Feline Testicular Biometry and Gonadosomatic Index: Associations Among Conventional Measurements, Mathematical Estimates, and Seminal Parameters
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Location and Animal Assessment
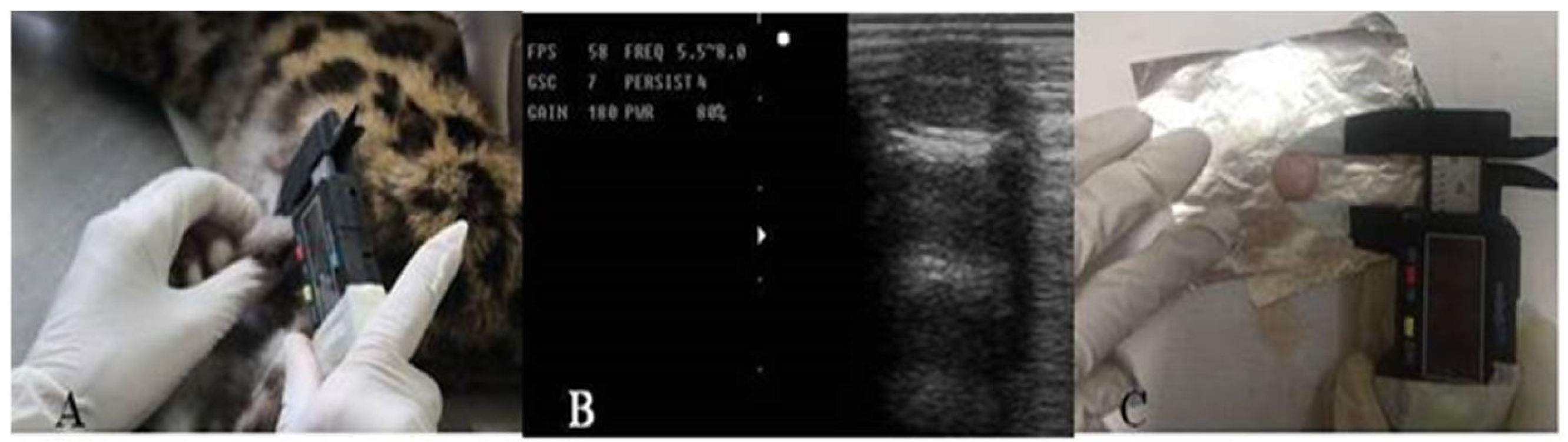
2.3. Anesthetic Procedures and Semen Collection
2.4. Body Biometry
2.5. Testicular Biometry and Testicular Parameters
Semen Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morato, R.G.; Verreschi, I.T.N.; Guimarães, M.A.B.V.; Cassaro, K.; Pessuti, C.; Barnabe, R.C. Seasonal variation in the endocrine–testicular function of captive jaguars (Panthera onca). Theriogenology 2004, 61, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, A.I.S.B.; Fioratti, E.G.; Ramos, R.S.; Neves, R.C.F.; Ferreira, J.C.P.; Cardoso, G.S.; Padilha, P.M.; Lopes, M.D. Blood and seminal plasma concentrations of selenium, zinc and testosterone and their relationship to sperm quality and testicular biometry in domestic cats. Anim. Reprod. Sci. 2014, 150, 50–55. [Google Scholar] [CrossRef] [PubMed]
- D’Francisco, F.; Lapuente, C.; Merlo, M.L.; Barbeito, C.; Gobello, C. Feline testicular ultrasonogram differentiates pre vs. postpubertal and normal vs. disrupted spermatogenesis. Theriogenology 2020, 157, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Lueders, I.; Reuken, J.; Luther, I.; Horst, G.; van der Kotze, A.; Tordiffe, A.; Sieme, H.; Jakop, U.; Mueller, K. Effect of age and body condition score on reproductive organ size and sperm parameters in captive male African lion (Panthera leo): Suggesting a prime breeding age. Theriogenol. Wild 2024, 5, 100093. [Google Scholar] [CrossRef]
- Swanson, W.F. The challenge of assisted reproduction for conservation of wild felids—A reality check. Theriogenology 2023, 197, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Valverde, M.; Bittencourd, R.F.; de Lisboa Ribeiro Filho, A.; Lents, M.P.; de Azevedo, M.C.; Barreto, R.O.; Teixeira, N.A.; Mattos, P.M.S.; Curvelo, V.P.; Gomes, M.C.; et al. Biometría testicular y características seminales en felinos neotropicales (Carnivora: Felidae) sometidos a cateterismo uretral. Rev. Biol. Trop. 2019, 67, 975–988. [Google Scholar] [CrossRef]
- Madrigal-Valverde, M.; Bittencourt, R.F.; Ribeiro Filho, A.D.L.; Vieira, C.A.; Romão, E.A.; Carneiro, I.B.; Azevedo, M.C.; Araujo, G.R. Quality of domestic cat semen collected by urethral catheterization after the use of different alpha 2-adrenergic agonists. J. Feline. Med. Surg. 2021, 23, 745–750. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, M.H.F.; de Paula, T.A.R.; da Matta, S.L.P.; Fonseca, C.C.; da Costa, E.P.; Costa, D.S.; Peixoto, J.V. Cell population indexes of spermatogenic yield and testicular sperm reserves in adult jaguars (Panthera onca). Anim. Reprod. Sci. 2010, 118, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.G.; Brown, J.L.; Bush, M.; Wildt, D.E. Teratospermic and normospermic domestic cats: Ejaculate traits, pituitary–gonadal hormones, and improvement of spermatozoal motility and morphology after swim-up processing. J. Androl. 1990, 11, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Lambert, B. The frequency of mumps and of mumps orchitis and the consequences for sexuality and fertility. Acta Genet. Stat. Med. 1951, 11, 1–166. [Google Scholar] [PubMed]
- Hansen, P.F.; With, T.K. Clinical measurements of the testes in boys and men. Acta Med. Scand. 1952, 142, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Petty, C.S.; Neaves, W.B. A new approach to quantification of spermatogenesis and its application to germinal cell attrition during human spermiogenesis. Biol. Reprod. 1981, 25, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Cedenho, A.; Tokunaga, I. Supravital sperm staining compares eosin and eosin-nigrosin techniques. J. Bras. Urol. 1995, 16, 234–236. [Google Scholar]
- Karun, K.M.; Puranik, A. BA.plot: An R function for Bland-Altman analyses. Clin. Epidemiol. Glob. Health 2021, 12, 100831. [Google Scholar] [CrossRef] [PubMed]
- Stremnitzer, M. Angiodynography of the Tom Cat Testis. Ph.D. Thesis, University of Vienna, Vienna, Austria, 2002. [Google Scholar]
- Kumar, K.; Kannan, T.A.; Ramesh, G.; Rao, G.V.S.; Rengasamy, S.; Ravali, K.S.; Asmitha, S. Gross morphometric analysis of the testis of tom cats (Felis catus). Pharma Innov. J. 2023, 12, 4174–4176. [Google Scholar] [CrossRef]
- Homola, S.; Sendag, S.; Koca, D.; Wehrend, A. Methods for evaluating testicular function in domestic cats. Reprod. Domest. Anim. 2025, 60, e70042. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.H. Morphofunctional Analysis of the Adult Jaguar (Panthera onca) testis. Master’s Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2004. Available online: https://www.carnivoreconservation.org/files/thesis/azevedo_2004_msc.pdf (accessed on 21 July 2025). (In Portuguese).
- Zambelli, D.; Raccagni, R.; Cunto, M.; Andreani, G.; Isani, G. Sperm evaluation and biochemical characterization of cat seminal plasma collected by electroejaculation and urethral catheterization. Theriogenology 2010, 74, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Jelinkova, K.; Vitasek, R.; Novotny, R.; Bartoskova, A. A comparison of quality parameters of fresh feline ejaculates collected by three different collection techniques. Reprod. Domest. Anim. 2018, 53, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Filliers, M.; Rijsselaere, T.; Bossaert, P.; Zambelli, D.; Anastasi, P.; Hoogewijs, M.; Van Soom, A. In vitro evaluation of fresh sperm quality in tomcats: A comparison of two collection techniques. Theriogenology 2010, 74, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Prochowska, S.; Niżański, W.; Partyka, A. Comparative analysis of in vitro characteristics of fresh and frozen–thawed urethral and epididymal spermatozoa from cats (Felis domesticus). Theriogenology 2016, 86, 2063–2072. [Google Scholar] [CrossRef] [PubMed]
- Morais, R.N.; Mucciolo, R.G.; Gomes, M.L.F.; Lacerda, O.; Moraes, W.; Moreira, N.; Graham, L.H.; Swanson, W.F.; Brown, J.L. Seasonal analysis of semen characteristics, serum testosterone and fecal androgens in the ocelot (Leopardus pardalis), margay (L. wiedii) and tigrina (L. tigrinus). Theriogenology 2002, 57, 2027–2041. [Google Scholar] [CrossRef] [PubMed]
- Kenagy, G.J.; Trombulak, S.C. Size and function of mammalian testes in relation to body size. J. Mammal. 1986, 67, 1–22. [Google Scholar] [CrossRef]
- Costa, D.S.; Paula, T.A.; Matta, S.L. Cat, cougar, and jaguar spermatogenesis: A comparative analysis. Braz. Arch. Biol. Technol. 2006, 49, 725–731. [Google Scholar] [CrossRef]



| Method 1 | Method 2 | Method 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Length | Height | Width | Length | Height | Width | Length | Height | Width | |
| Mean ± SD (mm) | 14.42 ± 3.89 | 7 ± 3.89 | 9.31 ± 3.89 | 17.14 ± 2.54 | 12.28 ± 1 | 10.2 ± 0.86 | 15.15 ± 1.49 | 12.42 ± 1.26 | 22.56 ± 2.78 |
| Median (mm) | 14.7 | 9.3 | 9.3 | 18 | 18 | 10 | 15.5 | 12.6 | 21.5 |
| Method 1 | Method 2 | Method 3 | Real | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Equation | Testicular Area | Testicular Volume | Testicular Weight | Testicular Area | Testicular Volume | Testicular Weight | Testicular Area | Testicular Volume | Testicular Weight | Scale Testicular Weight | ||||||||||
| (1) | 2.10 | ±0.47 | 4.45 | ±0.72 | 4.65 | ±0.75 | 3.77 | ±0.49 | 2.60 | ±0.84 | 2.72 | ±0.88 | 2.24 | ±1.03 | 9.00 | ±2.62 | 9.42 | ±2.74 | 2.16 | ±0.67 |
| (2) | 2.10 | ±0.47 | 1.30 | ±0.43 | 1.36 | ±0.45 | 3.77 | ±0.49 | 2.46 | ±0.61 | 2.57 | ±0.64 | 2.24 | ±1.03 | 2.87 | ±0.83 | 3.00 | ±0.87 | 2.16 | ±0.67 |
| (3) | 2.10 | ±0.47 | 1.51 | ±0.24 | 1.58 | ±0.25 | 3.77 | ±0.49 | 0.88 | ±0.29 | 0.88 | ±0.29 | 2.24 | ±1.03 | 1.57 | ±0.37 | 1.64 | ±0.39 | 2.16 | ±0.67 |
| (4) | 2.10 | ±0.47 | 1.12 | ±0.18 | 1.11 | ±0.30 | 3.77 | ±0.49 | 0.65 | ±0.21 | 1.11 | ±0.30 | 2.24 | ±1.03 | 1.16 | ±0.27 | 1.11 | ±0.30 | 2.16 | ±0.67 |
| (5) | 2.10 | ±0.47 | 1.34 | ±0.22 | 1.40 | ±0.23 | 3.77 | ±0.49 | 0.65 | ±0.21 | 0.68 | ±0.22 | 2.24 | ±1.03 | 1.27 | ±0.28 | 1.33 | ±0.29 | 2.16 | ±0.67 |
| Method 1 | Method 2 | Method 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Formulae | Mean (cm3) ± (SD) | Absolute Differences Between Limits (cm3) | Mean (cm3) ± (SD) | Absolute Differences Between Limits (cm3) | Mean (cm3) ± (SD) | Absolute Differences Between Limits (cm3) | |||
| 1 and 2 | −0.01 | ±−0.01 | 0.00 | 0.23 | ±−0.27 | 0.04 | 0.55 | ±−0.39 | 0.16 |
| (LSC to LIC) | (−0.76 | to −0.17) | 0.59 | (−0.31 | to 0.76) | 0.32 | (−0.21 | to 1.30) | 1.09 |
| 1 and 3 | 0.46 | ±−0.15 | 0.31 | 0.79 | ±−0.19 | 0.60 | −0.82 | ±−0.20 | 0.60 |
| (LSC to LIC) | (0.17 | to 0.76) | 0.59 | (0.42 | to 1.16) | 0.74 | (−0.95 | to 0.36) | 0.59 |
| 1 and 4 | 0.00 | ±−0.00 | 0.00 | 0.00 | ±−0.00 | 0 | 0.00 | ±−0.00 | 0 |
| (LSC to LIC) | (−0.76 | to −0.17) | 0.59 | (0.00 | to 0.00) | 0 | (0.00 | to 0.00) | 0 |
| 1 and 5 | −0.01 | ±−0.01 | 0.00 | 0.21 | ±−0.27 | 0.05 | 0.53 | ±−0.39 | 0.16 |
| (LSC to LIC) | (−0.76 | to −0.17) | 0.59 | (−0.32 | to 0.74) | 0.32 | (−0.22 | to 1.28) | 1.06 |
| 2 and 3 | 0.46 | ±−0.15 | 0.31 | 0.57 | ±−0.32 | 0.25 | 0.28 | ±−0.34 | 0.06 |
| (LSC to LIC) | (0.17 | to 0.76) | 0.59 | (−0.06 | to 1.20) | 0.06 | (−0.38 | to 0.94) | 0.56 |
| 2 and 4 | 0.00 | ±−0.01 | 0.00 | −0.22 | ±−0.27 | 0.05 | −0.55 | ±−0.39 | 0.16 |
| (LSC to LIC) | (−0.76 | to −0.17) | 0.59 | (−0.76 | to 0.31) | 0.31 | (−1.30 | to 0.21) | 1.09 |
| 2 and 5 | −0.01 | ±−0.00 | 0.00 | −0.02 | ±−0.00 | 0 | 0.00 | ±−0.01 | 0 |
| (LSC to LIC) | (−0.01 | to 0.00) | 0.01 | (−0.03 | to −0.01) | 0.03 | (0.00 | to 0.00) | 0 |
| 3 and 4 | −0.46 | ±−0.15 | 0.31 | −0.79 | ±−0.19 | 0.60 | (−0.82 | ±−0.20 | 0.62 |
| (LSC to LIC) | (−0.78 | to −0.17) | 0.59 | (−1.16 | to −0.42) | 0.41 | −0.95 | to 0.36) | −0.59 |
| 3 and 5 | −0.47 | ±−0.15 | 0.31 | −0.59 | ±−0.32 | 0.27 | (−0.30 | ±−0.33 | 0.03 |
| (LSC to LIC) | (−0.78 | to −0.17) | 0.59 | (−1.21 | to 0.04) | 1.16 | −0.95 | to 0.36) | 0.59 |
| 4 and 5 | −0.01 | ±−0.00 | 0.00 | 0.21 | ±−0.27 | 0.06 | (0.53 | ±−0.39 | 0.14 |
| (LSC to LIC) | (−0.02 | to 0.00) | 0.02 | (−0.32 | to 0.74) | 0.42 | −0.22 | to 1.27) | 1.05 |
| Area (cm2) | Volume (cm3) | Weight (g) | GSI | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | Median | |
| Method 1 | 2.10 ± 0.47 | 2.00 | 1.30 ± 0.43 | 1.22 | 1.36 ± 0.45 | 1.28 | 0.03 ± 0.01 | 0.03 |
| Method 2 | 3.77 ± 0.49 | 3.68 | 2.46 ± 0.61 | 2.28 | 2.57 ± 0.64 | 2.38 | 0.06 ± 0.01 | 0.06 |
| Method 3 | 2.24 ± 1.03 | 2.93 | 2.87 ± 0.83 | 2.94 | 3.00 ± 0.87 | 3.08 | 0.07 ± 0.02 | 0.07 |
| Variable | Volume (μL) * | Vigor * | MT (%) | MP (%) | CN/mL * | EOS (%) | CH (%) | EMN (%) |
|---|---|---|---|---|---|---|---|---|
| Mean ± DP | 37.50 ± 28.41 | 3.85 ± 0.41 | 81.50 ± 8.51 | 75.00 ± 10.27 | 473.31 ± 491.61 | 78.60 ± 11.16 | 80.70 ± 9.01 | 67.00 ± 20.74 |
| Minimum | 20.00 | 3.00 | 70.00 | 60.00 | 10.00 | 64.00 | 66.00 | 31.00 |
| Q25 | 20.00 | 3.50 | 73.75 | 67.50 | 41.52 | 68.75 | 70.00 | 50.75 |
| Median | 30.00 | 4.00 | 80.00 | 75.00 | 323.90 | 77.50 | 84.50 | 68.50 |
| Q75 | 37.50 | 4.00 | 90.00 | 85.00 | 881.25 | 88.50 | 88.25 | 83.50 |
| Maximum | 115.00 | 4.50 | 95.00 | 90.00 | 1333.33 | 95.00 | 90.00 | 96.00 |
| Measurement (cm) | Mean ± SD (cm) | Minimum (cm) | Q25 | Median (cm) | Q75 | Maximum (cm) |
|---|---|---|---|---|---|---|
| Head length | 12.58 ± 1.17 | 11.00 | 11.75 | 12.00 | 13.50 | 15.00 |
| Head circumference | 23.04 ± 1.75 | 20.00 | 21.75 | 23.00 | 24.00 | 27.00 |
| Thoracic diameter | 32.54 ± 1.51 | 30.00 | 32.00 | 32.50 | 33.00 | 35.50 |
| Tail + body lengths | 55.77 ± 1.52 | 53.00 | 54.75 | 56.00 | 57.00 | 58.00 |
| Tail length | 27.38 ± 4.58 | 14.50 | 25.75 | 28.00 | 30.25 | 33.00 |
| Total length | 83.15 ± 4.72 | 70.50 | 81.50 | 83.00 | 86.75 | 89.50 |
| Tail + Body Lengths | Tail Length | T. Diameter | Head Circ | Total Length | Head Length | Sp.Vol | Vigor | TM | PM | Cn | EOS | CH | MA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tail + body lengths | 1.00 | −0.11 | 0.00 | −0.06 | 0.32 | 0.28 | 0.13 | 0.55 | 0.50 | −0.03 | −0.26 | 0.08 | −0.57 | −0.25 |
| Tail length | −0.11 | 1.00 | 0.28 | 0.72 * | 0.90 ** | 0.04 | −0.03 | −0.36 | −0.26 | 0.22 | 0.51 | 0.21 | −0.15 | 0.55 |
| T. diameter | 0.00 | 0.28 | 1.00 | 0.51 | 0.26 | −0.12 | −0.23 | −0.12 | −0.15 | 0.05 | 0.17 | 0.21 | −0.29 | −0.03 |
| Head circ | −0.06 | 0.72 * | 0.51 | 1.00 | 0.70 * | −0.14 | −0.31 | −0.54 | −0.33 | 0.07 | 0.70 * | 0.29 | −0.18 | 0.43 |
| Total length | 0.32 | 0.90 ** | 0.26 | 0.69 * | 1.00 | 0.12 | −0.08 | −0.18 | −0.13 | 0.17 | 0.44 | 0.24 | −0.45 | 0.47 |
| Head length | 0.28 | 0.04 | −0.12 | −0.14 | 0.12 | 1.00 | 0.46 | 0.66 * | 0.57 | 0.65 * | −0.02 | 0.30 | 0.41 | 0.39 |
| Vol | 0.13 | −0.03 | −0.23 | −0.31 | −0.08 | 0.46 | 1.00 | 0.74* | 0.72 * | −0.13 | -0.677 * | −0.56 | 0.22 | 0.18 |
| Vigor | 0.55 | −0.36 | −0.12 | −0.54 | −0.18 | 0.658 * | 0.74 * | 1.00 | 0.86 ** | 0.75 * | -0.698 * | 0.75* | 0.02 | −0.13 |
| TM | 0.50 | −0.26 | −0.15 | −0.33 | −0.13 | 0.57 | 0.72 * | 0.86 ** | 1.00 | 0.95 ** | −0.49 | −0.28 | 0.18 | −0.24 |
| PM | −0.03 | 0.22 | 0.05 | 0.07 | 0.17 | 0.65 * | −0.13 | 0.75 * | 0.95 ** | 1.00 | 0.49 | 0.72 * | 0.56 | 0.22 |
| Cn | −0.26 | 0.51 | 0.17 | 0.695 * | 0.44 | −0.02 | −0.68 | −0.70 | −0.49 | 0.49 | 1.00 | 0.60 | 0.17 | 0.36 |
| EOS | 0.08 | 0.21 | 0.21 | 0.29 | 0.24 | 0.30 | −0.56 | 0.75 * | −0.28 | 0.72 * | 0.60 | 1.00 | 0.19 | 0.02 |
| CH | −0.57 | −0.15 | −0.29 | −0.18 | −0.45 | 0.41 | 0.22 | 0.02 | 0.18 | 0.56 | 0.17 | 0.19 | 1.00 | 0.16 |
| Sp.Vol | −0.25 | 0.55 | −0.03 | 0.43 | 0.47 | 0.39 | 0.18 | −0.13 | −0.24 | 0.22 | 0.36 | 0.02 | 0.16 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madrigal-Valverde, M.; Bittencourt, R.F.; Ribeiro Filho, A.L.; de Bittencourt, T.C.C.; de Matos Brandão Carneiro, I.; Maggitti, L.D.P., Jr.; de Menezes, G.F.O.; de Almeida Biscarde, C.E.; Mendes Xavier, G.; Snoeck, P.P.d.N.; et al. Feline Testicular Biometry and Gonadosomatic Index: Associations Among Conventional Measurements, Mathematical Estimates, and Seminal Parameters. Animals 2025, 15, 2191. https://doi.org/10.3390/ani15152191
Madrigal-Valverde M, Bittencourt RF, Ribeiro Filho AL, de Bittencourt TCC, de Matos Brandão Carneiro I, Maggitti LDP Jr., de Menezes GFO, de Almeida Biscarde CE, Mendes Xavier G, Snoeck PPdN, et al. Feline Testicular Biometry and Gonadosomatic Index: Associations Among Conventional Measurements, Mathematical Estimates, and Seminal Parameters. Animals. 2025; 15(15):2191. https://doi.org/10.3390/ani15152191
Chicago/Turabian StyleMadrigal-Valverde, Mónica, Rodrigo F. Bittencourt, Antonio Lisboa Ribeiro Filho, Thereza Cristina Calmon de Bittencourt, Isabella de Matos Brandão Carneiro, Luiz Di Paolo Maggitti, Jr., Gabriel Felipe Oliveira de Menezes, Carmo Emanuel de Almeida Biscarde, Gleice Mendes Xavier, Paola Pereira das Neves Snoeck, and et al. 2025. "Feline Testicular Biometry and Gonadosomatic Index: Associations Among Conventional Measurements, Mathematical Estimates, and Seminal Parameters" Animals 15, no. 15: 2191. https://doi.org/10.3390/ani15152191
APA StyleMadrigal-Valverde, M., Bittencourt, R. F., Ribeiro Filho, A. L., de Bittencourt, T. C. C., de Matos Brandão Carneiro, I., Maggitti, L. D. P., Jr., de Menezes, G. F. O., de Almeida Biscarde, C. E., Mendes Xavier, G., Snoeck, P. P. d. N., & Barbosa, L. P. (2025). Feline Testicular Biometry and Gonadosomatic Index: Associations Among Conventional Measurements, Mathematical Estimates, and Seminal Parameters. Animals, 15(15), 2191. https://doi.org/10.3390/ani15152191






