Assessing the Potential of Fecal NIRS for External Marker and Digestibility Predictions in Broilers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Origin
2.2. Reference Analysis
2.3. Spectral Collection
2.4. Chemometric Analysis
3. Results
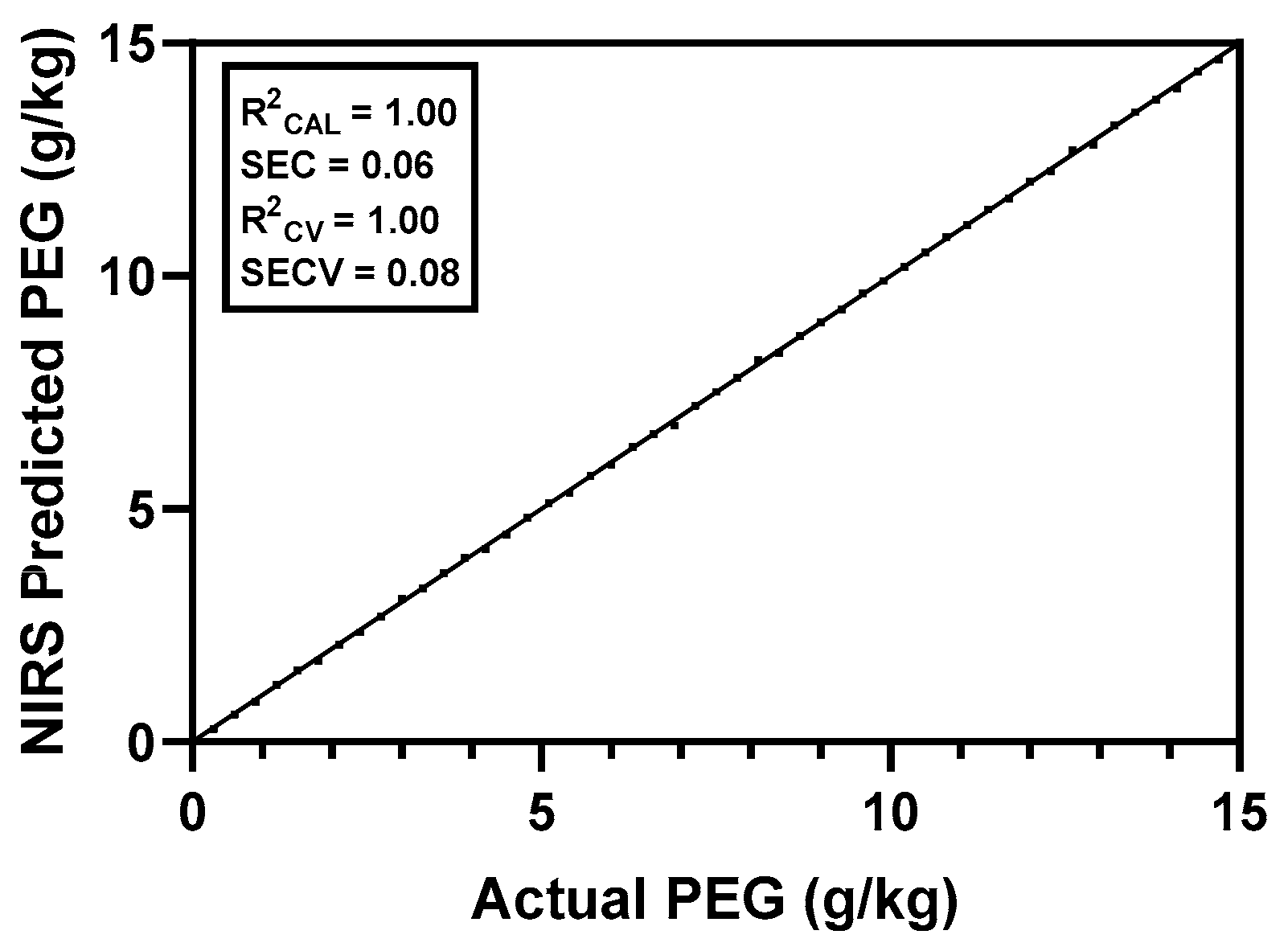
3.1. Ad Hoc PEG Calibration in Excreta
3.2. NIRS Predictive Models to Quantify Yb and Ti
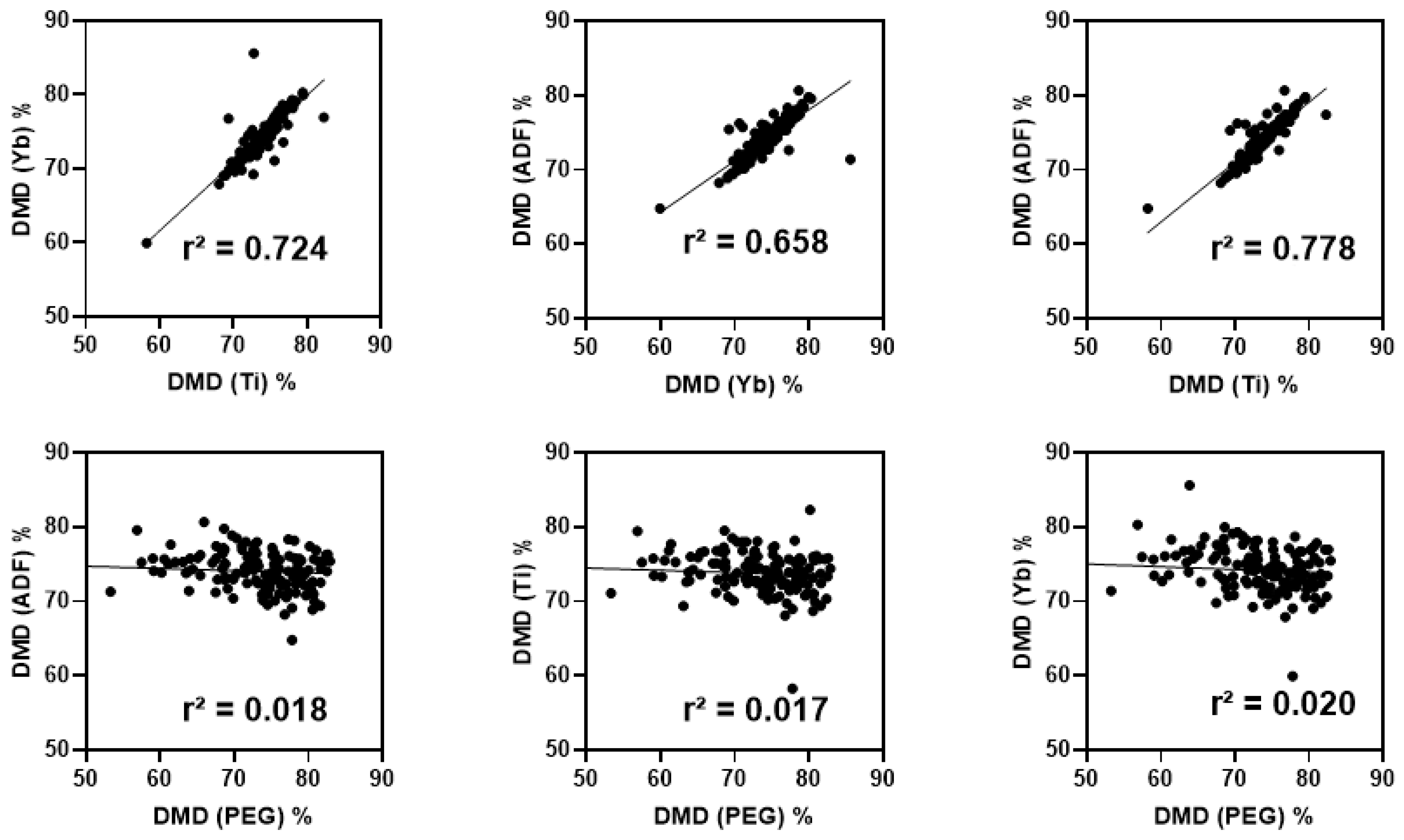
3.3. NIRS Predictive Models to Quantify Digestibility
4. Discussion
4.1. Feasibility of PEG in Broilers for Digestibility
4.2. Accuracy of NIRS Models for Yb, Ti, and Fiber Fractions, and DMD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meschy, F.; Jondreville, C.; Dourmad, J.Y.; Narcy, A.; Nys, Y. Maîtrise des rejets de phosphore dans les effluents d’élevage. Prod. Anim. 2008, 21, 79–86. [Google Scholar] [CrossRef]
- Short, F.J.; Gorton, P.; Wiseman, J.; Boorman, K.N. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996, 59, 215–221. [Google Scholar] [CrossRef]
- Scott, T.A.; Boldaji, F. Comparison of inert markers [chromic oxide or insoluble ash (Celite)] for determining apparent metabolizable energy of wheat- or barley-based broiler diets with or without enzymes. Poult. Sci. 1997, 76, 594–598. [Google Scholar] [CrossRef]
- Sales, J.; Janssens, G.P.J. The use of markers to determine energy metabolizability and nutrient digestibility in avian species. World’s Poult. Sci. J. 2003, 59, 314–327. [Google Scholar] [CrossRef]
- Jurjanz, S.; Germain, K.; Dziurla, M.A.; Juin, H.; Jondreville, C. Use of acid-insoluble ash and n-alkanes as markers of soil and plant ingestion by chickens. Anim. Feed Sci. Technol. 2014, 188, 92–101. [Google Scholar] [CrossRef]
- Hassoun, P.; Bastianelli, D.; Autran, P.; Bocquier, F. Polyethylene glycol compared with ytterbium oxide as a total faecal output marker to predict organic matter intake of dairy ewes fed indoors or at pasture. Animal 2014, 8, 1420–1426. [Google Scholar] [CrossRef]
- EFSA. EFSA statement on the review of the risks related to the exposure to the food additive titanium dioxide (E 171) performed by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES). EFSA J. 2019, 17, e05714. [Google Scholar] [CrossRef]
- Delagarde, R.; Perez-Ramirez, E.; Peyraud, J.L. Ytterbium oxide has the same accuracy as chromic oxide for estimating variations of faecal dry matter output in dairy cows fed a total mixed ration at two feeding levels. Anim. Feed Sci. Technol. 2010, 161, 121–131. [Google Scholar] [CrossRef]
- Kaikat, I.; Piquer, I.; Cerisuelo, A.; Pérez, J.F. Comparison of ytterbium oxide and acid-insoluble ash for digestibility assessment in growing pigs. In Proceedings of the 2024 International Workshop on Measurements and Applications in Veterinary and Animal Sciences (MeAVeAS 2024), Torino, Italy, 22–24 April 2024. [Google Scholar]
- Hassoun, P.; Viudes, G.; Autran, P.; Bastianelli, D.; Bocquier, F. A method for estimating dry forage intake by sheep using polyethylene glycol as a faecal marker measured with NIRS. Animal 2013, 7, 1280–1288. [Google Scholar] [CrossRef]
- Landau, S.; Friedman, S.; Devash, L.; Mabjeesh, S.J. Polyethylene glycol, determined by near-infrared reflectance spectroscopy, as a marker of fecal output in goats. J. Agric. Food Chem. 2002, 50, 1374–1378. [Google Scholar] [CrossRef]
- Decandia, M.; Giovanetti, V.; Boe, F.; Scanu, G.; Cabiddu, A.; Molle, G.; Cannas, A.; Landau, S. Faecal NIRS to assess the chemical composition and the nutritive value of dairy sheep diets. In Nutritional and Foraging Ecology of Sheep and Goats; Papachristou, T.G., Parissi, Z.M., Ben Salem, H., Morand-Fehr, P., Eds.; Options Méditerranéennes; CIHEAM: Zaragoza, Spain, 2007; Volume 85, pp. 135–139. [Google Scholar]
- Cruz-Conesa, A.; Ferré, J.; Pérez-Vendrell, A.M.; Callao, M.P.; Ruisánchez, I. Use of visible-near infrared spectroscopy to predict nutrient composition of poultry excreta. Anim. Feed Sci. Technol. 2022, 283, 115169. [Google Scholar] [CrossRef]
- Ahvenjärvi, S.; Nyholm, L.; Nousiainen, J.; Mäntysaari, E.A.; Lidauer, M. Polyethylene glycol as an indigestible marker to estimate fecal output in dairy cows. J. Dairy Sci. 2018, 101, 4245–4258. [Google Scholar] [CrossRef]
- Bastianelli, D.; Bonnal, L.; Barre, P.; Nabeneza, S.; Salgado, P.; Andueza, D. La spectrométrie dans le proche infrarouge pour la caractérisation des ressources alimentaires. INRA Prod. Anim. 2018, 31, 237–254. [Google Scholar] [CrossRef]
- Garnsworthy, P.C.; Unal, Y. Estimation of dry-matter intake and digestibility in group-fed dairy cows using near infrared reflectance spectroscopy. Anim. Sci. 2004, 79, 327–334. [Google Scholar] [CrossRef]
- Byron, B. Use of n-alkane and fecal Near Infrared Reflectance Spectroscopy (fNIRS) Methods and Traditional Prediction Equations to Estimate Intake of RFI-Divergent Beef Cattle Grazing Annual and Perennial Pastures. Master’s Thesis, University of Manitoba, Manitoba, Canada, 2018. [Google Scholar]
- Keli, A.; Andueza, D.; De Vega, A.; Guada, J.A. Validation of the n-alkane and NIRS techniques to estimate intake, digestibility and diet composition in sheep fed mixed lucerne: Ryegrass diets. Livest. Sci. 2008, 119, 42–54. [Google Scholar] [CrossRef]
- Landau, S.; Glasser, T.; Dvash, L.; Perevolotsky, A. Faecal NIRS to monitor the diet of Mediterranean goats. S. Afr. J. Anim. Sci. 2004, 34, 76–80. [Google Scholar]
- Ferreira, L.; Machado, N.; Gouvinhas, I.; Santos, S.; Celaya, R.; Rodrigues, M.; Barros, A. Application of Fourier transform infrared spectroscopy (FTIR) techniques in the mid-IR (MIR) and near-IR (NIR) spectroscopy to determine n-alkane and long-chain alcohol contents in plant species and faecal samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 280, 121544. [Google Scholar] [CrossRef]
- Landau, S.; Glasser, T.; Dvash, L. Monitoring nutrition in small ruminants with the aid of near-infrared reflectance spectroscopy (NIRS) technology: A review. Small Rumin. Res. 2006, 61, 1–11. [Google Scholar] [CrossRef]
- Acamovic, T.; Murray, I.; Paterson, R.M. The use of NIR in predicting chromic oxide in diets. In Making Light Work: Advances in Near Infrared Spectroscopy; Murray, I., Cowe, I.A., Eds.; Ian Michael Publications: Aberdeen, UK, 1992; pp. 250–252. [Google Scholar]
- Casasús, I.; Albanell, E. Prediction of faecal output and hay intake by cattle from NIRS estimates of faecal concentrations of orally-dosed polyethyleneglycol. Anim. Feed Sci. Technol. 2014, 192, 48–61. [Google Scholar] [CrossRef]
- Caja, G.; Ralha, V.M.; Albanell, E. Evaluación del polietilenglicol (PEG6000) como marcador indigestible para ovejas lecheras en estabulación o pastoreo. In XIII Jornadas Sobre Producción Animal AIDA; Congresos y Jornadas. Serie Producción Animal Asociación Interprofesional para el Desarrollo Agrario (AIDA): Zaragoza, Spain, 2009; Volume I, pp. 358–360. [Google Scholar]
- Hassoun, P.; Bastianelli, D.; Foulquié, D.; Bonnal, L.; Bocquier, F. Polyethylene glycol marker measured with NIRS gives a reliable estimate of the rangeland intake of grazing sheep. Animal 2015, 10, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, I.; Métayer, J.P.; Chartrin, P.; Mahaut, B.; Bouvarel, I.; Hogrel, P.; Bastianelli, D. La combinaison des informations issues des aliments et des fientes améliore la prédiction par SPIR de la digestibilité chez le poulet (JRA-JRFG 2013-165). In Journées de la Recherche Avicole et Palmipèdes à Foie Gras; World Poultry Science Association: La Rochelle, France, 2013. [Google Scholar]
- Kaikat, I.; Solà-Oriol, D.; Pérez, J.F. Assessing the Variability of Energy Metabolisability in Barley, Rye, and Wheat Varieties for Broiler Diets. Animals 2024, 14, 3559. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Williams, P. The RPD Statistic: A Tutorial Note. NIR News 2014, 25, 22–26. [Google Scholar] [CrossRef]
- Williams, P.C.; Sobering, D.C. How do we do it: A brief summary of the methods we use in developing near infrared calibrations. In Near Infrared Spectroscopy: The Future Waves; Davies, A.M.C., Williams, P., Eds.; NIR Publications: Chichester, UK, 1996; pp. 185–188. [Google Scholar]
- Saeys, W.; Mouazen, A.M.; Ramon, H. Potential for Onsite and Online Analysis of Pig Manure Using Visible and Near Infrared Reflectance Spectroscopy. Biosyst. Eng. 2005, 91, 393–402. [Google Scholar] [CrossRef]
- Landau, S.; Xue, B.; Dvash, L.; Friedman, S.; Mabjeesh, S.J. Polyethylene Glycol, Used to Alleviate the Negative Effects of Dietary Tannins, Can Also Serve as a Marker of Fecal Output in Goats. Small Rumin. Res. 2003, 48, 37–43. [Google Scholar] [CrossRef]
- Labussière, E.; Ganier, P.; Conde, J.A.; Janvier, E.; van Milgen, J.J. Development of a NIRS Method to Assess the Digestive Ability in Growing Pigs. In Book of Abstracts, Proceedings of the 70th Annual Meeting of the European Federation of Animal Science, Ghent, Belgium, 26–30 August 2019; European Federation of Animal Science: Rome, Italy; Volume 25, p. 604.
- Treviño, J.; Ortiz, L.; Centeno, C. Effect of Tannins from Faba Beans (Vicia faba) on the Digestion of Starch by Growing Chicks. Anim. Feed Sci. Technol. 1992, 37, 345–349. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Blümmel, M.; Becker, K. Formation of Complexes Between Polyvinyl Pyrrolidones or Polyethylene Glycols and Tannins, and Their Implication in Gas Production and True Digestibility in In Vitro Techniques. Br. J. Nutr. 1995, 73, 897–913. [Google Scholar] [CrossRef]
- De Marchi, M.; Penasa, M.; Zidi, A.; Manuelian, C.L. Invited Review: Use of Infrared Technologies for the Assessment of Dairy Products—Applications and Perspectives. J. Dairy Sci. 2018, 101, 10589–10604. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications; Wiley: Chichester, UK, 2004. [Google Scholar]
- Rinnan, Å.; van den Berg, F.; Engelsen, S.B. Review of the Most Common Pre-Processing Techniques for Near-Infrared Spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Karoui, R.; Mouazen, A.M.; Dufour, É.; Pillonel, L.; Picque, D.; Bosset, J.O.; De Baerdemaeker, J. Mid-Infrared Spectrometry: A Tool for the Determination of Chemical Parameters in Emmental Cheeses Produced During Winter. Lait 2006, 86, 83–97. [Google Scholar] [CrossRef]
- Williams, P.; Dardenne, P.; Flinn, P. Tutorial: Items to Be Included in a Report on a Near Infrared Spectroscopy Project. J. Near Infrared Spectrosc. 2017, 25, 85–90. [Google Scholar] [CrossRef]
- Fearn, T. Assessing Calibrations: SEP, RPD, RER and R2. NIR News 2002, 13, 12–13. [Google Scholar] [CrossRef]
- Yakubu, H.G.; Kovacs, Z.; Toth, T.; Bazar, G. The Recent Advances of Near-Infrared Spectroscopy in Dairy Production—A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 810–831. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, T.; Pan, X. Potential of Visible and Near-Infrared Reflectance Spectroscopy for the Determination of Rare Earth Elements in Soil. Geoderma 2017, 306, 120–126. [Google Scholar] [CrossRef]
- Chen, Z.; Świsłocka, R.; Choińska, R.; Marszałek, K.; Dąbrowska, A.; Lewandowski, W.; Lewandowska, H. Exploring the Correlation between the Molecular Structure and Biological Activities of Metal–Phenolic Compound Complexes: Research and Description of the Role of Metal Ions in Improving the Antioxidant Activities of Phenolic Compounds. Int. J. Mol. Sci. 2024, 25, 11775. [Google Scholar] [CrossRef] [PubMed]
- Bastianelli, D.; Bonnal, L.; Jaguelin-Peyraud, Y.; Noblet, J. Predicting Feed Digestibility from NIRS Analysis of Pig Faeces. Animal 2015, 9, 781–786. [Google Scholar] [CrossRef]
- Meineri, G.; Peiretti, P.G.; Masoero, G. Valutazione dell’ingestione e della digeribilità di mangimi in conigli in accrescimento tramite spettroscopia NIR del mangime e delle feci. Ital. J. Anim. Sci. 2009, 8, 75–82. [Google Scholar] [CrossRef]
- Núñez-Sánchez, N.; Marín, A.L.M.; Hernández, M.P.; Carrion, D.; Castro, G.G.; Alba, L.M.P. Faecal Near Infrared Spectroscopy (NIRS) as a Tool to Assess Rabbit’s Feed Digestibility. Livest. Sci. 2012, 150, 386–390. [Google Scholar] [CrossRef]
- Glasser, T.; Landau, S.; Ungar, E.D.; Perevolotsky, A.; Dvash, L.; Muklada, H.; Kababya, D.; Walker, J.W. A Fecal Near-Infrared Reflectance Spectroscopy-Aided Methodology to Determine Goat Dietary Composition in a Mediterranean Shrubland. J. Anim. Sci. 2008, 86, 1345–1356. [Google Scholar] [CrossRef]
- Coates, D.B.; Dixon, R.M. Developing Robust Fecal Near-Infrared Spectroscopy Calibrations to Predict Diet Dry Matter Digestibility in Cattle Consuming Tropical Forages. J. Near Infrared Spectrosc. 2011, 19, 507–519. [Google Scholar] [CrossRef]
| Item | Adaptation Diet (d 1–15) | Experimental Diets (d 16–25) |
|---|---|---|
| Ingredients | ||
| Corn | 32.28 | 32.28 |
| Wheat | 40 | - |
| Test ingredient 1 | - | 40 |
| Extruded soybean | 18 | 18 |
| Processed animal protein 2 | 8 | 8 |
| L-Lysine | 0.34 | 0.34 |
| DL-Methionine | 0.31 | 0.31 |
| L-Threonine | 0.19 | 0.19 |
| Isoleucine | 0.13 | 0.13 |
| Tryptophan | 0.02 | 0.02 |
| Salt | 0.33 | 0.33 |
| Vitamin and mineral premix 3 | 0.4 | 0.4 |
| Titanium dioxide | - | 2 g/kg |
| Ytterbium oxide | - | 50 mg/kg |
| Polyethylene glycol | - | 5 g/kg |
| In enzyme-supplemented diets | ||
| Phytase (FTU) | 1000 FTU/kg | |
| Xylanase (BXU) | 16,000 BXU/kg | |
| β-glucanase (BU). | 20,000 BU/kg | |
| Calculated composition | ||
| AME (kcal/kg) | 3246 | |
| Crude protein | 19.6 | |
| Calcium | 0.48 | |
| Phosphorus | 0.5 |
| Constituent 2 | Calibration Set | Validation Set | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | CV | Range | n | Mean | SD | CV | Range | ||
| Yb, g/kg | 147 | 0.019 | 0.002 | 10.53 | 0.013–0.032 | 45 | 0.018 | 0.002 | 11.11 | 0.012–0.023 | |
| Ti, g/kg | 147 | 0.503 | 0.057 | 11.33 | 0.320–0.730 | 45 | 0.485 | 0.054 | 11.13 | 0.310–0.800 | |
| DMDYb, % | 147 | 74.7 | 3.01 | 4.03 | 61.94–85.60 | 45 | 73.72 | 3.50 | 4.75 | 59.92–79.61 | |
| DMDTi, % | 147 | 74.20 | 2.89 | 3.89 | 59.96–82.27 | 45 | 73.35 | 3.37 | 4.59 | 58.25–78.23 | |
| DMDADF, % | 115 | 74.24 | 2.46 | 3.31 | 68.22–80.64 | 29 | 73.43 | 2.76 | 3.76 | 64.77–78.84 | |
| Constituent 2 | Calibration Set | Validation Set | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mathematical Treatment 3 | Scatter Correction 4 | R2CAL | SEC | R2cv | SECV | R2VAL | SEP | Bias | Slope | RPD | RER | ||
| Yb | 2.5.5.1 | MSC | 0.87 | 0.001 | 0.74 | 0.001 | 0.67 | 0.001 | 0 | 0.862 | 2.00 | 19.00 | |
| Ti | 2.4.4.1 | SNV + D | 0.90 | 0.016 | 0.78 | 0.023 | 0.73 | 0.025 | 0 | 0.885 | 2.28 | 16.40 | |
| DMDYb | 2.4.4.1 | SNV + D | 0.89 | 0.960 | 0.75 | 1.43 | 0.68 | 1.56 | 0.019 | 0.863 | 1.93 | 15.17 | |
| DMDTi | 2.4.4.1 | MSC | 0.91 | 0.851 | 0.80 | 1.22 | 0.77 | 1.20 | 0.014 | 0.900 | 2.41 | 18.59 | |
| DMDADF | 2.4.4.1 | SNV + D | 0.90 | 0.793 | 0.66 | 1.41 | 0.25 | 2.06 | 0.440 | 0.477 | 1.19 | 6.62 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tej, O.; Albanell, E.; Kaikat, I.; Manuelian, C.L. Assessing the Potential of Fecal NIRS for External Marker and Digestibility Predictions in Broilers. Animals 2025, 15, 2181. https://doi.org/10.3390/ani15152181
Tej O, Albanell E, Kaikat I, Manuelian CL. Assessing the Potential of Fecal NIRS for External Marker and Digestibility Predictions in Broilers. Animals. 2025; 15(15):2181. https://doi.org/10.3390/ani15152181
Chicago/Turabian StyleTej, Oussama, Elena Albanell, Ibtissam Kaikat, and Carmen L. Manuelian. 2025. "Assessing the Potential of Fecal NIRS for External Marker and Digestibility Predictions in Broilers" Animals 15, no. 15: 2181. https://doi.org/10.3390/ani15152181
APA StyleTej, O., Albanell, E., Kaikat, I., & Manuelian, C. L. (2025). Assessing the Potential of Fecal NIRS for External Marker and Digestibility Predictions in Broilers. Animals, 15(15), 2181. https://doi.org/10.3390/ani15152181






