Two Cases of Feather Dystrophy in Free-Living Griffon Vultures (Gyps fulvus fulvus) Associated with Viral-like Inclusion Bodies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Serological Examination
2.3. Microscopical and Immunohistochemical Examination
2.4. Ultrastructural Examination
2.5. Viral Genome Extraction and NGS Sequencing
3. Results
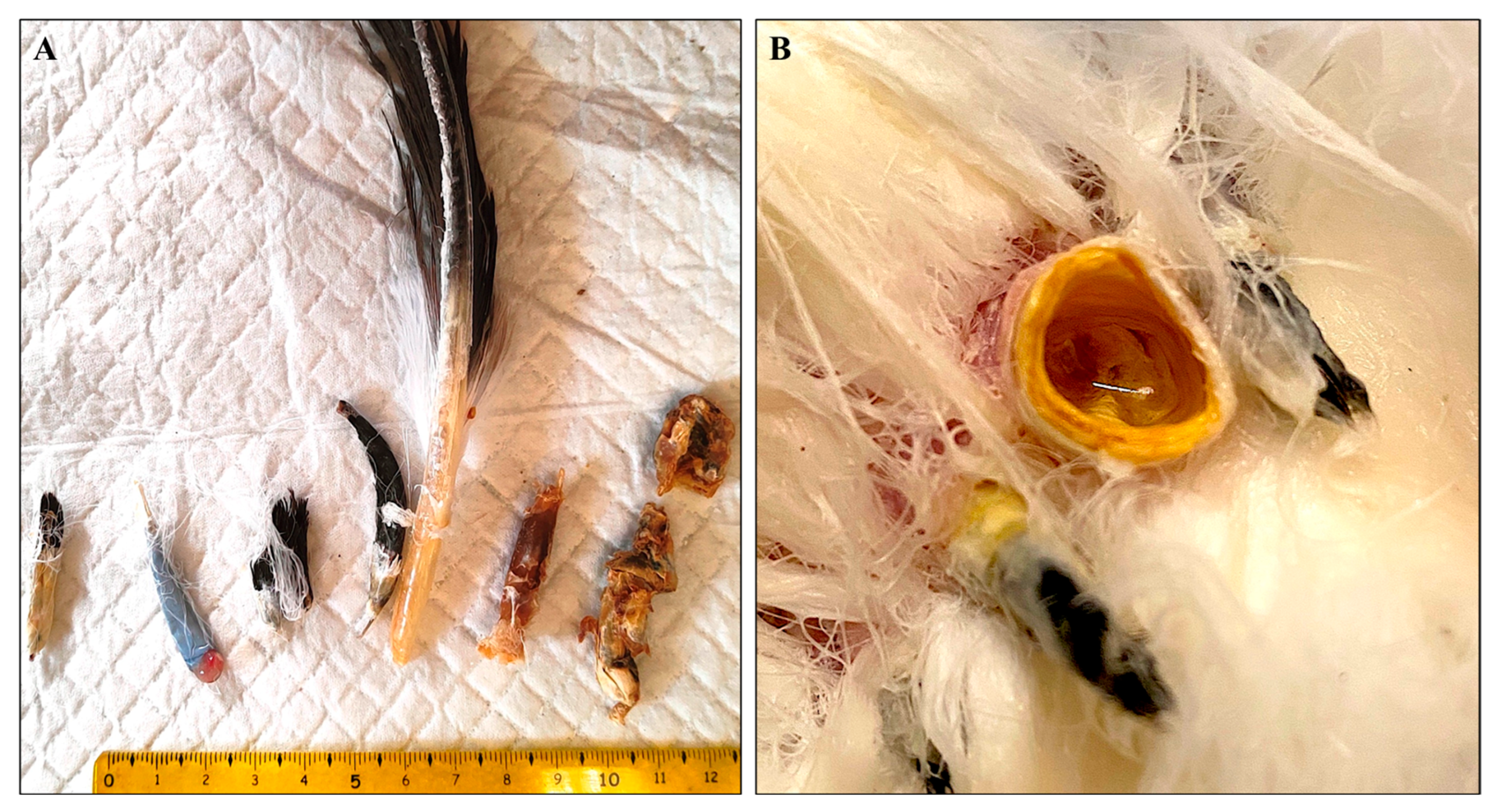
3.1. Macroscopic Examination
3.2. Serological Examination
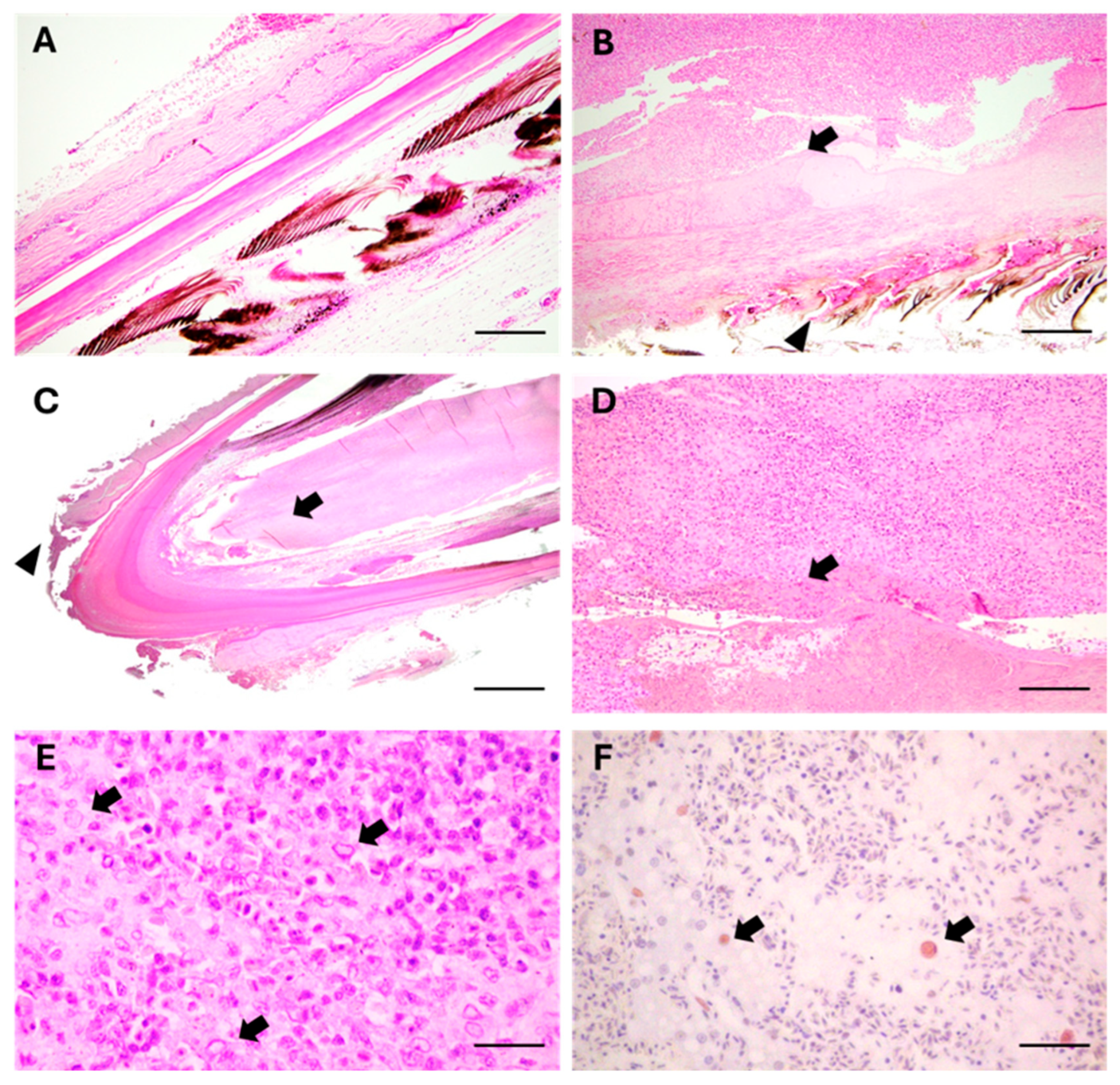
3.3. Microscopical Examination
3.4. Immunohistochemical Examination
3.5. Ultrastructural Examination
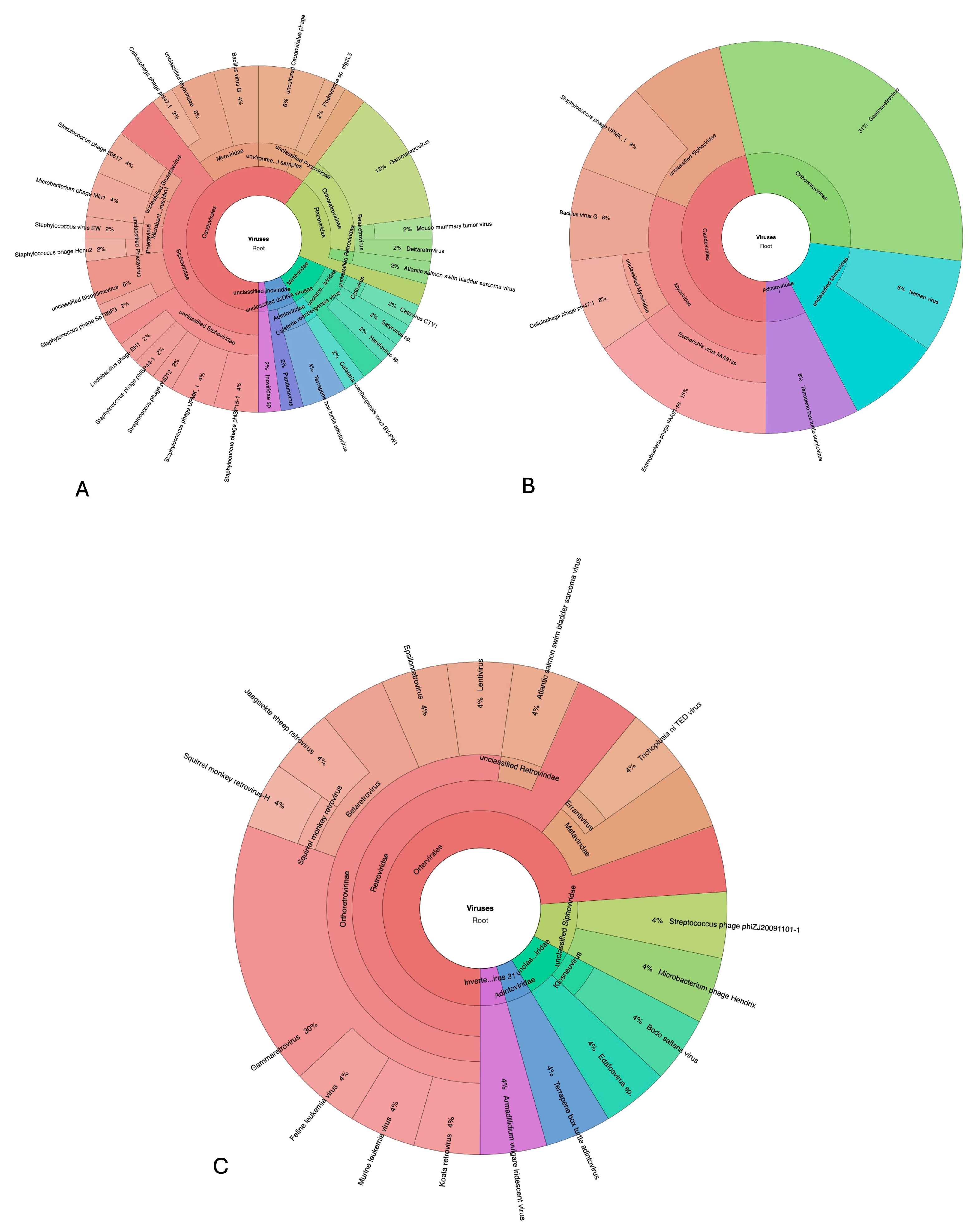
3.6. Virome Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| POS | Pinching Off Syndrome |
| APV | Avian polyomavirus |
| VP | Viral protein |
| TEM | Transmission electron microscopy |
| PBFD | Psittacine Beak and Feather Disease |
| WNV | West Nile Virus |
| ILTV | Laryngotracheitis alpha1-herpesvirus |
| RT | Room temperature |
| HE | Hematoxylin and Eosin |
References
- Foth, C. Introduction to the Morphology, Development, and Ecology of Feathers. In The Evolution of Feathers; Foth, C., Rauhut, O.W.M., Eds.; Fascinating Life Sciences; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–11. ISBN 978-3-030-27222-7. [Google Scholar]
- Ritchie, B.W.; Harrison, L.R. Avian Medicine: Principles and Application; Wingers Publishing: Lake Worth, FL, USA, 1994. [Google Scholar]
- Oglesbee, B.L. Hypothyroidism in a Scarlet Macaw. J. Am. Vet. Med. Assoc. 1992, 201, 1599–1601. [Google Scholar] [CrossRef] [PubMed]
- Greenacre, C.B.; Latimer, K.S.; Niagro, F.D.; Campagnoli, R.P.; Pesti, D.; Ritchie, B.W. Psittacine Beak and Feather Disease in a Scarlet Macaw (Ara macao). J. Assoc. Avian Vet. 1992, 6, 95–98. [Google Scholar] [CrossRef]
- Perry, R.A. A Psittacine Combined Beak and Feather Disease Syndrome with Particular Reference to the Australian Cockatoos Cacatua galerita (Sulphur-Crested Cockatoo), Cacatua leadbeateri (Major Mitchell or Pink Cockatoo), Cacatua roseicapella (Galah or Rose-Breasted Cockatoo) and Cacatua Sanguinea (Little Corella). Proceedings 1981, 55, 81–108. [Google Scholar]
- McOrist, S.; Black, D.G.; Pass, D.A.; Scott, P.C.; Marshall, J. Beak and Feather Dystrophy in Wild Sulphur-Crested Cockatoos (Cacatua galerita). J. Wildl. Dis. 1984, 20, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Melville, D.S.; Schuckard, R. New Zealand King Shag (Leucocarbo carunculatus) with Deformed Primary Feathers. Notornis 2021, 68, 274–277. [Google Scholar] [CrossRef]
- Pass, D.A.; Plant, S.L.; Sexton, N. Natural Infection of Wild Doves (Streptopelia senegalensis) with the Virus of Psittacine Beak and Feather Disease. Aust. Vet. J. 1994, 71, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Schettler, E.; Gerlach, H.; Brunnberg, L.; Hafez, H.M.; Hattermann, K.; Johne, R.; Kollmann, R.; Krone, O.; Lierz, M.; et al. Investigations on the Aetiology of Pinching off Syndrome in Four White-Tailed Sea Eagles (Haliaeetus albicilla) from Germany. Avian Pathol. 2007, 36, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, N.M.; Kratz, G.E.; Bates, R.; Scherpelz, J.A.; Bowen, R.A.; Komar, N. Clinical Evaluation and Outcomes of Naturally Acquired West Nile Virus Infection in Raptors. J. Zoo Wildl. Med. 2009, 40, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Lloyd, C.; Forwood, J.; Raidal, S.R. Forensic Genetic Evidence of Beak and Feather Disease Virus Infection in a Powerful Owl, Ninox Strenua. Emu 2015, 116, 71–74. [Google Scholar] [CrossRef]
- Destro, G.F.G.; De Marco, P.; Terribile, L.C. Threats for Bird Population Restoration: A Systematic Review. Perspect. Ecol. Conserv. 2018, 16, 68–73. [Google Scholar] [CrossRef]
- Willette, M.; Rosenhagen, N.; Buhl, G.; Innis, C.; Boehm, J. Interrupted Lives: Welfare Considerations in Wildlife Rehabilitation. Animals 2023, 13, 1836. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Phylogeny of Old and New World Vultures (Aves: Accipitridae and Cathartidae) Inferred from Nucleotide Sequences of the Mitochondrial Cytochrome b Gene. Z. Naturforschung C 1995, 50, 868–882. [Google Scholar] [CrossRef] [PubMed]
- Griffon Vulture Gyps fulvus Species. Available online: https://datazone.birdlife.org/species/factsheet/griffon-vulture-gyps-fulvus (accessed on 20 February 2025).
- Dobrev, D.; Tsiakiris, R.; Skartsi, T.; Dobrev, V.; Arkumarev, V.; Stara, K.; Stamenov, A.; Probonas, N.; Kominos, T.; Galanaki, A.; et al. Long-Term Size and Range Changes of the Griffon Vulture Gyps fulvus Population in the Balkans: A Review. Bird Conserv. Int. 2022, 32, 206–221. [Google Scholar] [CrossRef]
- Khan, U.; Murn, C. Gyps Vulture Restoration Project—Role of Captive Breeding in Endangered Species Management. J. Anim. Plant Sci. 2011, 21, 405–409. [Google Scholar]
- Lorenzo-Betancor, O.; Galosi, L.; Bonfili, L.; Eleuteri, A.M.; Cecarini, V.; Verin, R.; Dini, F.; Attili, A.-R.; Berardi, S.; Biagini, L.; et al. Homozygous CADPS2 Mutations Cause Neurodegenerative Disease with Lewy Body-like Pathology in Parrots. Mov. Disord. 2022, 37, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Desantis, S.; Galosi, L.; Santamaria, N.; Roncarati, A.; Biagini, L.; Rossi, G. Modulation of Morphology and Glycan Composition of Mucins in Farmed Guinea Fowl (Numida meleagris) Intestine by the Multi-Strain Probiotic Slab51®. Animals 2021, 11, 495. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Taccini, E.; Tarantino, C. Outbreak of Avian Polyomavirus Infection with High Mortality in Recently Captured Crimson’s Seedcrackers (Pyrenestes sanguineus). J. Wildl. Dis. 2005, 41, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and Sensitive Protein Alignment Using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive Metagenomic Visualization in a Web Browser. BMC Bioinform. 2011, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.E. Veterinary Aspects of Captive Birds of Prey; The Hawk Trust: Newent, UK, 1985; Volume 11, pp. 156–160. [Google Scholar]
- Cooper, J.E. Feather Conditions in Birds of Prey. J. N. Am. Falconers’ Assoc. 1972, 11, 39–44. [Google Scholar]
- Yu, M.; Yue, Z.; Wu, P.; Wu, D.-Y.; Mayer, J.-A.; Medina, M.; Widelitz, R.B.; Jiang, T.-X.; Chuong, C.-M. The Developmental Biology of Feather Follicles. Int. J. Dev. Biol. 2004, 48, 181. [Google Scholar] [CrossRef]
- Phalen, D.N. Papillomaviruses and Polyomaviruses. In Infectious Diseases of Wild Birds; Blackwell Publishing: Oxford, UK, 2008; 206p. [Google Scholar]
- Couteaudier, M.; Trapp-Fragnet, L.; Auger, N.; Courvoisier, K.; Pain, B.; Denesvre, C.; Vautherot, J.-F. Derivation of Keratinocytes from Chicken Embryonic Stem Cells: Establishment and Characterization of Differentiated Proliferative Cell Populations. Stem Cell Res. 2015, 14, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Couteaudier, M.; Courvoisier, K.; Trapp-Fragnet, L.; Denesvre, C.; Vautherot, J.-F. Keratinocytes Derived from Chicken Embryonic Stem Cells Support Marek’s Disease Virus Infection: A Highly Differentiated Cell Model to Study Viral Replication and Morphogenesis. Virol. J. 2016, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Johne, R.; Müller, H. Avian Polyomavirus in Wild Birds: Genome Analysis of Isolates from Falconiformes and Psittaciformes. Arch. Virol. 1998, 143, 1501–1512. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesaro, S.; Volpatti, D.; Baggio, A.; Verin, R.; Genero, F.; Sicuro, L.; Galosi, L.; Biagini, L.; Perlin, I.; Robino, P.; et al. Two Cases of Feather Dystrophy in Free-Living Griffon Vultures (Gyps fulvus fulvus) Associated with Viral-like Inclusion Bodies. Animals 2025, 15, 2190. https://doi.org/10.3390/ani15152190
Pesaro S, Volpatti D, Baggio A, Verin R, Genero F, Sicuro L, Galosi L, Biagini L, Perlin I, Robino P, et al. Two Cases of Feather Dystrophy in Free-Living Griffon Vultures (Gyps fulvus fulvus) Associated with Viral-like Inclusion Bodies. Animals. 2025; 15(15):2190. https://doi.org/10.3390/ani15152190
Chicago/Turabian StylePesaro, Stefano, Donatella Volpatti, Alice Baggio, Ranieri Verin, Fulvio Genero, Luca Sicuro, Livio Galosi, Lucia Biagini, Isabella Perlin, Patrizia Robino, and et al. 2025. "Two Cases of Feather Dystrophy in Free-Living Griffon Vultures (Gyps fulvus fulvus) Associated with Viral-like Inclusion Bodies" Animals 15, no. 15: 2190. https://doi.org/10.3390/ani15152190
APA StylePesaro, S., Volpatti, D., Baggio, A., Verin, R., Genero, F., Sicuro, L., Galosi, L., Biagini, L., Perlin, I., Robino, P., Colitti, B., Avanzato, D., & Rossi, G. (2025). Two Cases of Feather Dystrophy in Free-Living Griffon Vultures (Gyps fulvus fulvus) Associated with Viral-like Inclusion Bodies. Animals, 15(15), 2190. https://doi.org/10.3390/ani15152190





