Simple Summary
Male fertility depends greatly on the quality and function of spermatozoa. Spermatozoa need a lot of energy to move and fertilize the egg, and this energy is mainly produced by mitochondria. However, mitochondria can also produce harmful substances called reactive oxygen species, especially when the balance in the cell is disturbed. While small amounts of ROS are necessary for normal sperm function, too much can damage sperm quality. In this review, we summarize how mitochondria produce energy in sperm, how excess ROS can lead to problems, and how damage to mitochondrial DNA can affect sperm health. We also discuss promising treatments by antioxidants that specifically target mitochondria to protect spermatozoa from damage. Understanding how to keep mitochondria healthy in spermatozoa could lead to better treatments for male infertility and help improve reproductive success in both humans and animals.
Abstract
Mitochondria are central to energy production and redox regulation in spermatozoa, supporting key functions such as progressive motility, capacitation, and the acrosome reaction. These processes are essential for successful fertilization and embryo development. However, species-specific differences exist in the reliance on oxidative phosphorylation versus glycolysis. Mitochondria also generate reactive oxygen species, which at physiological levels aid in sperm function but can cause oxidative stress and damage when overproduced. Mitochondrial dysfunction and excessive ROS can impair membrane potential, induce apoptosis, and damage nuclear and mitochondrial DNA, ultimately compromising sperm quality. Sperm mitochondrial DNA is highly susceptible to mutations and deletions, contributing to reduced motility and fertility. Targeted antioxidant strategies have emerged as promising therapeutic interventions to mitigate oxidative damage. This article provides a comprehensive overview of mitochondrial regulation in spermatozoa, the consequences of redox imbalance, and the potential of mitochondria-targeted antioxidants to improve sperm function and male fertility outcomes. The paper aims to deepen our understanding of mitochondrial roles in sperm physiology and contribute to the advancement of strategies for addressing male infertility.
1. Introduction
Infertility affects approximately 10% to 15% of couples worldwide, with male factors contributing to nearly 50% of cases. Male infertility is most commonly associated with poor semen quality, including reduced sperm count (e.g., oligozoospermia or azoospermia), decreased motility (asthenozoospermia), abnormal morphology (teratozoospermia), and increased DNA fragmentation [1]. In livestock breeding, male fertility is also a key determinant of reproductive success, influencing artificial insemination efficiency and overall productivity. For example, the quality of boar spermatozoa significantly affects the conception rate of sows [2,3], and studies have revealed a considerable variation in acrosome integrity among bulls with differing fertility levels [4], indicating the crucial role of sperm quality in determining the reproductive success of male livestock.
Mitochondria play a central role in sperm function by generating ATP through oxidative phosphorylation (OXPHOS), regulating redox balance, and triggering apoptosis [5]. OXPHOS can also generate reactive oxygen species (ROS) as natural byproducts, linking energy metabolism to oxidative stress (OS) regulation. However, excessive ROS production or insufficient antioxidant defenses can disrupt mitochondrial redox balance and lead to sperm damage [6,7]. Mitochondrial ROS are also involved in the fertilization processes such as sperm maturation, capacitation and acrosome reaction. Mitochondrial dysfunction can trigger intrinsic apoptotic pathways, leading to loss of mitochondrial membrane potential (MMP) and the activation of caspases, ultimately compromising sperm viability and fertilization potential. Additionally, mitochondrial DNA (mtDNA) lacks histone protection, making it more susceptible to oxidative damage from ROS, which can lead to mutations that impair OXPHOS and reduce sperm motility [8].
Although several studies have reviewed some aspects of mitochondria in sperm, a comprehensive synthesis that integrates species-specific energy metabolism strategies, mitochondrial redox imbalance, mtDNA vulnerabilities, and therapeutic interventions remains lacking. The article aims to give a comprehensive summary of sperm mitochondrial energy metabolism, outlining the key factors that affect it, discussing how mitochondrial ROS and mtDNA damage contribute to sperm dysfunction, and highlighting current advances in mitochondria-targeted antioxidants as a potential strategy to improve sperm quality and male fertility. This may help to enrich our understanding of mitochondria’s function in sperm, expanding our knowledge of male reproductive physiology, and inspiring new research ideas for addressing male infertility, as well as enhancing the reproductive performance of male animals.
2. Relevance of Mitochondrial Energy Metabolism in Spermatozoa
2.1. The Mitochondrial Energy Metabolism in Spermatozoa
Spermatozoa produce ATP mainly through glycolysis and OXPHOS [9]. Glycolysis occurs in the fiber sheath of sperm flagella [9,10], converting glucose into pyruvic acid. This process mainly involves a series of enzyme-catalyzed reactions, including hexokinase, phosphofructokinase, and glyceraldehyde-3-phosphate dehydrogenase, etc. [11]. Glycolysis is important in sperm ATP production; inhibition of glycolysis not only reduces ATP levels but also decreases protein tyrosine phosphorylation, a critical signaling event associated with sperm capacitation and hyperactivated motility [12]. Studies have shown that mouse spermatozoa primarily produce ATP by glycolysis [13]. In the medium mainly constituted of glucose, mouse spermatozoa are capable of producing a considerable amount of ATP, which is crucial for maintaining their motility. Notably, any disruption to glycolysis severely impairs mouse sperm motility, even when provided with alternative substrates necessary for OXPHOS [14]. And also, Takei et al. analyzed the distribution of adenosine monophosphate (AMP) in mouse sperm flagella, they found that glycolysis may transport ATP from mitochondria to the distal end of the flagella, thereby maintaining ATP concentration at the distal end of the flagella, which means that glycolysis could also play a role in energy transfer in mouse spermatozoa [15]. Glycolysis is also important in the energy metabolism of human spermatozoa [11,16]. Nascimento et al. performed inhibition treatments targeting both OXPHOS and glycolysis in human spermatozoa, which indicated that OXPHOS did not yield enough ATP to maintain sperm motility, and motility decreases progressively if glycolysis is inhibited [17]. And also, the hyperactivation of human spermatozoa is marked by a requirement for glycolytic substrates such as glucose or fructose, highlighting that the glycolysis pathway is the predominant energy supplier for human sperm function [18]. In the realm of livestock species, the energy metabolism of spermatozoa in ram is similar to that of mice and humans; the inhibition of the glycolysis pathway in ram spermatozoa would result in a decrease of sperm motility and compromised fertilization ability [19]. These findings indicate the critical role of glycolysis across these species.
Another pathway for ATP production is OXPHOS, a mitochondria-dependent process in which ATP is generated via electron transfer through the respiratory chain complexes I–IV and ATP synthase. In this process, NADH+, H+ and FADH2 are oxidized through a series of enzyme-catalyzed reactions and a continuous electron transfer, resulting in the production of H2O and ATP [20]. The glycolysis and OXPHOS pathways jointly contribute to ATP production in spermatozoa, but the primary means of ATP production differs among species. Davila et al. inhibited OXPHOS in stallion spermatozoa; the intervention led to a notable reduction in sperm viability, motility, and membrane integrity, illustrating the crucial role of OXPHOS in ATP synthesis for stallion spermatozoa [21]. And also, the preference for OXPHOS over glycolysis in stallion sperm has been confirmed by the Agilent Seahorse XFp Technology [22]. Intriguingly, there are studies that show that stallion spermatozoa also require glycolysis to maintain high sperm velocities, suggesting that stallion sperm not only rely on OXPHOS for the production of ATP but also maintain the capacity to generate ATP via glycolysis [23,24]. Bovine spermatozoa are capable of harnessing both OXPHOS and glycolysis for energy production [25]. Bulkeley et al. have demonstrated that impeding the electron transport chain (ETC) resulted in a marked decline in both the motility and vitality of bovine spermatozoa [26], which highlights the significance of OXPHOS in ATP provision for these cells. Nevertheless, in circumstances where ample glycolytic substrates are available, bovine spermatozoa retain the ability to produce ATP via the glycolytic pathway, showing a flexible energy metabolism in these cells [27]. In addition, pig spermatozoa were thought to predominantly rely on glycolysis for ATP production [28,29], while Prieto et al. found that mitochondrial OXPHOS is the primary source of ATP in fresh boar semen, however, with extended storage under in vitro conditions, the OXPHOS capacity of pig spermatozoa progressively deteriorates, which shifts their energy production towards an enhanced reliance on glycolysis to sustain ATP synthesis [30]. This indicates a temporal adaptation of energy metabolism in pig spermatozoa in response to extrinsic storage conditions. Spermatozoa differ significantly from somatic cells in terms of energy metabolism. While most somatic cells rely predominantly on mitochondrial OXPHOS for steady ATP production [31], sperm cells demonstrate a compartmentalized and species-dependent balance between glycolysis and OXPHOS. Exploring the intricacies of sperm energy metabolism not only elucidates these disparities but also paves the way for refining semen preservation methodologies, thereby contributing to advancements in reproductive science and technology.
2.2. Proteins Affecting Mitochondrial Energy Metabolism in Spermatozoa
Many proteins are involved in the spermatozoa energy metabolism process [32]. The oxidative respiratory chain, mainly composed of respiratory chain protein complexes I–IV, ATP synthase, cytochrome C, and coenzyme Q10, is located on the inner membrane of mitochondria and serves as the main site for ATP production and plays a key role in mitochondrial energy metabolism [33,34]. Complex I, which has been known as NADH dehydrogenase, primarily catalyzes the oxidation of NADH within mitochondria [35]. It also serves as a proton pump that transports protons from the matrix into the intermembrane space as electrons pass through, thus forming a proton gradient, and then drives ATP synthase to produce ATP from ADP and inorganic phosphate [36]. Complex II, also known as succinate dehydrogenase (SDH), consists of four subunits (SDHA, SDHB, SDHC, and SDHD) [37]. This complex could transfer electrons from succinate to ubiquinone via its iron-sulfur (Fe–S) clusters, and oxidizes succinate to fumarate in the tricarboxylic acid (TCA) cycle [37,38,39], which is important in reprogramming of metabolic and respiratory adaptation. Complex III is the cytochrome reductase complex, which is responsible for transferring electrons to the cytochrome C receptor [40]. Complex IV is a cytochrome oxidase complex that serves as the final component of the respiratory chain by converting oxygen molecules into water molecules [41]. Complex III and complex IV also possess the function of proton transfer [40], which generates a transmembrane potential that promotes ATP synthase for ATP synthesis [42]. Research has revealed that oligozoospermia patients exhibit significantly lower expression levels of mitochondrial cytochrome oxidase in their spermatozoa compared to normal individuals [43,44], suggesting a direct correlation between the complex III and IV function and sperm quality. Additionally, ATP synthase, a component of the mitochondrial respiratory chain, converts ADP and Pi into ATP by harnessing the transmembrane proton electrochemical gradient created by the electron transfer chain [45,46,47]. Coenzyme Q10, known as ubiquinone, can promote energy production and neutralize the ROS in sperm mitochondria. Deficiency of coenzyme Q10 can lead to decreased sperm motility [48,49,50]. These results demonstrated that the mitochondrial respiratory chain is indispensable in sperm function; impaired mitochondrial respiratory chain protein complexes would destroy sperm energy metabolism.
Besides the respiratory chain proteins, many other proteins have been confirmed to participate in the sperm mitochondria’s energy metabolism. We summarized these proteins and their function in energy metabolism in Table 1. Studying the effects of these molecules on sperm metabolism may help us to better understand the physiological processes of sperm energy metabolism.

Table 1.
Sperm proteins are involved in mitochondrial energy metabolism.
3. Effects of Mitochondrial ROS on Spermatozoa
3.1. Sources of Mitochondrial ROS in Spermatozoa
Mitochondria are the primary source of ROS produced in spermatozoa [65]. The main types of mitochondrial ROS are O2−, ·OH and H2O2; they are oxygen-containing substances with high activity [66]. Mitochondrial ROS primarily occurs during OXPHOS in the inner membrane of mitochondria [67]. Spermatozoa possess antioxidant mechanisms that help maintain ROS balance. Controlled levels of ROS regulate physiological functions and support normal sperm performance, while excessive ROS can cause OS, leading to sperm damage and reduced sperm quality. ROS production in sperm mitochondria is influenced by various factors [6,68]. Spermatogenesis, which includes mitosis, meiosis, and cell differentiation, significantly contributes to ROS generation [69]. The energy metabolic activity of mitochondria also produces substantial ROS as a byproduct [70]. Under stressful conditions, the electron transport during mitochondrial OXPHOS is imperfect, resulting in the formation of superoxide anion (O2−). These superoxide anions are then converted into hydrogen peroxide (H2O2) through the action of superoxide dismutase [66]. Both superoxide anions and hydrogen peroxide have short half-lives and are harmless to sperm under normal circumstances. For example, leukocytes and immature or morphologically abnormal spermatozoa in semen contribute significantly to ROS generation, exacerbating OS and impairing mitochondrial function [71]. Additionally, diseases, nutrient deficiencies, unhealthy lifestyle habits, and environmental pollution are notable contributors to ROS production [72].
3.2. Consequences of Mitochondrial ROS Overproduction and Redox Imbalance on Sperm Quality
ROS-mediated damage compromises the structural and functional integrity of spermatozoa [69]. The mitochondrial ETC is involved in the phosphorylation of ADP into ATP [73]. Notably, ROS are primarily generated in respiratory complexes I and III of the ETC [74], highlighting their direct impact on mitochondrial energy metabolism. Indeed, elevated ROS levels have been shown to negatively correlate with the activity of ETC complexes and sperm motility [75,76]. Excessive ROS can reduce cytochrome C oxidase activity in spermatozoa, causing impaired mitochondrial ATP synthesis and disrupted energy metabolism [77]. Excessive ROS also causes lipid peroxide (LP), damaging the polyunsaturated fatty acids (PUFA) in spermatozoa [78], and resulting in mitochondrial electron transport dysfunction and disruption of MMP.
Apart from affecting ATP production, ROS affect the molecular components in spermatozoa. Spermatozoa carry a variety of macromolecules, among which proteins and lipids are especially susceptible to ROS [69] (Chianese and Pierantoni, 2021). ROS regulates the sperm proteins, especially those within the nucleus. ROS can oxidize nuclear proteins in sperm, particularly protamines, which are essential for compacting DNA during spermatogenesis. Oxidative modifications, such as carbonylation or disulfide bond disruption, can impair chromatin packaging and compromise DNA integrity, potentially affecting fertilization and embryo development [79,80]. ROS also increases sperm histone methylation and impairs histone acetylation, resulting in double-stranded DNA breaks, reduced sperm quality, and epigenetic dysregulation [81]. Additionally, some enzymes involved in ATP production and ion channel regulation, as well as sperm proteins modified by tyrosine nitration, exhibit physiological or pathological effects that are closely linked to the levels of ROS generated [82]. In spermatozoa, disulfide bonds exist between cysteine residues of protamine, which contribute to the stability of chromatin. Moderate levels of mitochondrial ROS can facilitate the formation of disulfide bonds, which ensure chromatin stability, protecting DNA from damage, and safeguarding mitochondria against proteolytic hydrolysis [83]. Subsequently, ROS can activate adenylate cyclase and induce intracellular cyclic adenosine monophosphate (cAMP) production, which in turn activates protein kinase A (PKA), extracellular signal-regulated kinase (MEK)-like proteins, threonine-glutamic acid-tyrosine, and fibronectin to enable spermatozoa to achieve final capacitation [84,85,86]. The capacitated spermatozoa undergo phosphorylation of tyrosine proteins, calcium influx, leading to an increase in intracellular cAMP and PKA levels. This triggers the release of proteolytic enzymes, which enable sperm to penetrate and fuse with the egg [83]. Thus, it can be seen that ROS is involved in sperm maturation, capacitation and acrosome reaction, and plays an important role in these processes.
Excessive mitochondrial ROS can also cause epigenetic alterations, telomere shortening, Y chromosome microdeletions, and the activation of apoptotic pathways [87,88]. Due to the limited DNA repair capacity in sperm cells, DNA damage often remains unrepaired, leading to increased phosphorylation and activation of p53, which in turn triggers the mitochondrial-dependent apoptotic pathway [89]. This process also induces the opening of a permeability transition pore, the extrusion of cytochrome c and the activation of a caspase cascade, ultimately resulting in apoptosis-like phenomena [90]. The resulting apoptosis helps eliminate structurally or functionally compromised sperm, thereby preserving overall semen quality. While apoptosis is essential for maintaining sperm homeostasis, excessive activation can contribute to subfertility by reducing the viable sperm population [87]. 8-hydroxy-2′-deoxyguanosine (8-OHdG) is a key indicator reflecting DNA OS damage [91]. 8-OHdG induced incomplete base pairing, which disrupts the ribose-phosphate backbone of DNA and contributes to DNA fragmentation [92]. Comparing the normal fertile men with those experiencing infertility revealed that higher levels of 8-OHdG were found in the infertile group. This elevation suggests that ROS induces oxidative damage to sperm DNA, potentially contributing to male infertility [93,94]. And also, telomeric DNA, composed of guanine-rich repeat sequences, is highly vulnerable to oxidative radical attacks, which can impede the repair and elongation processes of telomeres [95,96]. Additionally, the ROS-induced DNA mutations may transfer and exert effects on the offspring [97,98]. The effects of mitochondrial ROS on spermatozoa have been illustrated in Figure 1.
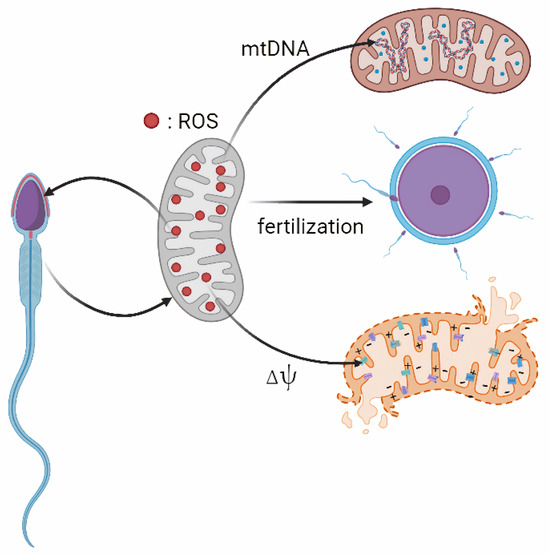
Figure 1.
Effects of mitochondrial ROS on spermatozoa. Note: Mitochondria are the major source of ROS in sperm. Excessive ROS can damage sperm MMP (Δψ), as well as sperm nuclear DNA and mtDNA damage. At the same time, ROS also plays an important role in sperm maturation, capacitation and acrosome reaction.
4. The Importance of mtDNA Stability in Mitochondrial Function Maintenance
4.1. The Characteristics of Sperm mtDNA
Sperm mtDNA possesses distinct genetic characteristics, notably its maternal inheritance pattern [99]. Both humans and animals have evolved a series of mechanisms to eliminate sperm mitochondria and mtDNA. As a result, the number of mitochondria and mtDNA in spermatozoa is progressively reduced during spermatogenesis and fertilization [100]. Consequently, mtDNA primarily functions in the metabolic processes of spermatozoa before fertilization. And also, the susceptibility to mutation of mtDNA is notable [101]. Sperm mtDNA has a smaller molecular weight and lacks introns; they are in a constant state of synthesis throughout the entire cell cycle, making it less stable and more susceptible to interference from various factors [102]. What is more, mtDNA replication enzymes have poor proofreading capabilities, leading to a higher probability of errors during the duplication process. And the absence of effective repair systems contributes significantly to an escalated risk of mtDNA mutations, which stands at multiple-fold higher frequencies compared to those observed in nuclear DNA [103]. In addition, mtDNA lacks protection from histones and DNA-binding proteins, leaving it directly exposed to the mitochondrial matrix. This makes it susceptible to damage from ROS, leading to a decline of normal physiological functions such as sperm motility, as we described above [104].
4.2. Effects of mtDNA Mutation on Sperm Function
The mtDNA abnormalities associated with male infertility have gained much attention [105]. MtDNA mutations in spermatozoa can be generally categorized into point mutations, insert/deletion mutations and mtDNA copy number variation, all of which are known to adversely affect sperm motility and morphology, impairing sperm function and leading to male infertility [106]. There is increasing evidence suggesting that single-nucleotide polymorphisms (SNPs) in mtDNA may significantly influence male fertility [107]. For instance, high levels of A3243G point mutation in mtDNA are strongly linked to decreased sperm motility [108]. And also, some SNPs in mtDNA genes, such as MT-CYB, MT-CO3 and MT-ATP6, have been proven to be associated with male fertility [105,109].
In addition, there are large deletion mutations in mtDNA, which mostly occur in gene fragments with tandem repeat sequences on both sides [102]. The presence of this specific mtDNA deletion in spermatozoa is linked with various male infertility conditions [110]. A higher frequency of 4977, 7599 and 7491 bp mtDNA deletion mutations is associated with asthenozoospermia, asthenoteratospermia and oligasthenospermia in males [111,112,113]. The 4977 bp deletion in mtDNA is among the most extensively researched genetic mutations in spermatozoa. The spermatozoa harboring this mutation show significantly lower motility and may also display abnormalities in morphology, such as head deformities or tail twisting, which render them incapable of successfully fertilizing the eggs [114]. The frequency of spermatozoa with 4977 bp deletion mutation in mtDNA was found to be negatively correlated with the fertilization rate during IVF [115], and the occurrence rate of the 4977bp deletion mutation in mtDNA was higher in males with asthenospermia, oligospermia, and primary infertility [116]. For example, the rate of 4977 bp deletions mutation in patients with asthenospermia was 85.93%; whereas the deletion rate was 14% in normal controls, with a significant difference between the two groups [113]. What is more, a comparative study of 60 infertile men and 60 healthy controls has shown significant associations between the 4977 and 7599 bp deletion mutations and male infertility [112]. These findings indicated that the 4977 bp deletion mutation in sperm mtDNA is significantly higher in male infertility patients compared with the normal group. This prompts the consideration that the 4977 bp deletion mutation has the potential to become a biomarker of sperm fertility. What is more, the deletion mutation resulted in functional defects of respiratory chain proteins encoded by sperm mtDNA, and then impaired ATP generation. This, in turn, can increase the production of mitochondrial ROS or free radicals, ultimately damaging sperm mtDNA [117].
MtDNA copy number is recognized as a sensitive biomarker of mitochondrial integrity and function. In spermatozoa, abnormal mtDNA has been implicated in defective spermatogenesis, heightened mitophagy, and the accumulation of dysfunctional mitochondria. Elevated sperm mtDNA copy number has also been correlated with impaired semen quality, including reduced concentration, total count, viability, and abnormal morphology, and is significantly associated with an increased risk of clinical infertility, Nguyen et al. have summarized the quantitative variations in mitochondrial content and mtDNA copy number across different semen quality categories in various species [7,118]. Importantly, mtDNA copy number in sperm exhibits strong diagnostic potential for predicting male infertility across clinical evaluations [119]. However, investigations into the specific types of sperm mtDNA mutations and their associations with sperm functionality remain limited. Further studies are needed to elucidate the mechanisms through which mtDNA mutations regulate sperm fertilization.
5. Mitochondria-Targeted Antioxidant Therapeutic Approaches That Improve Sperm Quality
Given that mitochondria are the origin of ROS, antioxidant-based strategies targeting mitochondria have emerged as promising interventions to restore redox balance and improve sperm function. The common mitochondria-target antioxidants include ubiquinone (MitoQ), melatonin, quercetin, MitoTEMPO, etc. Mitochondrial-target antioxidants block oxidative damage through covalent attachment of lipophilic cations such as lipophilic cation triphenylphosphonium (TPP+) in mitochondria [120]. The TPP+ of mitochondrial-target antioxidants can easily penetrate the mitochondrial membrane’s lipid bilayer and deliver the antioxidant to the mitochondrial matrix [121]. Once inside the mitochondria, these compounds can accumulate in large amounts, exhibiting significant mitochondrial antioxidant effects [122].
MitoQ is a widely studied mitochondrial-target antioxidant [123]. It accepts electrons from mitochondrial respiratory chain protein complex I or II, and reduces to ubiquinol, which then transfers these electrons to mitochondrial complex III [122]. Ubiquinol also acts as an antioxidant by providing hydrogen atoms to lipid peroxyl radicals, thereby preventing LP [124]. Supplementation of MitoQ in human spermatozoa resulted in improved sperm function, attenuated DNA damage, and concurrently induced the upregulation of antioxidant gene expression, thereby conferring supplementary benefits for sperm cryopreservation [125]. Studies have been conducted to assess the comparative effects of MitoQ and a cytoplasmic antioxidant RESV on buffalo semen subjected to cryopreservation. Results showed that MitoQ significantly improved sperm motility and viability prior to freezing compared to RESV (p ≤ 0.05), and MitoQ-treated sperm displayed heightened acrosome integrity and a greater proportion of viable spermatozoa post-freezing and thawing [126]. Furthermore, MitoQ treatment was found to boost the motility of freshly collected fish sperm and decrease LP levels; it may be attributed to its effective reduction of ROS generation in fish sperm following the freeze-thaw process [127]. These results indicate that MitoQ exerts a mitochondrial-targeted antioxidant effect and can mitigate ROS-induced sperm cryodamage in frozen-thawed sperm.
Melatonin is also an antioxidant that targets mitochondria [128]. Melatonin is predominantly synthesized within mitochondria and exerts its principal effects [129]. Melatonin maintains optimal MMP by scavenging ROS, activating uncoupling proteins, and inhibiting 1 methyl-4-phenyl-1,2,3,6- tetrahydropyridine formation [130]. Melatonin can also optimize the distribution of enzymes required for OXPHOS in spermatozoa, enhancing the activity of respiratory chain protein complexes, ultimately boosting the oxygen utilization capacity of cryopreserved spermatozoa upon thawing [131]. In human spermatozoa, melatonin can improve mitochondrial function by reducing mitochondrial OS [132]. And melatonin demonstrates a protective effect against environmental toxicity and cold-induced damage [133]. The addition of melatonin at varying concentrations has been shown to enhance sperm viability and motility, concomitant with a decrease in intracellular ROS levels. However, the optimal melatonin concentration for these beneficial effects varies across different studies [134,135,136]. Moreover, melatonin supplementation in cryopreservation medium for farm animals such as bovine [137], rams [138], buffalo [139], and pigs [140] has produced similar positive outcomes. Thus, melatonin supplementation plays a protective role in safeguarding both human and livestock spermatozoa, preserving their viability, and diminishing abnormal morphology and DNA fragmentation during the cryopreservation process. As a result, it has gained widespread application as an antioxidant additive in sperm cryopreservation [124,141].
Quercetin is a mitochondria-targeted flavone; it can reduce lipid peroxide accumulation by neutralizing harmful free radicals and attaching to transition metal ions. This activity alters the fluidity of the mitochondrial membrane and affects the oxidative proteins within the mitochondrial matrix [142,143]. It also modulates mitochondrial biogenesis, MMP, OXPHOS and ATP production, mitochondrial redox states, and ultimately triggers mitochondria-mediated apoptosis [144]. Quercetin could modulate sperm mitochondrial respiration efficiency in asthenozoospermic men [145], and decreases ram sperm motility within the first two hours by acidifying the incubation medium, while subsequently stimulates sperm motility during the following three to four hours by maintaining mitochondrial respiration [146]. Quercetin is also beneficial for spermatozoa during cryopreservation due to its antioxidative properties, as the freezing and thawing process typically induces high levels of OS. The antioxidant capability of quercetin has been confirmed across various animal species, demonstrating its efficacy in safeguarding sperm from oxidative stress during the cryopreservation procedure [147]. The addition of quercetin to the freezing solution was found to decrease oxidative damage and thereby enhance the sperm quality after thawing in human [148], pig [149], goat [150], rooster [151], dog [152] and so on. Despite its benefits, the precise molecular mechanisms behind quercetin’s actions are yet to be fully understood, and there are also studies showing that quercetin has no significant effects on frozen sperm quality. Further research is necessary to elucidate its protective effects in semen cryopreservation.
MitoTEMPO is a ROS scavenger composed of piperidine nitroxide TEMPOL and lipophilic triphenylphosphine (TPP). As a cell-permeable novel antioxidant, its lipophilic properties enable it to rapidly permeate through the lipid bilayer membrane of mitochondria and accumulate in high concentrations within these organelles [153]. Research has demonstrated that MitoTEMPO selectively targets mitochondrial superoxide, thereby enhancing mitochondrial function and the cell’s antioxidant capabilities [154]. Currently, research on MitoTEMPO on sperm quality is predominantly centered on semen cryopreservation. This emphasis is placed because OS has been recognized as a key factor affecting sperm quality, which could lead to the increase of ROS and LP during the cryopreservation process [155]. Research has shown that the addition of MitoTEMPO significantly enhanced the human post-thaw sperm motility, membrane integrity and MMP, and reduced the ROS level in sperm mitochondria, suggesting that MitoTEMPO could serve as an effective cryoprotectant for semen samples, and also, MitoTEMPO could alleviate cryodamage of asthenozoospermic spermatozoa after cryopreservation [155,156,157]. Additionally, research has extended to examine the impact of MitoTEMPO on post-thaw spermatozoa from various animal species, including bulls [158,159], rams [160,161,162,163], roosters [164,165], and tomcats [166]. These studies revealed that supplementation of MitoTEMPO significantly improved sperm quality in comparison to the untreated control group. However, further studies are required regarding MitoTEMPO’s impact on non-cryopreserved semen, as well as its effects on sperm quality in species such as pigs. Moreover, in-depth exploration of the mechanisms by which MitoTEMPO influences sperm quality can enhance our comprehension of sperm antioxidant systems, thereby facilitating the discovery of more efficacious mitochondria-targeted antioxidants to boost sperm quality.
The mitochondrial-target antioxidants have gained considerable attention, highlighting the critical role of mitochondrial antioxidant activity in maintaining sperm function. However, their effects have yielded inconsistent results, which may be explained by a biphasic, concentration-dependent response of sperm cells to the antioxidants. In addition to the previously introduced mitochondria-targeted antioxidants, an increasing number of compounds with mitochondrial antioxidant properties have also been investigated for their roles in sperm function. It can be deduced that mitochondria-targeted antioxidants are potentially useful in ameliorating OS in spermatozoa, particularly demonstrating favorable outcomes in mitigating mitochondrial OS induced by cryopreservation. Future research and endeavors should be taken to examine the effectiveness of mitochondrial antioxidants, elucidating the underlying mechanisms of their action, and developing additional potent mitochondria-specific antioxidants. Table 2 provides a summary of studies that have investigated the use of mitochondria-targeted antioxidants in spermatozoa.

Table 2.
Applications of mitochondria-targeted antioxidants on sperm quality.
6. Conclusions
Mitochondria play a central role in regulating sperm energy metabolism, redox balance, and overall function. The role of mitochondria in sperm energy metabolism varies across species, and proteins within sperm are among the key molecules influencing mitochondrial function. While physiological levels of mitochondrial ROS are essential for normal sperm processes such as capacitation and acrosome reaction, excessive ROS production leads to oxidative stress, mitochondrial dysfunction, and damage to nuclear and mitochondrial DNA. Mitochondria-targeted antioxidants have shown promising results in preserving sperm motility, viability, and fertilization potential by restoring redox balance and protecting mitochondrial integrity. However, more research is needed to optimize antioxidant type, dosage, and delivery methods across species. Future studies should also focus on refining diagnostic tools for assessing mitochondrial health in sperm and evaluating antioxidant strategies in clinical and agricultural settings. Such approaches may provide effective interventions for improving male fertility and reproductive efficiency.
Author Contributions
Conceptualization, Z.X.; methodology, Z.X.; validation, Z.X., X.L. and F.W.; investigation, Z.X., K.Z., Q.Y., Y.L., X.L. and F.W.; writing—original draft preparation, Z.X., K.Z., Q.Y. and Y.L.; writing—review and editing, C.Z., T.R., F.W., N.G. and X.L.; supervision, Z.X., X.L. and F.W.; project administration, Z.X., X.L. and F.W.; funding acquisition, Z.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (32302742), the Doctoral Scientific Research Foundation of Henan University of Science and Technology (13480096), and the Science and Technology Key Project of Henan Provincial Education Department in China (Project No. 24A230002).
Data Availability Statement
There are no new data associated with this article.
Acknowledgments
Figures for this review were created with BioRender.com.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Eisenberg, M.L.; Esteves, S.C.; Lamb, D.J.; Hotaling, J.M.; Giwercman, A.; Hwang, K.; Cheng, Y.; Faculty, O.M.; Medicinska, F.; Strategiska, F.S.; et al. Male Infertility. Nature reviews. Dis. Primers 2023, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Michos, I.; Tsantarliotou, M.; Boscos, C.M.; Tsousis, G.; Basioura, A.; Tzika, E.D.; Tassis, P.D.; Lymberopoulos, A.G.; Tsakmakidis, I.A. Effect of Boar Sperm Proteins and Quality Changes on Field Fertility. Animals 2021, 11, 1813. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, M.J.; Minton, A.M.; Schwab, C.; Herickhoff, L.A. Effects of Boar Sperm Antioxidant Supplementation on Fertility. Anim. Reprod. Sci. 2022, 237, 106923. [Google Scholar] [CrossRef]
- Narud, B.; Klinkenberg, G.; Khezri, A.; Zeremichael, T.T.; Stenseth, E.B.; Nordborg, A.; Haukaas, T.H.; Morrell, J.M.; Heringstad, B.; Myromslien, F.D.; et al. Differences in Sperm Functionality and Intracellular Metabolites in Norwegian Red Bulls of Contrasting Fertility. Theriogenology 2020, 157, 24–32. [Google Scholar] [CrossRef]
- Simonik, O.; Bryndova, B.; Sur, V.P.; Ded, L.; Cockova, Z.; Benda, A.; Qasemi, M.; Pecina, P.; Pecinova, A.; Spevakova, D.; et al. Bioenergetics of Human Spermatozoa in Patients with Testicular Germ Cell Tumours. Mol. Hum. Reprod. 2025, 31, gaaf005. [Google Scholar] [CrossRef]
- Mai, Z.; Yang, D.; Wang, D.; Zhang, J.; Zhou, Q.; Han, B.; Sun, Z. A Narrative Review of Mitochondrial Dysfunction and Male Infertility. Transl. Androl. Urol. 2024, 13, 2134–2145. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Yan, H.; Han, P.; Zhang, J.; Liu, Y. Deciphering the Role of Oxidative Stress in Male Infertility: Insights from Reactive Oxygen Species to Antioxidant Therapeutics. Front. Biosci. 2025, 30, 27046. [Google Scholar] [CrossRef]
- Dhillon, V.S.; Shahid, M.; Husain, S.A. Associations of Mthfr Dnmt3B 4977 Bp Deletion in Mtdna and Gstm1 Deletion, and Aberrant Cpg Island Hypermethylation of Gstm1 in Non-Obstructive Infertility in Indian Men. Mol. Hum. Reprod. 2007, 13, 213–222. [Google Scholar] [CrossRef]
- Ferramosca, A.; Zara, V. Bioenergetics of Mammalian Sperm Capacitation. Biomed Res. Int. 2014, 2014, 902953. [Google Scholar] [CrossRef]
- Shi, L.Z.; Nascimento, J.; Botvinick, E.; Durrant, B.; Berns, M.W. An Interdisciplinary Systems Approach to Study Sperm Physiology and Evolution. Wiley Interdiscip. Rev. Syst. Biol. Med. 2011, 3, 36–47. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, S.S.; Agarwal, A.; Mohanty, G.; van der Linde, M. Oxidative Phosphorylation Versus Glycolysis: What Fuel Do Spermatozoa Use? Asian J. Androl. 2015, 17, 230–235. [Google Scholar] [CrossRef]
- Hereng, T.H.; Elgstoen, K.B.; Cederkvist, F.H.; Eide, L.; Jahnsen, T.; Skalhegg, B.S.; Rosendal, K.R. Exogenous Pyruvate Accelerates Glycolysis and Promotes Capacitation in Human Spermatozoa. Hum. Reprod. 2011, 26, 3249–3263. [Google Scholar] [CrossRef]
- Sanchez-Guevara, Y.; Oliver, E.I.; Nishigaki, T. Ca2+ Concentrations in Mouse Sperm Mitochondria Fluctuate According to the Cytosol. Reproduction 2023, 167, REP-23-0237. [Google Scholar] [CrossRef]
- Mukai, C.; Okuno, M. Glycolysis Plays a Major Role for Adenosine Triphosphate Supplementation in Mouse Sperm Flagellar Movement. Biol. Reprod. 2004, 71, 540–547. [Google Scholar] [CrossRef]
- Takei, G.L.; Miyashiro, D.; Mukai, C.; Okuno, M. Glycolysis Plays an Important Role in Energy Transfer from the Base to the Distal End of the Flagellum in Mouse Sperm. J. Exp. Biol. 2014, 217 Pt 11, 1876–1886. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The Role of Mitochondria in Energy Production for Human Sperm Motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, J.M.; Shi, L.Z.; Tam, J.; Chandsawangbhuwana, C.; Durrant, B.; Botvinick, E.L.; Berns, M.W. Comparison of Glycolysis and Oxidative Phosphorylation as Energy Sources for Mammalian Sperm Motility, Using the Combination of Fluorescence Imaging, Laser Tweezers, and Real-Time Automated Tracking and Trapping. J. Cell. Physiol. 2008, 217, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Ford, W.C. The Role of Glucose in Supporting Motility and Capacitation in Human Spermatozoa. J. Androl. 2001, 22, 680–695. [Google Scholar] [CrossRef] [PubMed]
- Losano, J.; Angrimani, D.; Dalmazzo, A.; Rui, B.R.; Brito, M.M.; Mendes, C.M.; Kawai, G.; Vannucchi, C.I.; Assumpcao, M.; Barnabe, V.H.; et al. Effect of Mitochondrial Uncoupling and Glycolysis Inhibition on Ram Sperm Functionality. Reprod. Domest. Anim. 2017, 52, 289–297. [Google Scholar] [CrossRef]
- Wang, J.J.; Wang, S.X.; Tehmina; Feng, Y.; Zhang, R.F.; Li, X.Y.; Sun, Q.; Ding, J. Age-Related Decline of Male Fertility: Mitochondrial Dysfunction and the Antioxidant Interventions. Pharmaceuticals 2022, 15, 519. [Google Scholar] [CrossRef]
- Davila, M.P.; Muñoz, P.M.; Bolaños, J.M.G.; Stout, T.A.E.; Gadella, B.M.; Tapia, J.A.; Da Silva, C.B.; Ferrusola, C.O.; Peña, F.J. Mitochondrial Atp is Required for the Maintenance of Membrane Integrity in Stallion Spermatozoa, Whereas Motility Requires Both Glycolysis and Oxidative Phosphorylation. Reproduction 2016, 152, 683–694. [Google Scholar] [CrossRef]
- Ortiz-Rodriguez, J.M.; Bucci, D.; Tovar-Pascual, L.; Granata, S.; Spinaci, M.; Nesci, S. Analysis of Stallion Spermatozoa Metabolism Using Agilent Seahorse Xfp Technology. Anim. Reprod. Sci. 2024, 271, 107633. [Google Scholar] [CrossRef]
- Pena, F.J.; Martin-Cano, F.E.; Becerro-Rey, L.; Da, S.E.; Gaitskell-Phillips, G.; Aparicio, I.M.; Gil, M.C.; Ortega-Ferrusola, C. Redox Regulation and Glucose Metabolism in the Stallion Spermatozoa. Antioxidants 2025, 14, 225. [Google Scholar] [CrossRef]
- Plaza, D.M.; Martin, M.P.; Tapia, J.A.; Ortega, F.C.; Balao, D.S.C.C.; Pena, F.J. Inhibition of Mitochondrial Complex I Leads to Decreased Motility and Membrane Integrity Related to Increased Hydrogen Peroxide and Reduced Atp Production, while the Inhibition of Glycolysis Has Less Impact on Sperm Motility. PLoS ONE 2015, 10, e0138777. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Prieto, O.; Mislei, B.; Martinez-Pastor, F.; Spinaci, M.; Mari, G.; Bucci, D. Study of Mitochondrial Function in Thawed Bull Spermatozoa Using Selective Electron Transfer Chain Inhibitors. Theriogenology 2023, 208, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Bulkeley, E.A.; Foutouhi, A.; Wigney, K.; Santistevan, A.C.; Collins, C.; McNabb, B.; Meyers, S. Effects from Disruption of Mitochondrial Electron Transport Chain Function on Bull Sperm Motility. Theriogenology 2021, 176, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Hutson, S.M.; Van Dop, C.; Lardy, H.A. Mitochondrial Metabolism of Pyruvate in Bovine Spermatozoa. J. Biol. Chem. 1977, 252, 1309–1315. [Google Scholar] [CrossRef]
- Henning, H.; Nguyen, Q.T.; Luther, A.M.; Wallner, U.; Beyerbach, M.; Waberski, D. In Vitro Storage of Boar Spermatozoa Increases the Demand of Adenosine Triphosphate for Reactivation of Motility. Andrology 2022, 10, 1426–1440. [Google Scholar] [CrossRef]
- Nesci, S.; Spinaci, M.; Galeati, G.; Nerozzi, C.; Pagliarani, A.; Algieri, C.; Tamanini, C.; Bucci, D. Sperm Function and Mitochondrial Activity: An Insight on Boar Sperm Metabolism. Theriogenology 2020, 144, 82–88. [Google Scholar] [CrossRef]
- Prieto, O.B.; Algieri, C.; Spinaci, M.; Trombetti, F.; Nesci, S.; Bucci, D. Cell Bioenergetics and Atp Production of Boar Spermatozoa. Theriogenology 2023, 210, 162–168. [Google Scholar] [CrossRef]
- Haran, M.; Gross, A. Balancing Glycolysis and Mitochondrial Oxphos: Lessons From the Hematopoietic System and Exercising Muscles. Mitochondrion 2014, 19 Pt A, 3–7. [Google Scholar] [CrossRef]
- Castello-Ruiz, M.; Gacem, S.; Sanchez, D.P.M.; Hidalgo, C.O.; Tamargo, C.; Alvarez-Rodriguez, M.; Yaniz, J.L.; Silvestre, M.A. Effect of Capacitation on Proteomic Profile and Mitochondrial Parameters of Spermatozoa in Bulls. J. Proteome Res. 2025, 24, 1817–1831. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial Electron Transport Chain: Oxidative Phosphorylation, Oxidant Production, and Methods of Measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Vercellino, I.; Sazanov, L.A. The Assembly, Regulation and Function of the Mitochondrial Respiratory Chain. Nat. Rev. Mol. Cell Biol. 2022, 23, 141–161. [Google Scholar] [CrossRef]
- Hirst, J. Mitochondrial Complex I. Annu. Rev. Biochem. 2013, 82, 551–575. [Google Scholar] [CrossRef]
- Braun, H.P.; Klusch, N. Promotion of Oxidative Phosphorylation by Complex I-Anchored Carbonic Anhydrases? Trends Plant Sci. 2024, 29, 64–71. [Google Scholar] [CrossRef]
- Bezawork-Geleta, A.; Rohlena, J.; Dong, L.; Pacak, K.; Neuzil, J. Mitochondrial Complex II: At the Crossroads. Trends Biochem. Sci. 2017, 42, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, G. Function and Structure of Complex II of the Respiratory Chain. Annu. Rev. Biochem. 2003, 72, 77–109. [Google Scholar] [CrossRef] [PubMed]
- Miyadera, H.; Shiomi, K.; Ui, H.; Yamaguchi, Y.; Masuma, R.; Tomoda, H.; Miyoshi, H.; Osanai, A.; Kita, K.; Omura, S. Atpenins, Potent and Specific Inhibitors of Mitochondrial Complex II (Succinate-Ubiquinone Oxidoreductase). Proc. Natl. Acad. Sci. USA 2003, 100, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Purhonen, J.; Kallijärvi, J. The Mitochondrial Coenzyme Q Junction and Complex III: Biochemistry and Pathophysiology. FEBS J. 2022, 289, 6936–6958. [Google Scholar] [CrossRef]
- Kadenbach, B. Complex IV—the Regulatory Center of Mitochondrial Oxidative Phosphorylation. Mitochondrion 2021, 58, 296–302. [Google Scholar] [CrossRef]
- Juhaszova, M.; Kobrinsky, E.; Zorov, D.B.; Nuss, H.B.; Yaniv, Y.; Fishbein, K.W.; de Cabo, R.; Montoliu, L.; Gabelli, S.B.; Aon, M.A.; et al. Atp Synthase K(+)- And H(+)-Fluxes Drive Atp Synthesis and Enable Mitochondrial K(+)-“Uniporter” Function: I. Characterization of Ion Fluxes. Function 2022, 3, zqab065. [Google Scholar] [CrossRef] [PubMed]
- Bucak, M.N.; Ataman, M.B.; Baspinar, N.; Uysal, O.; Taspinar, M.; Bilgili, A.; Ozturk, C.; Gungor, S.; Inanc, M.E.; Akal, E. Lycopene and Resveratrol Improve Post-Thaw Bull Sperm Parameters: Sperm Motility, Mitochondrial Activity and Dna Integrity. Andrologia 2015, 47, 545–552. [Google Scholar] [CrossRef]
- Mughal, I.A.; Irfan, A.; Hameed, A.; Jahan, S. Sperm Mitochondrial Dna 15Bp Deletion of Cytochrome C Oxidase Subunit III is Significantly Associated with Human Male Infertility in Pakistan. J. Pak. Med. Assoc. 2016, 66, 3–7. [Google Scholar]
- Capaldi, R.A.; Aggeler, R.; Turina, P.; Wilkens, S. Coupling Between Catalytic Sites and the Proton Channel in F1F0-Type Atpases. Trends Biochem. Sci. 1994, 19, 284–289. [Google Scholar] [CrossRef]
- Nijtmans, L.G.; Klement, P.; Houstek, J.; van den Bogert, C. Assembly of Mitochondrial Atp Synthase in Cultured Human Cells: Implications for Mitochondrial Diseases. Biochim. Biophys. Acta 1995, 1272, 190–198. [Google Scholar] [CrossRef]
- Ramio-Lluch, L.; Yeste, M.; Fernandez-Novell, J.M.; Estrada, E.; Rocha, L.; Cebrian-Perez, J.A.; Muino-Blanco, T.; Concha, I.I.; Ramirez, A.; Rodriguez-Gil, J.E. Oligomycin a-Induced Inhibition of Mitochondrial Atp-Synthase Activity Suppresses Boar Sperm Motility and in Vitro Capacitation Achievement without Modifying Overall Sperm Energy Levels. Reprod. Fertil. Dev. 2014, 26, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, I.; Heaton, R.A.; Mantle, D. Disorders of Human Coenzyme Q10 Metabolism: An Overview. Int. J. Mol. Sci. 2020, 21, 6695. [Google Scholar] [CrossRef]
- Lewin, A.; Lavon, H. The Effect of Coenzyme Q10 on Sperm Motility and Function. Mol. Aspects Med. 1997, 18, 213–219. [Google Scholar] [CrossRef]
- Littarru, G.P.; Tiano, L. Bioenergetic and Antioxidant Properties of Coenzyme Q10: Recent Developments. Mol. Biotechnol. 2007, 37, 31–37. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Fang, Y.; Zhang, F.; Liu, Y.; Cheng, X.; Wang, J.; Li, D.; Chen, D.; Wu, F. Adenine Nucleotide Translocase 2 (Ant2) is Required for Individualization of Spermatogenesis of Drosophila Melanogaster. Insect Sci. 2024, 31, 1055–1072. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Shi, M.; Wan, Y.; Li, H.; Zhang, M.; Wang, Z.; Wang, S.; Lv, Y.; Lu, G.; et al. Morn2 Regulates the Morphology and Energy Metabolism of Mitochondria and is Required for Male Fertility in Mice. J. Transl. Med. 2024, 22, 240. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Xiao, Y.; Gu, J.; Zhao, P.; Hu, Z.; Zheng, J.; Hua, R.; Hai, Z.; Su, J.; Zhang, J.V.; et al. Clpp/Clpx Deficiency Impairs Mitochondrial Functions and Mtorc1 Signaling During Spermatogenesis. Commun. Biol. 2023, 6, 1012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, C.; Wang, Y.; Zhang, H.; Xu, C.; Cheng, Y.; Yuan, Y.; Sha, J.; Guo, X.; Cui, Y. A Novel Protein Encoded by Circrsrc1 Regulates Mitochondrial Ribosome Assembly and Translation During Spermatogenesis. BMC Biol. 2023, 21, 94. [Google Scholar] [CrossRef] [PubMed]
- Kuang, W.; Zhang, J.; Lan, Z.; Deepak, R.; Liu, C.; Ma, Z.; Cheng, L.; Zhao, X.; Meng, X.; Wang, W.; et al. Slc22a14 is a Mitochondrial Riboflavin Transporter Required for Sperm Oxidative Phosphorylation and Male Fertility. Cell Rep. 2021, 35, 109025. [Google Scholar] [CrossRef]
- Zhu, F.; Li, W.; Zhou, X.; Chen, X.; Zheng, M.; Cui, Y.; Liu, X.; Guo, X.; Zhu, H. Prss55 Plays an Important Role in the Structural Differentiation and Energy Metabolism of Sperm and is Required for Male Fertility in Mice. J. Cell Mol. Med. 2021, 25, 2040–2051. [Google Scholar] [CrossRef]
- Ortiz-Rodriguez, J.M.; Martin-Cano, F.E.; Gaitskell-Phillips, G.; Silva, A.; Tapia, J.A.; Gil, M.C.; Redondo, E.; Masot, J.; Ortega-Ferrusola, C.; Pena, F.J. The Slc7a11: Sperm Mitochondrial Function and Non-Canonical Glutamate Metabolism. Reproduction 2020, 160, 803–818. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, X.; Zhao, L.; Zhang, Z.; Wang, Y.; Dai, Z.; Zhao, X.; Pu, X. Dj-1 Deficiency Causes Metabolic Abnormality in Ornidazole-Induced Asthenozoospermia. Reproduction 2020, 160, 931–941. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Wang, L.; Zheng, Y.; Hoque, S.; Lv, Y.; Zeng, W. Glycogen Synthase Kinase-3 Regulates Sperm Motility and Acrosome Reaction Via Affecting Energy Metabolism in Goats. Front. Physiol. 2019, 10, 968. [Google Scholar] [CrossRef]
- Zhu, F.; Yan, P.; Zhang, J.; Cui, Y.; Zheng, M.; Cheng, Y.; Guo, Y.; Yang, X.; Guo, X.; Zhu, H. Deficiency of Tppp2, a Factor Linked to Oligoasthenozoospermia, Causes Subfertility in Male Mice. J. Cell Mol. Med. 2019, 23, 2583–2594. [Google Scholar] [CrossRef]
- Tartarin, P.; Guibert, E.; Toure, A.; Ouiste, C.; Leclerc, J.; Sanz, N.; Briere, S.; Dacheux, J.L.; Delaleu, B.; McNeilly, J.R.; et al. Inactivation of Ampkalpha1 Induces Asthenozoospermia and Alters Spermatozoa Morphology. Endocrinology 2012, 153, 3468–3481. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Ma, G.; Bai, W.; Fan, X.; Lv, Y.; Luo, J.; Zeng, W. 5′-Amp-Activated Protein Kinase Regulates Goat Sperm Functions Via Energy Metabolism in Vitro. Cell Physiol. Biochem. 2018, 47, 2420–2431. [Google Scholar] [CrossRef]
- Odet, F.; Gabel, S.A.; Williams, J.; London, R.E.; Goldberg, E.; Eddy, E.M. Lactate Dehydrogenase C and Energy Metabolism in Mouse Sperm. Biol. Reprod. 2011, 85, 556–564. [Google Scholar] [CrossRef]
- Gawlik, V.; Schmidt, S.; Scheepers, A.; Wennemuth, G.; Augustin, R.; Aumuller, G.; Moser, M.; Al-Hasani, H.; Kluge, R.; Joost, H.G.; et al. Targeted Disruption of Slc2a8 (Glut8) Reduces Motility and Mitochondrial Potential of Spermatozoa. Mol. Membr. Biol. 2008, 25, 224–235. [Google Scholar] [CrossRef]
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of Mitochondrial Reactive Oxygen Species in the Generation of Oxidative Stress in Spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal Transduction by Reactive Oxygen Species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E.; Davies, K.J. Mitochondrial Free Radical Generation, Oxidative Stress, and Aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Hussain, T.; Kandeel, M.; Metwally, E.; Murtaza, G.; Kalhoro, D.H.; Yin, Y.; Tan, B.; Chughtai, M.I.; Yaseen, A.; Afzal, A.; et al. Unraveling the Harmful Effect of Oxidative Stress on Male Fertility: A Mechanistic Insight. Front. Endocrinol. 2023, 14, 1070692. [Google Scholar] [CrossRef]
- Chianese, R.; Pierantoni, R. Mitochondrial Reactive Oxygen Species (Ros) Production Alters Sperm Quality. Antioxidants 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Trocchia, S.; Abdel-Gawad, F.K.; Ciarcia, G. Roles of Reactive Oxygen Species in the Spermatogenesis Regulation. Front. Endocrinol. 2014, 5, 56. [Google Scholar] [CrossRef]
- Sikka, S.C. Relative Impact of Oxidative Stress on Male Reproductive Function. Curr. Med. Chem. 2001, 8, 851–862. [Google Scholar] [CrossRef]
- Sanocka, D.; Kurpisz, M. Reactive Oxygen Species and Sperm Cells. Reprod. Biol. Endocrinol. 2004, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Foutouhi, A.; Meyers, S. Comparative Oxidative Metabolism in Mammalian Sperm. Anim. Reprod. Sci. 2022, 247, 107095. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, C.L.; Perevoshchikova, I.V.; Hey-Mogensen, M.; Orr, A.L.; Brand, M.D. Sites of Reactive Oxygen Species Generation by Mitochondria Oxidizing Different Substrates. Redox Biol. 2013, 1, 304–312. [Google Scholar] [CrossRef]
- Kamali Sangani, A.; Masoudi, A.A.; Vaez Torshizi, R. Association of Mitochondrial Function and Sperm Progressivity in Slow- And Fast-Growing Roosters. Poult. Sci. 2017, 96, 211–219. [Google Scholar] [CrossRef]
- Zhu, Z.; Kawai, T.; Umehara, T.; Hoque, S.; Zeng, W.; Shimada, M. Negative Effects of Ros Generated During Linear Sperm Motility on Gene Expression and Atp Generation in Boar Sperm Mitochondria. Free Radic. Biol. Med. 2019, 141, 159–171. [Google Scholar] [CrossRef]
- Suen, D.F.; Norris, K.L.; Youle, R.J. Mitochondrial Dynamics and Apoptosis. Genes. Dev. 2008, 22, 1577–1590. [Google Scholar] [CrossRef]
- Ferramosca, A.; Zara, V. Diet and Male Fertility: The Impact of Nutrients and Antioxidants on Sperm Energetic Metabolism. Int. J. Mol. Sci. 2022, 23, 2542. [Google Scholar] [CrossRef] [PubMed]
- Lone, S.A.; Mohanty, T.K.; Baithalu, R.K.; Yadav, H.P. Sperm Protein Carbonylation. Andrologia 2019, 51, e13233. [Google Scholar] [CrossRef]
- Tirmarche, S.; Kimura, S.; Dubruille, R.; Horard, B.; Loppin, B. Unlocking Sperm Chromatin at Fertilization Requires a Dedicated Egg Thioredoxin in Drosophila. Nat. Commun. 2016, 7, 13539. [Google Scholar] [CrossRef]
- Montjean, D.; Ravel, C.; Benkhalifa, M.; Cohen-Bacrie, P.; Berthaut, I.; Bashamboo, A.; McElreavey, K. Methylation Changes in Mature Sperm Deoxyribonucleic Acid from Oligozoospermic Men: Assessment of Genetic Variants and Assisted Reproductive Technology Outcome. Fertil. Steril. 2013, 100, 1241–1247. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive Oxygen Species and Protein Modifications in Spermatozoa. Biol. Reprod. 2017, 97, 577–585. [Google Scholar] [CrossRef]
- Fujii, J.; Tsunoda, S. Redox Regulation of Fertilisation and the Spermatogenic Process. Asian J. Androl. 2011, 13, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Reactive Oxygen Species as Mediators of Sperm Capacitation and Pathological Damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A.; Nixon, B. Are Sperm Capacit. Apoptosis Opposite Ends A Contin. Driven By Oxidative Stress? Asian J. Androl. 2015, 17, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative Stress and Sperm Function: A Systematic Review on Evaluation and Management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Ghahremani, R.; Abdolmaleki, A.; Rajaei, F. Role of Sperm Apoptosis and Oxidative Stress in Male Infertility: A Narrative Review. Int. J. Reprod. Biomed. 2021, 19, 493–504. [Google Scholar] [CrossRef]
- Bui, A.D.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive Oxygen Species Impact on Sperm Dna and its Role in Male Infertility. Andrologia 2018, 50, e13012. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Z.; Tavana, O.; Gu, W. Understanding the Complexity of P53 in a New Era of Tumor Suppression. Cancer Cell 2024, 42, 946–967. [Google Scholar] [CrossRef]
- Amaral, A.; Lourenco, B.; Marques, M.; Ramalho-Santos, J. Mitochondria Functionality and Sperm Quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′ -Deoxyguanosine (8-Ohdg): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Envron. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Moor, N.A.; Vasil’Eva, I.A.; Kuznetsov, N.A.; Lavrik, O.I. Human Apurinic/Apyrimidinic Endonuclease 1 is Modified in Vitro by Poly(Adp-Ribose) Polymerase 1 Under Control of the Structure of Damaged Dna. Biochimie 2020, 168, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Moazamian, A.; Gharagozloo, P.; Aitken, R.J.; Drevet, J.R. Oxidative Stress and Reproductive Function: Sperm Telomeres, Oxidative Stress, and Infertility. Reproduction 2022, 164, F125–F133. [Google Scholar] [CrossRef]
- Stuppia, L.; Franzago, M.; Ballerini, P.; Gatta, V.; Antonucci, I. Epigenetics and Male Reproduction: The Consequences of Paternal Lifestyle on Fertility, Embryo Development, and Children Lifetime Health. Clin. Epigenetics 2015, 7, 120. [Google Scholar] [CrossRef]
- Bonetti, D.; Martina, M.; Falcettoni, M.; Longhese, M.P. Telomere-End Processing: Mechanisms and Regulation. Chromosoma 2014, 123, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kumar, R.; Malhotra, N.; Singh, N.; Dada, R. Mild Oxidative Stress is Beneficial for Sperm Telomere Length Maintenance. World J. Methodol. 2016, 6, 163–170. [Google Scholar] [CrossRef]
- Aitken, R.J. Human Spermatozoa: Revelations on the Road to Conception. F1000Prime Rep. 2013, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.B.; Chawla, B.; Bisht, S.; Yadav, R.K.; Dada, R. Tobacco Use Increases Oxidative Dna Damage in Sperm—Possible Etiology of Childhood Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 6967–6972. [Google Scholar] [CrossRef]
- Lee, W.; Zamudio-Ochoa, A.; Buchel, G.; Podlesniy, P.; Marti, G.N.; Puigros, M.; Calderon, A.; Tang, H.Y.; Li, L.; Mikhalchenko, A.; et al. Molecular Basis for Maternal Inheritance of Human Mitochondrial Dna. Nat. Genet. 2023, 55, 1632–1639. [Google Scholar] [CrossRef]
- Nishimura, Y.; Yoshinari, T.; Naruse, K.; Yamada, T.; Sumi, K.; Mitani, H.; Higashiyama, T.; Kuroiwa, T. Active Digestion of Sperm Mitochondrial Dna in Single Living Sperm Revealed by Optical Tweezers. Proc. Natl. Acad. Sci. USA 2006, 103, 1382–1387. [Google Scholar] [CrossRef]
- Yan, C.; Duanmu, X.; Zeng, L.; Liu, B.; Song, Z. Mitochondrial Dna: Distribution, Mutations, and Elimination. Cells 2019, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Durairajanayagam, D.; Singh, D.; Agarwal, A.; Henkel, R. Causes and Consequences of Sperm Mitochondrial Dysfunction. Andrologia 2021, 53, e13666. [Google Scholar] [CrossRef]
- Vertika, S.; Singh, K.K.; Rajender, S. Mitochondria, Spermatogenesis, and Male Infertility—An Update. Mitochondrion 2020, 54, 26–40. [Google Scholar] [CrossRef]
- Shamsi, M.B.; Kumar, R.; Bhatt, A.; Bamezai, R.N.; Kumar, R.; Gupta, N.P.; Das, T.K.; Dada, R. Mitochondrial Dna Mutations in Etiopathogenesis of Male Infertility. Indian. J. Urol. 2008, 24, 150–154. [Google Scholar] [CrossRef]
- Amor, H.; Hammadeh, M.E. A Systematic Review of the Impact of Mitochondrial Variations on Male Infertility. Genes 2022, 13, 1182. [Google Scholar] [CrossRef] [PubMed]
- Copeland, W.C. Defects of Mitochondrial Dna Replication. J. Child. Neurol. 2014, 29, 1216–1224. [Google Scholar] [CrossRef]
- Holyoake, A.J.; McHugh, P.; Wu, M.; O’Carroll, S.; Benny, P.; Sin, I.L.; Sin, F.Y.T. High Incidence of Single Nucleotide Substitutions in the Mitochondrial Genome is Associated with Poor Semen Parameters in Men. Int. J. Androl. 2001, 24, 175–182. [Google Scholar] [CrossRef]
- Spiropoulos, J.; Turnbull, D.M.; Chinnery, P.F. Can Mitochondrial Dna Mutations Cause Sperm Dysfunction? Mol. Hum. Reprod. 2002, 8, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Saleh Jaweesh, M.; Hammadeh, M.E.; Dahadhah, F.W.; Al Zoubi, M.S.; Amor, H. Association Between the Single Nucleotide Variants of the Mitochondrial Cytochrome B Gene (Mt-Cyb) and the Male Infertility. Mol. Biol. Rep. 2022, 49, 3609–3616. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.; Chao, H.T.; Wei, Y.H. Mitochondrial Deoxyribonucleic Acid 4977-Bp Deletion is Associated with Diminished Fertility and Motility of Human Sperm. Biol. Reprod. 1995, 52, 729–736. [Google Scholar] [CrossRef]
- Bahrehmand, N.I.; Vaziri, H. Sperm Mitochondrial Dna Deletion in Iranian Infertiles with Asthenozoospermia. Andrologia 2017, 49, e12627. [Google Scholar] [CrossRef]
- Karimian, M.; Babaei, F. Large-Scale Mtdna Deletions as Genetic Biomarkers for Susceptibility to Male Infertility: A Systematic Review and Meta-Analysis. Int. J. Biol. Macromol. 2020, 158, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Talebi, E.; Karimian, M.; Nikzad, H. Association of Sperm Mitochondrial Dna Deletions with Male Infertility in an Iranian Population. Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2018, 29, 615–623. [Google Scholar] [CrossRef]
- Ieremiadou, F.; Rodakis, G.C. Correlation of the 4977 Bp Mitochondrial Dna Deletion with Human Sperm Dysfunction. BMC Res. Notes 2009, 2, 18. [Google Scholar] [CrossRef]
- Mirabutalebi, S.H.; Karami, N.; Ashrafzadeh, H.R.; Akhvansales, Z.; Tavakoli, M.; Ghasemi, N. Detection of 4977-Bp Deletion of Mitochondrial Dna in in Vitro Fertilization Failure Women: A Case-Control Study. Int. J. Reprod. Biomed. 2018, 16, 571–576. [Google Scholar] [CrossRef]
- Gashti, N.G.; Salehi, Z.; Madani, A.H.; Dalivandan, S.T. 4977-Bp Mitochondrial Dna Deletion in Infertile Patients with Varicocele. Andrologia 2014, 46, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H. Primary and Secondary Defects of the Mitochondrial Respiratory Chain. J. Inherit. Metab. Dis. 2002, 25, 207–214. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Do, S.Q.; Wakai, T.; Funahashi, H. Mitochondrial Content and Mtdna Copy Number in Spermatozoa and Penetrability into Oocytes. Theriogenology 2025, 234, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huffman, A.M.; Whitcomb, B.W.; Josyula, S.; Labrie, S.; Tougias, E.; Rahil, T.; Sites, C.K.; Pilsner, J.R. Sperm Mitochondrial Dna Measures and Semen Parameters Among Men Undergoing Fertility Treatment. Reprod. Biomed. Online 2019, 38, 66–75. [Google Scholar] [CrossRef]
- Teixeira, J.; Deus, C.M.; Borges, F.; Oliveira, P.J. Mitochondria: Targeting Mitochondrial Reactive Oxygen Species with Mitochondriotropic Polyphenolic-Based Antioxidants. Int. J. Biochem. Cell Biol. 2018, 97, 98–103. [Google Scholar] [CrossRef]
- Wang, J.Y.; Li, J.Q.; Xiao, Y.M.; Fu, B.; Qin, Z.H. Triphenylphosphonium (Tpp)-Based Antioxidants: A New Perspective on Antioxidant Design. ChemMedChem 2020, 15, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Escada-Rebelo, S.; Cristo, M.I.; Ramalho-Santos, J.; Amaral, S. Mitochondria-Targeted Compounds to Assess and Improve Human Sperm Function. Antioxid. Redox Signal. 2022, 37, 451–480. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, X.; Zhang, F.; Wang, M.; Xu, Y.; Tang, D.; Wang, J.; Qin, Y.; Liu, Y.; Tang, C.; et al. The Mitochondria-Targeted Antioxidant Mitoq Ameliorated Tubular Injury Mediated by Mitophagy in Diabetic Kidney Disease Via Nrf2/Pink1. Redox Biol. 2017, 11, 297–311. [Google Scholar] [CrossRef]
- Alevra, A.I.; Exadactylos, A.; Mente, E.; Papadopoulos, S. The Protective Role of Melatonin in Sperm Cryopreservation of Farm Animals and Human: Lessons for Male Fish Cryopreservation. Animals 2022, 12, 791. [Google Scholar] [CrossRef]
- Moradi Gardeshi, T.; Shahandeh, E.; Tavakolpoor Saleh, N.; Karami, S.; Mirzaei Azandaryani, Z.; Mazaheri, F.; Mohammadi, H. Evaluation of the Effect of Mitoquinone on Functional Parameters, Dna Structure, and Genes Expression Related to the Apoptotic and Antioxidants of Human Sperm After Freezing–Thawing. Mol. Biol. Rep. 2024, 51, 183. [Google Scholar] [CrossRef]
- Tiwari, S.; Mohanty, T.K.; Bhakat, M.; Kumar, N.; Baithalu, R.K.; Nath, S.; Yadav, H.P.; Dewry, R.K. Comparative Evidence Support Better Antioxidant Efficacy of Mitochondrial-Targeted (Mitoquinone) than Cytosolic (Resveratrol) Antioxidant in Improving in-Vitro Sperm Functions of Cryopreserved Buffalo (Bubalus bubalis) Semen. Cryobiology 2021, 101, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Bai, C.; Chen, Y.; Dai, J.; Xiang, Y.; Ji, X.; Huang, C.; Dong, Q. Inhibition of Ros Production through Mitochondria-Targeted Antioxidant and Mitochondrial Uncoupling Increases Post-Thaw Sperm Viability in Yellow Catfish. Cryobiology 2014, 69, 386–693. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.M.; Dong, X.; Xue, X.D.; Xu, S.; Zhang, X.; Xu, Y.L.; Wang, Z.S.; Wang, Y.; Gao, H.; Liang, Y.X.; et al. Melatonin Attenuates Diabetic Cardiomyopathy and Reduces Myocardial Vulnerability to Ischemia-Reperfusion Injury by Improving Mitochondrial Quality Control: Role of Sirt6. J. Pineal Res. 2021, 70, e12698. [Google Scholar] [CrossRef]
- Govender, J.; Loos, B.; Marais, E.; Engelbrecht, A.M. Mitochondrial Catastrophe During Doxorubicin-Induced Cardiotoxicity: A Review of the Protective Role of Melatonin. J. Pineal Res. 2014, 57, 367–680. [Google Scholar] [CrossRef]
- Acuna-Castroviejo, D.; Escames, G.; Rodriguez, M.I.; Lopez, L.C. Melatonin Role in the Mitochondrial Function. Front Biosci. 2007, 12, 947–963. [Google Scholar] [CrossRef]
- Fang, Y.; Zhao, C.; Xiang, H.; Zhao, X.; Zhong, R. Melatonin Inhibits Formation of Mitochondrial Permeability Transition Pores and Improves Oxidative Phosphorylation of Frozen-Thawed Ram Sperm. Front. Endocrinol. 2019, 10, 896. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, Y.; Tan, Y.; Wang, L.; Li, R.; Zhang, Y.; Liu, X.; Lin, X.; Jin, L.; Hu, Y.; et al. Melatonin Rescues Impaired Penetration Ability of Human Spermatozoa Induced by Mitochondrial Dysfunction. Reproduction 2019, 158, 465–475. [Google Scholar] [CrossRef]
- Niu, F.W.; Liu, M.D.; Yao, K.; Yang, R.; Gao, L.; Zhai, J.X.; Wang, C.; Zhang, S.H.; Xu, D.X.; Zhang, Z.H. Mitochondrial Ros-Associated Integrated Stress Response is Involved in Arsenic-Induced Blood-Testis Barrier Disruption and Protective Effect of Melatonin. Environ. Int. 2025, 197, 109346. [Google Scholar] [CrossRef]
- Deng, S.L.; Sun, T.C.; Yu, K.; Wang, Z.P.; Zhang, B.L.; Zhang, Y.; Wang, X.X.; Lian, Z.X.; Liu, Y.X. Melatonin Reduces Oxidative Damage and Upregulates Heat Shock Protein 90 Expression in Cryopreserved Human Semen. Free Radic. Biol. Med. 2017, 113, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Karimfar, M.H.; Niazvand, F.; Haghani, K.; Ghafourian, S.; Shirazi, R.; Bakhtiyari, S. The Protective Effects of Melatonin Against Cryopreservation-Induced Oxidative Stress in Human Sperm. Int. J. Immunopathol. Pharmacol. 2015, 28, 69–76. [Google Scholar] [CrossRef]
- Najafi, A.; Adutwum, E.; Yari, A.; Salehi, E.; Mikaeili, S.; Dashtestani, F.; Abolhassani, F.; Rashki, L.; Shiasi, S.; Asadi, E. Melatonin Affects Membrane Integrity, Intracellular Reactive Oxygen Species, Caspase3 Activity and Akt Phosphorylation in Frozen Thawed Human Sperm. Cell Tissue Res. 2018, 372, 149–159. [Google Scholar] [CrossRef]
- Perumal, P.; Chang, S.; Baruah, K.K.; Srivastava, N. Administration of Slow Release Exogenous Melatonin Modulates Oxidative Stress Profiles and in Vitro Fertilizing Ability of the Cryopreserved Mithun (Bos Frontalis) Spermatozoa. Theriogenology 2018, 120, 79–90. [Google Scholar] [CrossRef]
- Pool, K.R.; Rickard, J.P.; Tumeth, E.; de Graaf, S.P. Treatment of Rams with Melatonin Implants in the Non-Breeding Season Improves Post-Thaw Sperm Progressive Motility and Dna Integrity. Anim. Reprod. Sci. 2020, 221, 106579. [Google Scholar] [CrossRef] [PubMed]
- Inyawilert, W.; Rungruangsak, J.; Liao, Y.J.; Tang, P.C.; Paungsukpaibool, V. Melatonin Supplementation Improved Cryopreserved Thai Swamp Buffalo Semen. Reprod. Domest. Anim. 2021, 56, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hidalgo, D.; Barón, F.J.; Bragado, M.J.; Carmona, P.; Robina, A.; García-Marín, L.J.; Gil, M.C. The Effect of Melatonin on the Quality of Extended Boar Semen After Long-Term Storage at 17 °C. Theriogenology 2011, 75, 1550–1560. [Google Scholar] [CrossRef]
- Ofosu, J.; Qazi, I.H.; Fang, Y.; Zhou, G. Use of Melatonin in Sperm Cryopreservation of Farm Animals: A Brief Review. Anim. Reprod. Sci. 2021, 233, 106850. [Google Scholar] [CrossRef]
- Esplugues, J.V.; Rocha, M.; Nunez, C.; Bosca, I.; Ibiza, S.; Herance, J.R.; Ortega, A.; Serrador, J.M.; D’Ocon, P.; Victor, V.M. Complex I Dysfunction and Tolerance to Nitroglycerin: An Approach Based on Mitochondrial-Targeted Antioxidants. Circ. Res. 2006, 99, 1067–1075. [Google Scholar] [CrossRef]
- Veiko, A.G.; Sekowski, S.; Lapshina, E.A.; Wilczewska, A.Z.; Markiewicz, K.H.; Zamaraeva, M.; Zhao, H.C.; Zavodnik, I.B. Flavonoids Modulate Liposomal Membrane Structure, Regulate Mitochondrial Membrane Permeability and Prevent Erythrocyte Oxidative Damage. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183442. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Nabavi, S.M.; Braidy, N.; Setzer, W.N.; Ahmed, T.; Nabavi, S.F. Quercetin and the Mitochondria: A Mechanistic View. Biotechnol. Adv. 2016, 34, 532–549. [Google Scholar] [CrossRef]
- Ferramosca, A.; Lorenzetti, S.; Di Giacomo, M.; Lunetti, P.; Murrieri, F.; Capobianco, L.; Dolce, V.; Coppola, L.; Zara, V. Modulation of Human Sperm Mitochondrial Respiration Efficiency by Plant Polyphenols. Antioxidants 2021, 10, 217. [Google Scholar] [CrossRef]
- Nass-Arden, L.; Breitbart, H. Modulation of Mammalian Sperm Motility by Quercetin. Mol. Reprod. Dev. 1990, 25, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, S.A.; Sindi, R.A.; Abd, E.M.; Khalifa, N.E.; Khafaga, A.F.; Noreldin, A.E.; Samir, H.; Tufarelli, V.; Losacco, C.; Gamal, M.; et al. Quercetin: Putative Effects on the Function of Cryopreserved Sperms in Domestic Animals. Reprod. Domest. Anim. 2023, 58, 191–206. [Google Scholar] [CrossRef]
- Salehi, E.; Shadboorestan, A.; Mohammadi-Bardbori, A.; Mousavi, A.; Kargar-Abargouei, E.; Sarkoohi, P.; Omidi, M. Effect of Crocin and Quercetin Supplementation in Cryopreservation Medium on Post-Thaw Human Sperm Quality. Cell Tissue Bank. 2024, 25, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Tanga, B.M.; Fang, X.; Seong, G.; Saadeldin, I.M.; Qamar, A.Y.; Lee, S.; Kim, K.J.; Park, Y.J.; Nabeel, A.; et al. Cryopreservation of Pig Semen Using a Quercetin-Supplemented Freezing Extender. Life 2022, 12, 1155. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Han, C.; Wei, B.; Zhao, L.; Zhang, Q.; Deng, R.; Liu, J.; Luo, Y.; Zhang, Y. Protective Effects of Quercetin Against Cadmium Chloride-Induced Oxidative Injury in Goat Sperm and Zygotes. Biol. Trace Elem. Res. 2018, 185, 344–355. [Google Scholar] [CrossRef]
- Najafi, A.; Kia, H.D.; Mehdipour, M.; Hamishehkar, H.; Alvarez-Rodriguez, M. Effect of Quercetin Loaded Liposomes or Nanostructured Lipid Carrier (Nlc) on Post-Thawed Sperm Quality and Fertility of Rooster Sperm. Theriogenology 2020, 152, 122–128. [Google Scholar] [CrossRef]
- Bang, S.; Qamar, A.Y.; Tanga, B.M.; Fang, X.; Seong, G.; Nabeel, A.; Yu, I.J.; Saadeldin, I.M.; Cho, J. Quercetin Improves the Apoptotic Index and Oxidative Stress in Post-Thaw Dog Sperm. Environ. Sci. Pollut. Res. Int. 2022, 29, 21925–21934. [Google Scholar] [CrossRef]
- Bharati, S.; Shetty, S. Mitochondria-Targeted Antioxidants and Cancer; Springer: Singapore, 2022; pp. 1167–1188. [Google Scholar]
- Wang, P.F.; Xie, K.; Cao, Y.X.; Zhang, A. Hepatoprotective Effect of Mitochondria-Targeted Antioxidant Mito-Tempo Against Lipopolysaccharide-Induced Liver Injury in Mouse. Mediat. Inflamm. 2022, 2022, 6394199. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, Y.; Bai, H.; Liu, J.; Li, J.; Wu, B. Mitochondria-Targeted Antioxidant Mitotempo Improves the Post-Thaw Sperm Quality. Cryobiology 2018, 80, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Liu, J.; Zheng, Q.Y.; Liu, N.; Huang, X.L.; Wu, Y.Y.; Yao, X.F.; Tan, Q.Y.; Huang, Y.; Hu, C.H.; et al. The Effect of the Mitochondria-Targeted Antioxidant Mito-Tempo During Sperm Ultra-Rapid Freezing. Cryobiology 2024, 114, 104860. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, X.; Li, J.; Xia, Q.; Gao, J.; Wu, B. Mito-Tempo Alleviates Cryodamage by Regulating Intracellular Oxidative Metabolism in Spermatozoa from Asthenozoospermic Patients. Cryobiology 2019, 91, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ghosh, S.K.; Katiyar, R.; Rautela, R.; Bisla, A.; Ngou, A.A.; Pande, M.; Srivastava, N.; Bhure, S.K. Effect of Mito-Tempo Incorporated Semen Extender on Physico-Morphological Attributes and Functional Membrane Integrity of Frozen Thawed Buffalo Spermatozoa. Cryo Lett. 2021, 42, 111–119. [Google Scholar]
- Kumar, A.; Kumar, G.S.; Katiyar, R.; Gemeda, A.E.; Rautela, R.; Bisla, A.; Srivastava, N.; Kumar, B.S.; Devi, H.L.; Chandra, V. Supplementation of Mito Tempo and Acetovanillone in Semen Extender Improves Freezability of Buffalo Spermatozoa. Andrology 2022, 10, 775–788. [Google Scholar] [CrossRef]
- Asadzadeh, N.; Abdollahi, Z.; Esmaeilkhanian, S.; Masoudi, R. Fertility and Flow Cytometry Evaluations of Ram Frozen Semen in Plant-Based Extender Supplemented with Mito-Tempo. Anim. Reprod. Sci. 2021, 233, 106836. [Google Scholar] [CrossRef]
- Shi, L.; Shi, J.; Feng, J.; Zhang, P.; Ren, Y. Proteomic Analysis Reveals the Potential Positive Effects of Mito-Tempo on Ram Sperm Motility and Fertility During Cryopreservation. Theriogenology 2023, 205, 27–39. [Google Scholar] [CrossRef]
- Zarei, F.; Daghigh-Kia, H.; Masoudi, R. Supplementation of Ram’s Semen Extender with Mito-Tempo II: Quality Evaluation and Flow Cytometry Study of Post-Thawed Spermatozoa. Andrologia 2022, 54, e14299. [Google Scholar] [CrossRef]
- Zarei, F.; Kia, H.D.; Masoudi, R.; Moghaddam, G.; Ebrahimi, M. Supplementation of Ram’s Semen Extender with Mito-Tempo I: Improvement in Quality Parameters and Reproductive Performance of Cooled-Stored Semen. Cryobiology 2021, 98, 215–218. [Google Scholar] [CrossRef]
- Masoudi, R.; Asadzadeh, N.; Sharafi, M. Effects of Freezing Extender Supplementation with Mitochondria-Targeted Antioxidant Mito-Tempo on Frozen-Thawed Rooster Semen Quality and Reproductive Performance. Anim. Reprod. Sci. 2021, 225, 106671. [Google Scholar] [CrossRef]
- Masoudi, R.; Asadzadeh, N.; Sharafi, M. The Mitochondria-Targeted Antioxidant Mito-Tempo Conserves Rooster’s Cooled Semen Quality and Fertility Potential. Theriogenology 2020, 156, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.H.; Banchi, P.; Domain, G.; Vanderheyden, L.; Prochowska, S.; Nizanski, W.; Van Soom, A. Mito-Tempo Improves Acrosome Integrity of Frozen-Thawed Epididymal Spermatozoa in Tomcats. Front. Vet. Sci. 2023, 10, 1170347. [Google Scholar] [CrossRef]
- Alipour-Jenaghard, P.; Daghigh-Kia, H.; Masoudi, R.; Hatefi, A. Mitoq Preserves Epigenetic Modifications and Quality Parameters of Rooster Sperm During Cryopreservation Process. Reprod. Domest. Anim. 2025, 60, e70012. [Google Scholar] [CrossRef]
- Nateghi, R.; Masoudi, R.; Asadzadeh, N. Supplementing the Beltsville Extender with Mitoquinol Improves the Quality and Fertility Potential of the Rooster’s Cooled Sperm. Reprod. Domest. Anim. 2024, 59, e14740. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, J.; Yang, Q.; Xie, Y.; Li, X.; Chen, Z.; Xiong, Y.; Fu, W.; He, H.; Yin, S.; et al. Mitochondria-Targeted Antioxidant Mitoq Improves the Quality of Low Temperature-Preserved Yak Semen Via Alleviating Oxidative Stress. Anim. Reprod. Sci. 2025, 273, 107680. [Google Scholar] [CrossRef]
- Gonzalez, M.; Prashar, T.; Connaughton, H.; Barry, M.; Robker, R.; Rose, R. Restoring Sperm Quality Post-Cryopreservation Using Mitochondrial-Targeted Compounds. Antioxidants 2022, 11, 1808. [Google Scholar] [CrossRef]
- Elkhawagah, A.R.; Donato, G.G.; Poletto, M.; Martino, N.A.; Vincenti, L.; Conti, L.; Necchi, D.; Nervo, T. Effect of Mitoquinone on Sperm Quality of Cryopreserved Stallion Semen. J. Equine Vet. Sci. 2024, 141, 105168. [Google Scholar] [CrossRef]
- Hatami, M.; Masoudi, R.; Hatefi, A.; Alipour-Jenaghard, P.; Esmaeili, V. The Effects of Mitoq as a Mitochondrial-Targeted Antioxidant in a Plant-Based Extender on Buck Sperm Quality Parameters During Cryopreservation. Anim. Reprod. Sci. 2024, 266, 107517. [Google Scholar] [CrossRef]
- Camara, D.R.; Ibanescu, I.; Siuda, M.; Bollwein, H. Mitoquinone Does Not Improve Sperm Cryo-Resistance in Bulls. Reprod. Domest. Anim. 2022, 57, 10–18. [Google Scholar] [CrossRef]
- Tvrda, E.; Debacker, M.; Duracka, M.; Kovac, J.; Bucko, O. Quercetin and Naringenin Provide Functional and Antioxidant Protection to Stored Boar Semen. Animals 2020, 10, 1930. [Google Scholar] [CrossRef]
- Mehdipour, M.; Mohammadi, H.; Salih, S.A.; Rashidi, A. Mitochondrial Specific Antioxidant Mitopbn Mitigates Oxidative Stress and Improves Mitochondrial Function in Cryopreserved Ram Sperm. Sci. Rep. 2025, 15, 10526. [Google Scholar] [CrossRef]
- Boussabbeh, M.; Haddar, M.; Sallem, A.; Chaieb, A.; Khdhiri, R.; Abid Essefi, S.; Mehdi, M. Enhancing Male Fertility: The Role of Crocin in Boosting Sperm Motility through Antioxidant Activity and Mitochondrial Pathways. J. Biochem. Mol. Toxicol. 2025, 39, e70275. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.S.; Tahir, M.Z.; Zahoor, M.Y.; Rahman, H.U.; Riaz, A. Ameliorative Effect of Crocin on Post-Thaw Quality, Fertility-Associated Gene Expression and Fertilization Potential of Buffalo (Bubalus bubalis) Bull Sperm. Reprod. Domest. Anim. 2024, 59, e14519. [Google Scholar] [CrossRef]
- Lv, Y.Q.; Ji, S.; Chen, X.; Xu, D.; Luo, X.T.; Cheng, M.M.; Zhang, Y.Y.; Qu, X.L.; Jin, Y. Effects of Crocin on Frozen-Thawed Sperm Apoptosis, Protamine Expression and Membrane Lipid Oxidation in Yanbian Yellow Cattle. Reprod. Domest. Anim. 2020, 55, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.; Caamano, J.N.; Fernandez-Alegre, E.; Hidalgo, C.O.; Nadri, T.; Tamargo, C.; Fueyo, C.; Fernandez, A.; Merino, M.J.; Martinez-Pastor, F. Supplementation of the Bioxcell Extender with the Antioxidants Crocin, Curcumin and Gsh for Freezing Bull Semen. Res. Vet. Sci. 2021, 136, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, M.; Daghigh, K.H.; Najafi, A.; Mohammadi, H.; Alvarez-Rodriguez, M. Effect of Crocin and Naringenin Supplementation in Cryopreservation Medium on Post-Thaw Rooster Sperm Quality and Expression of Apoptosis Associated Genes. PLoS ONE 2020, 15, e0241105. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.S.; Tahir, M.Z.; Zahoor, M.Y.; Hifz-Ul-Rahman; Riaz, A. Effect of Naringenin on Post-Thaw Quality, Fertility-Associated Gene Expression and Fertilization Potential of Buffalo (Bubalus bubalis) Bull Sperm. Cryobiology 2024, 116, 104953. [Google Scholar] [CrossRef]
- Usuga, A.; Vergara, A.K.; Tobon, M.C.; Vargas, S.; Rojano, B.; Restrepo, G. Metformin and Rosiglitazone Affect Motility, Lipid Peroxidation and Mitochondrial Activity of Thawed Equine Spermatozoa. J. Equine Vet. Sci. 2025, 149, 105570. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.M.; Zhu, D.W.; Li, Y.; Wen, F.; Xian, M.; Hu, Z.T.; Zou, Q.L.; Zhang, L.K.; Chen, Y.L.; et al. Metformin Improves Sheep Sperm Cryopreservation Via Vitalizing the Ampk Pathway. Theriogenology 2023, 208, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, C.; Ma, Y.; Wang, Y.; Zheng, L.; Jin, T.; He, S.; Yang, F.; Dong, W. Metformin Improves Fish Sperm Quality by Regulating Glucose Uptake Capacity During In Vitro Storage. J. Anim. Sci. 2023, 101, skad152. [Google Scholar] [CrossRef]
- Calle-Guisado, V.; Gonzalez-Fernandez, L.; Martin-Hidalgo, D.; Garcia-Marin, L.J.; Bragado, M.J. Metformin Inhibits Human Spermatozoa Motility and Signalling Pathways Mediated by Protein Kinase a and Tyrosine Phosphorylation without Affecting Mitochondrial Function. Reprod. Fertil. Dev. 2019, 31, 787–795. [Google Scholar] [CrossRef]
- Hurtado, D.L.A.; Martin-Hidalgo, D.; Garcia-Marin, L.J.; Bragado, M.J. Metformin Blocks Mitochondrial Membrane Potential and Inhibits Sperm Motility in Fresh and Refrigerated Boar Spermatozoa. Reprod. Domest. Anim. 2018, 53, 733–741. [Google Scholar] [CrossRef]
- Li, R.N.; Zhu, Z.D.; Zheng, Y.; Lv, Y.H.; Tian, X.E.; Wu, D.; Wang, Y.J.; Zeng, W.X. Metformin Improves Boar Sperm Quality Via 5′-Amp-Activated Protein Kinase-Mediated Energy Metabolism in Vitro. Zool. Res. 2020, 41, 527–538. [Google Scholar] [CrossRef]
- Grandhaye, J.; Partyka, A.; Ligocka, Z.; Dudek, A.; Nizanski, W.; Jeanpierre, E.; Estienne, A.; Froment, P. Metformin Improves Quality of Post-Thaw Canine Semen. Animals 2020, 10, 287. [Google Scholar] [CrossRef]
- Swegen, A.; Lambourne, S.R.; Aitken, R.J.; Gibb, Z. Rosiglitazone Improves Stallion Sperm Motility, Atp Content, and Mitochondrial Function. Biol. Reprod. 2016, 95, 107. [Google Scholar] [CrossRef] [PubMed]
- Losano, J.; Daigneault, B.W. Pharmacological Perturbation of Peroxisome-Proliferator-Activated Receptor Gamma Alters Motility and Mitochondrial Function of Bovine Sperm. Andrology 2023, 11, 155–166. [Google Scholar] [CrossRef]
- Mehdipour, M.; Daghigh-Kia, H.; Najafi, A.; Mehdipour, Z.; Mohammadi, H. Protective Effect of Rosiglitazone on Microscopic and Oxidative Stress Parameters of Ram Sperm After Freeze-Thawing. Sci. Rep. 2022, 12, 13981. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yang, K.; Guo, H.T.; Wang, J.R.; Sun, H.H.; Wang, S.W.; Sun, M.; Sun, L.Z.; Yue, S.L.; Zhou, J.B. Protective Influence of Rosiglitazone Against Time-Dependent Deterioration of Boar Spermatozoa Preserved at 17 Degrees C. Reprod. Domest. Anim. 2019, 54, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Q.; Min, L.; Cao, H.; Adetunji, A.O.; Zhou, K.; Zhu, Z. Pyrroloquinoline Quinone Improved Boar Sperm Quality Via Maintaining Mitochondrial Function During Cryopreservation. Antioxidants 2025, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, W.; Yang, Q.; Zhao, H.; Zhang, W.; Adetunji, A.O.; Hoque, S.; Kou, X.; Min, L. Pyrroloquinoline Quinone Improves Ram Sperm Quality through its Antioxidative Ability During Storage at 4 Degrees C. Antioxidants 2024, 13, 104. [Google Scholar] [CrossRef]
- Younus, A.M.; Yamanaka, T.; Shimada, M. The Protective Effects of Antioxidants Against Endogenous and Exogenous Oxidative Stress on Bull Sperm. Vitr. Cell Dev. Biol. Anim. 2024, 60, 969–982. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, B.X.; Long, C.; Heng, N.; Guo, Y.; Sheng, X.H.; Wang, X.G.; Xing, K.; Xiao, L.F.; Ni, H.M.; et al. Natural Astaxanthin Improves Testosterone Synthesis and Sperm Mitochondrial Function in Aging Roosters. Antioxidants 2022, 11, 1684. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Kang, H.Y.; Guo, Y.; Sheng, X.H.; Wang, X.G.; Xing, K.; Xiao, L.F.; Lv, X.Z.; Long, C.; Qi, X.L. Effect of Natural Astaxanthin on Sperm Quality and Mitochondrial Function of Breeder Rooster Semen Cryopreservation. Cryobiology 2024, 117, 104979. [Google Scholar] [CrossRef]
- Sohail, T.; Zhang, L.; Wang, X.; Jiang, C.; Wang, J.; Sun, X.; Li, Y. Astaxanthin Improved the Quality of Hu Ram Semen by Increasing the Antioxidant Capacity and Mitochondrial Potential and Mitigating Free Radicals-Induced Oxidative Damage. Animals 2024, 14, 319. [Google Scholar] [CrossRef]
- Qamar, A.Y.; Fang, X.; Bang, S.; Shin, S.T.; Cho, J. The Effect of Astaxanthin Supplementation on the Post-Thaw Quality of Dog Semen. Reprod. Domest. Anim. 2020, 55, 1163–1171. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, X.; Li, Y.; Cheng, Y.; Li, J. Ergothioneine Improves the Quality of Boar Sperm During In Vitro Liquid Preservation by Regulating Mitochondrial Respiratory Chain. Animals 2025, 15, 1450. [Google Scholar] [CrossRef]
- Usuga, A.; Tejera, I.; Gomez, J.; Restrepo, O.; Rojano, B.; Restrepo, G. Cryoprotective Effects of Ergothioneine and Isoespintanol on Canine Semen. Animals 2021, 11, 2757. [Google Scholar] [CrossRef]
- Abdalkarim, S.S.; Daghigh-Kia, H.; Mehdipour, M.; Najafi, A. Does Ergothioneine and Thawing Temperatures Improve Rooster Semen Post-Thawed Quality? Poult. Sci. 2021, 100, 101405. [Google Scholar] [CrossRef]
- Vandvali, F.; Kia, H.D.; Ebrahimi, M.; Moghaddam, G.; Najafi, A. Impact of Losartan-Supplemented Extender on Enhancing Rooster Sperm Quality Post-Freezing and Thawing. Poult. Sci. 2025, 104, 105179. [Google Scholar] [CrossRef]
- Sindi, R.A.; Abdelnour, S.A.; El-Haroun, E.; Alfattah, M.A.; Saber, Y.; Sheiha, A.M. Does Lagenaria Siceraria Seed Oil-Enriched Extender Regulate Sperm Quality, Oxidant/Antioxidant Markers, and Sperm Mitochondrial Enzymes in Chilled Diluted Rabbit Semen? BMC Vet. Res. 2025, 21, 345. [Google Scholar] [CrossRef] [PubMed]
- Jorge, M.; Ferreira, F.C.; Marques, C.C.; Batista, M.C.; Oliveira, P.J.; Lidon, F.; Duarte, S.C.; Teixeira, J.; Pereira, R. Effect of Urolithin a on Bovine Sperm Capacitation and in Vitro Fertilization. Animals 2024, 14, 2726. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.; Marques, C.C.; Baptista, M.C.; Pimenta, J.; Teixeira, J.; Montezinho, L.; Cagide, F.; Borges, F.; Oliveira, P.J.; Pereira, R. Effect of a Novel Hydroxybenzoic Acid Based Mitochondria Directed Antioxidant Molecule on Bovine Sperm Function and Embryo Production. Animals 2022, 12, 804. [Google Scholar] [CrossRef]
- Coyan, K.; Baspinar, N.; Bucak, M.N.; Akalin, P.P. Effects of Cysteine and Ergothioneine on Post-Thawed Merino Ram Sperm and Biochemical Parameters. Cryobiology 2011, 63, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiong, M.; Zhang, J.; Li, S.; Ma, S.; Jiang, S.; Jiang, Y.; Li, X. Polydopamine-Based Nano-Protectant for Prolonged Boar Semen Preservation by Eliminating Ros and Regulating Protein Phosphorylation Via D2Dr-Mediated Camp/Pka Signaling Pathway. J. Nanobiotechnol. 2025, 23, 151. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).