [33] |
| Accelerometer, Neck collar | Nedap (Nedap Livestock Management, Groenlo, The Netherlands) | 79–94% | Estrus and health tracking | Physical activity | [34] |
| Accelerometer, Leg sensor | Gyuho (Comtech, Tokyo, Japan) | 95% | Activity and estrus detection | Physical activity | [31] |
| Accelerometer, Neck collar | Heatime (SCR Engineers Ltd., Netanya, Israel) | 85–95% | Estrus and reproductive health | Activity, rest patterns, feeding | [21] |
| Ruminoreticular biocapsule sensor | LiveCare (uLikeKorea, Seoul, Republic of Korea) | 98–100% | Estrus and health tracking | Activity, body temperature | [18] |
| Multi-parameter Sensor, Neck collar | SenseHub Beef (Allflex Livestock Intelligence, Madison, WI, USA) | 92% | Estrus detection, health monitoring | Activity, temperature, health | [33] |
| Thermometer, Ruminoreticular bolus | SmartStock (Smartstock, Boston, MA, USA) | - | Estrus and calving detection | Temperature | [17] |
| Biosensor, Ear tag | Moocall HEAT (Moocall Ltd., Limerick, Ireland) | 88% | Estrus detection | Activity, health | [35] |
| Reference | IRT Camera | Breed of Cattle | Site of IRT Observation | Conclusion |
|---|---|---|---|---|
| Radigonda et al., 2017 [51] | FLIR T300 | Braford | Vulva | Infrared thermography was shown to be a reliable, non-invasive method for identifying estrus in Braford cows by detecting changes in vulvar temperature linked to ovarian activity. Cows in estrus displayed distinct temperature patterns compared to non-estrus cows. However, the accuracy of this technique can be affected by environmental conditions. |
| Ozaki et al., 2024 [52] | Video-based infrared camera ARGO P1-400 | Japanese Black cows | Vulva, eyes, and pelvic area | The study demonstrates that monitoring ocular temperature is an effective, non-invasive way to predict ovulation in cows, even when typical signs of estrus are minimal. However, its reliability decreases in the presence of follicular cysts. |
| De Ruediger et al., 2018 [53] | FLIR E40 | Murrah | Vulva, muzzle, and orbital area | The findings indicate that vulvar surface temperature is a consistent marker of hormonal changes linked to the estrous cycle in buffalo. In contrast, temperatures measured at the muzzle and around the eyes are more closely influenced by core body temperature and external environmental conditions. |
| George et al., 2014 [54] | FLIR ThermaCAM P65HS | Senepol | Eyes and muzzle | The study shows that eye temperature measurement using thermography offers a practical, non-invasive way to monitor cattle body temperature. Although cost currently limits widespread use, technological advancements and reduced prices could make it more accessible for routine health and welfare monitoring. |
| Kang et al., 2019 [55] | FLIR A615 | Hanwoo | Topographic body surface | The study demonstrates that a thermal imaging-based tracking system can accurately identify estrus in Hanwoo beef cattle by monitoring behavioral changes such as increased activity and decreased feed intake. This approach offers a precise, non-invasive solution for enhancing estrus detection in beef herds. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merkelytė, I.; Šiukščius, A.; Nainienė, R. The Role of Sensor Technologies in Estrus Detection in Beef Cattle: A Review of Current Applications. Animals 2025, 15, 2313. https://doi.org/10.3390/ani15152313
Merkelytė I, Šiukščius A, Nainienė R. The Role of Sensor Technologies in Estrus Detection in Beef Cattle: A Review of Current Applications. Animals. 2025; 15(15):2313. https://doi.org/10.3390/ani15152313
Chicago/Turabian StyleMerkelytė, Inga, Artūras Šiukščius, and Rasa Nainienė. 2025. "The Role of Sensor Technologies in Estrus Detection in Beef Cattle: A Review of Current Applications" Animals 15, no. 15: 2313. https://doi.org/10.3390/ani15152313
APA StyleMerkelytė, I., Šiukščius, A., & Nainienė, R. (2025). The Role of Sensor Technologies in Estrus Detection in Beef Cattle: A Review of Current Applications. Animals, 15(15), 2313. https://doi.org/10.3390/ani15152313






